Abstract
Background
Intracranial artery atherosclerotic stenosis (ICAS) is among the causes of intracranial large artery occlusion (LVO). The optimal treatment strategy for patients with ischemic stroke due to ICAS-related LVO remains unclear. In this retrospective case series, we discussed our experience with direct angioplasty as frontline therapy for ICAS-related LVO.
Methods
We extracted data for patients who had a known pre-existing ICAS and undergone direct angioplasty as frontline therapy for ICAS-related LVO in the anterior circulation at our institution between January 2019 and December 2019. We analysed procedural details, the degree of reperfusion, functional outcomes, and complications. Successful reperfusion was defined as a modified Treatment in Cerebral Ischemia (mTICI) score of 2 b − 3. Functional outcomes at 90 days were assessed using modified Rankin Scale (mRS) scores (good outcome: mRS of 0–2).
Results
We analysed data for five patients (mean age: 51.6 ± 11 years). The mean time from symptom onset to recanalization was 371 ± 38.6 min. Occlusions involved the first segment of the middle cerebral artery in four patients and the intracranial internal carotid artery in one patient. Successful reperfusion was achieved in four (80%) patients. The remaining patient (20%) underwent intracranial stenting as rescue therapy, achieving a final mTICI of 2a. No re-occlusion was observed on follow-up images. Four patients (80%) achieved good outcomes at 90 days. There were no cases of symptomatic intracranial hemorrhage, although asymptomatic intracranial haemorrhage was observed in one patient.
Conclusion
Direct angioplasty may represent an alternative treatment strategy in patients with acute ischemic stroke due to known ICAS-related LVO.
Keywords: Ischemic stroke, thrombectomy, atherosclerosis, stenosis, angioplasty
Introduction
Since the publication of findings from five randomised clinical trials, endovascular treatment has become standard for patients with acute ischemic stroke due to large vessel occlusion (LVO).1–5 Severe atherosclerotic stenosis of the intracranial arteries (ICAS) is among the major causes of large intracranial artery occlusion in Asian and Hispanic populations and accounts for approximately 12–30% of all strokes due to LVO.6–8 However, the optimal treatment strategy for patients with ischemic stroke due to ICAS-related LVO remains unclear.9 ICAS-related LVO is often refractory to stent-retriever thrombectomy.10 Rescue therapy such as angioplasty and/or stenting is usually necessary for successful reperfusion in patients with ICAS-related LVO.9 We assumed that skipping thrombectomy prior to angioplasty may lead to faster recanalization and improved outcomes. In the present study, we aimed to analyse data for five patients with acute ischemic stroke due to ICAS-related LVO, focusing on indications of angioplasty as frontline therapy in these patients.
Methods
The present study was approved by the appropriate institutional ethics committee. All patients provided written informed consent.
All patients undergoing endovascular treatment for ischemic stroke at a single institution were prospectively registered in an electronic database. For this study, we extracted data for patients with acute ischemic stroke due to ICAS-related LVO in the anterior circulation who had undergone angioplasty as frontline therapy between January 2019 and December 2019. All patients had a diagnosis of severe ICAS as determined via previous magnetic resonance angiography (MRA). ICAS-related LVO was defined as an occlusion located at the site of ICAS on previous MRA. Direct angioplasty was indicated when computed tomography (CT) angiography or digital subtraction angiography (DSA) revealed occlusions with short thrombus (clot burden score (CBS): 7 to 10).11
The following data were extracted and analysed: demographic characteristics, baseline National Institutes of Health Stroke Scale (NIHSS) scores, baseline Alberta Stroke Program Early CT Scores (ASPECTS), collateral status, CBS, previous stroke, intravenous thrombolysis (IV), time from stroke onset to groin puncture, and procedure duration.
Technical procedures
All interventional procedures were performed under conscious sedation without heparinisation. A microcatheter was navigated with a guidewire (Traxcess 14, Microvention, Aliso Viejo, CA, USA) to the distal lumen of the occlusion site. Following angiography performed, each patient underwent reperfusion therapy using a semi-compliant intracranial balloon catheter (Neuro RX, Sinomed, Tianjin, China). Balloon sizes were determined based on the surgeon’s discretion. The diameter of the balloon should approximate the lesser of the vessel diameters just proximal and distal to the occlusion. The balloon was inflated under nominal pressure for 30 seconds. Patients then received an intra-arterial infusion of tirofiban (Grandpharma, Wuhan, China), a glycoprotein IIb/IIIa inhibitor. Tirofiban was injected at a rate of 1.5 ml/min through the guiding catheter. The total dosage of tirofiban typically ranged from 0.5 mg to 1.0 mg during the procedure.12 DSA was performed immediately after the infusion and was repeated 20 minutes later to assess recanalization. Procedures were completed when we observed successful reperfusion without the tendency for re-occlusion. Stent-retriever thrombectomy or intracranial stenting was performed as a rescue therapy in cases of unsuccessful recanalization. IV tirofiban was administered at a maintenance dose of 0.25 mg/h for 24 h. Aspirin (100 mg/day) and clopidogrel (75 mg/day) were administered 4 hours prior to the discontinuation of tirofiban.
Outcomes and complications
The degree of reperfusion was assessed on the last DSA angiogram, based on the modified Treatment in Cerebral Ischemia (mTICI) score. Successful reperfusion was defined as an mTICI score of 2 b − 3 on the last angiogram. Functional outcomes at 90 days were assessed using modified Rankin Scale (mRS) scores. Scores of 0 to 2 were considered indicative of good outcomes. Complications included vessel perforation, arterial dissection, intracranial haemorrhage, progression of stroke, embolism in new territories, and recurrent stroke in the same territories at the 90-day follow-up. Intracranial hemorrhage was assessed via non-contrast CT 24 h following the procedure. Symptomatic hemorrhage was defined as an increase of 4 or more points on the NIHSS.
Statistical analysis
Data were analysed using descriptive statistics. Continuous variables were expressed as the mean ± standard deviation or as medians and quartiles. Categorical variables were expressed as absolute values (i.e., number of patients) and relative frequencies (percentages).
Results
Patient characteristics
A total of 192 patients with ischemic stroke underwent endovascular treatment between January 2019 and December 2019. LVO due to ICAS in the anterior circulation was observed in 12.8% (19/149) of patients. Among them, five patients (mean age: 57 ± 11.2 years) underwent angioplasty as frontline therapy and were included in the present series. All four patients (80%) with previous stroke exhibited preoperative mRS scores of 0. Occlusions were located on the left side in four patients (80%). Occlusions involved the first segment of the middle cerebral artery (M1) in four patients (80%) and the intracranial internal carotid artery in one patient (20%). The mean baseline NIHSS score was 17.6 (range: 11–26). Two patients received intravenous thrombolytic agents prior to endovascular treatment. The mean time from symptom onset to recanalization was 304 ± 62.6 min. The duration of the procedure ranged from 30 to 160 min. Detailed patient characteristics are presented in Table 1.
Table 1.
Clinical characteristics.
| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Occlusion location | Left M1 | Right M1 | Left M1 | Left M1 | Left ICA |
| Hypertension | Yes | Yes | Yes | Yes | Yes |
| Hypercholesterolemia | Yes | No | No | No | No |
| Diabetes mellitus | No | No | No | Yes | Yes |
| Previous stroke | Yes | Yes | No | Yes | Yes |
| Baseline NIHSS | 16 | 11 | 20 | 26 | 15 |
| Baseline ASPECTS | 10 | 7 | 9 | 9 | 10 |
| Intravenous thrombolysis | No | Yes | No | No | Yes |
| Time from onset to groin puncture (min) | 320 | 295 | 390 | 215 | 300 |
| Time from puncture to recanalization min) | 30 | 40 | 45 | 160 | 60 |
M1, first segment of the middle cerebral artery; ICA, internal carotid artery; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score.
Outcomes and complications
Successful reperfusion was achieved in four patients (80%), two patients (40%) exhibited complete reperfusion (mTICI: 3). The patient who did not exhibit successful reperfusion following angioplasty underwent stenting as rescue therapy (final mTICI: 2a). Good outcomes were observed in two patients (40%) at the 90-day follow-up. Four patients (80%) achieved favourable outcomes (mRS: 0–3). We observed no recurrence of ischemic stroke among these five patients, although asymptomatic intracranial haemorrhage was noted in one patient (20%). No other complications were reported (Table 2).
Table 2.
Outcomes and complications.
| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| mTICI score | 3 | 2b | 2b | 2a | 3 |
| Rescue therapy | No | No | No | Stenting | No |
| Asymptomatic hemorrhage | No | No | No | Yes | No |
| mRS score at 90 days | 0 | 1 | 3 | 4 | 3 |
mTICI, modified Treatment in Cerebral Ischemia; mRS, modified Rankin Scale.
Case illustration
Case 1
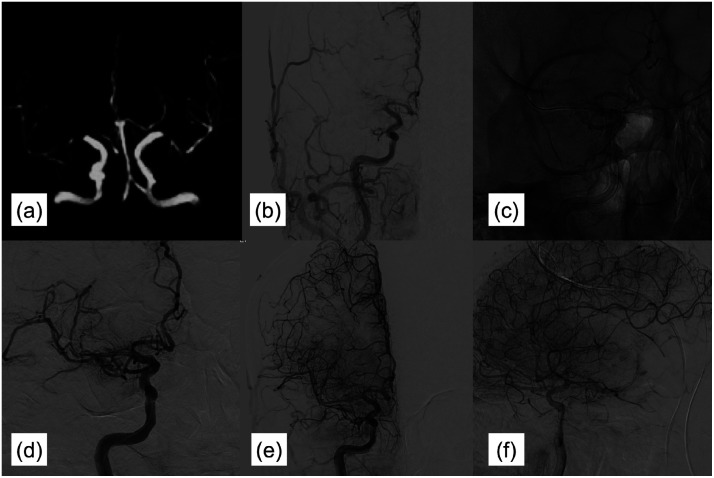
An adult male (patient No. 1) presented 5 hours after the onset of left hemispheric stroke and aphasia. His baseline NIHSS and ASPECTS scores were 16 and 10, respectively. Three months earlier, he had experienced a minor stroke accompanied by weakness in his right limbs. MRA performed 3 months prior to admission revealed severe stenosis in the left M1 (Figure 1(a)). DSA during the more recent attack indicated an occlusion of the left M1 with good collaterals. Retro-flow reconstituted the distal portion of M1 (Figure 1(b)), suggestive of ICAS-related LVO. Direct angioplasty was performed using a balloon catheter (Neuro RX 2.0*15, Sinomed, Tianjin, China) (Figure 1(c)). Following angioplasty, his mTICI score was 3 (Figure 1(d) to (f)). The time from onset to groin puncture was 320 min, while the duration of the procedure was 30 min. Tirofiban was administered for 24 h, followed by aspirin and clopidogrel. The patient recovered immediately after the procedure, and his mRS score at 90 days was 0. No recurrence of stroke or transient ischemic attack occurred within this 90-day period.
Figure 1.
Images of case 1. (a) MRA performed 3 months prior to admission revealed severe stenosis in the left M1. (b) DSA during the more recent attack indicated occlusion of the left M1 with good collaterals. Retro-flow reconstituted the distal portion of M1. (c) Direct angioplasty was performed using a balloon catheter. (d–f) Angioplasty achieved an mTICI score of 3. MRA, magnetic resonance angiography; M1, first segment of the middle cerebral artery; DSA, digital subtraction angiography; mTICI: modified Treatment in Cerebral Ischemia.
Case 2
An adult male (patient No. 2) presented 6.5 hours after the onset of right hemispheric stroke. His baseline NIHSS and ASPECTS scores were 11 and 7, respectively. Two months earlier, he had experienced a minor stroke presented with weakness in his left limbs. MRA performed 2 months prior to admission revealed severe stenosis in both M1 (Figure 2(a)). DSA during the more recent attack indicated an occlusion of the right M1 (Figure 2(b)), suggestive of ICAS-related LVO. Direct angioplasty was performed using a balloon catheter (Neuro RX 1.5*10, Sinomed, Tianjin, China) (Figure 2(c)). Following angioplasty, his mTICI score was 2 b (Figure 2(d) to (f)). The time from onset to groin puncture was 295 min, while the duration of the procedure was 40 min. Tirofiban was administered for 24 h, followed by aspirin and clopidogrel. The patient’s mRS score at 90 days was 1. No recurrence of stroke or transient ischemic attack occurred within this 90-day period.
Figure 2.
Images of case 2. (a) MRA performed 2 months prior to admission revealed severe stenosis in both M1. (b) DSA during the more recent attack indicated occlusion of the right M1. (c) Direct angioplasty was performed using a balloon catheter. (d–f) Angioplasty achieved an mTICI score of 2 b. MRA, magnetic resonance angiography; M1, first segment of the middle cerebral artery; DSA, digital subtraction angiography; mTICI: modified Treatment in Cerebral Ischemia.
Discussion
In the present study, we summarised data for patients with ischemic stroke due to ICAS-related LVO who had a known pre-existing stenosis in their artery and undergone direct angioplasty as frontline therapy at our institution. The rate of successful reperfusion following direct angioplasty was high, and no severe complications were observed.
ICAS-related LVO occurs when fixed intracranial stenosis is observed at the site of occlusion following thrombectomy.13 Although the diagnosis of ICAS-related LVO remains challenging, the presence of progressive symptoms and/or a history of atherosclerotic disease may aid in diagnosis. Eighty percent of patients in our study had previously experienced a stroke, and all patients exhibited intracranial artery stenosis on previous MRA images. Furthermore, more recent DSA images revealed occlusions at the site of previous ICAS in our patients. Such findings were used to make definitive diagnoses of ICAS-related LVO.
Current guidelines recommend stent-retriever or aspiration thrombectomy for LVO.14 However, the optimal treatment strategy for ICAS-related LVO remains to be determined, given that performing thrombectomy first may lead to thrombus removal without dilation of the atherosclerotic plaque. Furthermore, thrombectomy may lead to injury of the intima, resulting in immediate re-occlusion.15 Indeed, only a portion of patients with ICAS-related LVO achieve successful reperfusion following thrombectomy.16 Previous studies have noted that more than one third of patients exhibit immediate re-occlusion following thrombectomy, which typically necessitates rescue therapy.17 Jia et al.18 reported that 63.8% of patients accepted rescue therapy following re-occlusion, while another multicentre prospective cohort study reported that rescue therapy was performed in 71.7% of patients.19 When performed as rescue therapy, angioplasty and/or stenting can reduce the risk of re-occlusion in patients with ICAS-related LVO, without increasing the risk of intracranial haemorrhage. Although the final rate of successful reperfusion was similar, previous studies have indicated that clinical outcomes are poorer among patients with ICAS than among those without ICAS.20,21 This finding may be attributable to the long procedure duration in patients with ICAS: One study noted that mean procedure times (155 min vs. 40 min) were significantly longer in patients with ICAS-related LVO undergoing rescue stenting or angioplasty.22 Skipping thrombectomy prior to angioplasty may lead to faster recanalization and improved outcomes. In our study, the procedure duration was under 60 min in most cases, similar to findings reported in a previous study.23
Several studies have highlighted the safety and efficacy of angioplasty as rescue treatment following thrombectomy in patients with ICAS-related LVO.24,25 Use of a balloon catheter for angioplasty can increase the diameter of the lumen lesion and reduce the risk of intra-procedure re-occlusion. Tokunaga et al.26 reported that direct angioplasty with or without intra-arterial thrombolysis for acute LVO is associated with a low risk of haemorrhagic complications. Nakano et al.27 also reported a high rate of recanalization and good outcomes in patients with LVO who had undergone direct angioplasty. In accordance with these findings, successful recanalization was achieved in 80% of our patients without severe complications, with 40% exhibiting good outcomes.
A potential disadvantage of direct angioplasty for ICAS-related LVO is the risk of distal embolisation.28 Angioplasty can fragment relatively long thrombus, which can then migrate distally. To avoid distal embolization, thrombus length should be evaluated prior to angioplasty. A CBS of 7–10 on CT angiography indicates a short thrombus and has been associated with good outcomes following endovascular treatment.11 Thrombus length can also be assessed using DSA. Thrombus length is defined as the distance between the most distal filling of the blocked artery and the most proximal filling of the closest collateral vessel.29 When collaterals are absent, the distal margin of the thrombus is defined based on the proximal retrograde flow of contrast on a microcatheter angiogram. Long thrombus has also been associated with poor clinical outcomes.30 Thus, patients with longer thrombus were excluded from the present study and underwent thrombectomy first. No cases of distal embolization were observed in our series.
The present study possesses some limitations of note, including its small sample size. In addition, although we extracted data from a prospective database, the case series was retrospective in nature. As such, we were unable to examine follow-up images or the degree of long-term reperfusion. Further randomised studies are required to determine the optimal treatment strategy for patients with ischemic stroke due to ICAS-related LVO.
Conclusion
Direct angioplasty may represent an alternative treatment strategy in patients with acute ischemic stroke due to known ICAS-related LVO.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
This study was approved by the ethics committee of The First Affiliated Hospital of Harbin Medical University.
Declaration of conflicting interests
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Huaizhang Shi https://orcid.org/0000-0003-3150-6234
References
- 1.Berkhemer OA, Fransen PS, Beumer D, MR CLEAN Investigators et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Hong JM, Lee KS, et al. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke 2016; 18: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek JH, Kim BM, Yoo J, et al. Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke 2017; 48: 2746–2752. [DOI] [PubMed] [Google Scholar]
- 9.Al Kasab S, Almadidy Z, Spiotta AM, et al. Endovascular treatment for AIS with underlying ICAD. J Neurointerv Surg 2017; 9: 948–951. [DOI] [PubMed] [Google Scholar]
- 10.Baek JH, Kim BM, Heo JH, et al. Outcomes of endovascular treatment for acute intracranial atherosclerosis-related large vessel occlusion. Stroke 2018; 49: 2699–2705. [DOI] [PubMed] [Google Scholar]
- 11.Treurniet KM, Yoo AJ, Berkhemer OA, et al. Clot burden score on baseline computerized tomographic angiography and intra-arterial treatment effect in acute ischemic stroke. Stroke 2016; 47: 2972–2978. [DOI] [PubMed] [Google Scholar]
- 12.Yu T, Lin Y, Jin A, et al. Safety and efficiency of low dose intra-arterial tirofiban in mechanical thrombectomy during acute ischemic stroke. Curr Neurovasc Res 2018; 15: 145–150. [DOI] [PubMed] [Google Scholar]
- 13.Baek JH, Kim BM. Angiographical identification of intracranial, atherosclerosis-related, large vessel occlusion in endovascular treatment. Front Neurol 2019; 10: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Yoon W, Kim SK, et al. Endovascular treatment for emergent large vessel occlusion due to severe intracranial atherosclerotic stenosis. J Neurosurg 2018; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Lee SJ, Hong JM, et al. Solitaire thrombectomy for acute stroke due to intracranial atherosclerosis-related occlusion: Rose assist study. Front Neurol 2018; 9: 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang ACO, Orru E, Klostranec JM, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke 2019; 50: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 18.Jia B, Feng L, Liebeskind DS, EAST Study Group et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018; 10: 746–750. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Chang W, Wu D, et al. Angioplasty and/or stenting after thrombectomy in patients with underlying intracranial atherosclerotic stenosis. Neuroradiology 2019; 61: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 20.Kim GE, Yoon W, Kim SK, et al. Incidence and clinical significance of acute reocclusion after emergent angioplasty or stenting for underlying intracranial stenosis in patients with acute stroke. AJNR Am J Neuroradiol 2016; 37: 1690–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JS, Lee SJ, Yoo JS, et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J Stroke 2018; 20: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrocky T, Kaesmacher J, Bellwald S, et al. Stent-retriever thrombectomy and rescue treatment of m1 occlusions due to underlying intracranial atherosclerotic stenosis: cohort analysis and review of the literature. Cardiovasc Intervent Radiol 2019; 42: 863–872. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Lin M, Wang S, et al. Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion. Interv Neuroradiol 2018; 24: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R, Vora NA, Horowitz MB, et al. Multimodal reperfusion therapy for acute ischemic stroke: factors predicting vessel recanalization. Stroke 2006; 37: 986–990. [DOI] [PubMed] [Google Scholar]
- 25.Baek JH, Kim BM, Kim DJ, et al. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 2016; 47: 2360–2363. [DOI] [PubMed] [Google Scholar]
- 26.Tokunaga K, Sugiu K, Yoshino K, et al. Percutaneous balloon angioplasty for acute occlusion of intracranial arteries. Neurosurgery 2010; 67: ons189–196; discussion ons, 196–197. [DOI] [PubMed] [Google Scholar]
- 27.Nakano S, Iseda T, Yoneyama T, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke 2002; 33: 2872–2876. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery 2002; 51: 1319–1327. discussion 27-29. [DOI] [PubMed] [Google Scholar]
- 29.Polito V, La Piana R, Del Pilar Cortes M, et al. Assessment of clot length with multiphase CT angiography in patients with acute ischemic stroke. Neuroradiol J 2017; 30: 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganeshan R, Nave AH, Scheitz JF, et al. Assessment of thrombus length in acute ischemic stroke by post-contrast magnetic resonance angiography. J Neurointerv Surg 2018; 10: 756–760. [DOI] [PubMed] [Google Scholar]