Abstract
Introduction
Endovascular dural venous sinus stenting (DVSS) has emerged as a safe and effective therapy for idiopathic intracranial hypertension (IIH) in patients with transverse-sinus stenosis associated with an elevated mean pressure gradient (MPG). The typical antegrade approach, jugular to sigmoid to transverse, is not always technically feasible due to the degree of stenosis and other anatomic factors. To this point, there has been no reported cases of contralateral DVSS. We describe four cases of DVSS from a contralateral transverse-sigmoid sinus approach.
Methods
We describe 4 patients presenting with symptoms of IIH between 2019 and 2020 who we treated with contralateral transverse-sigmoid sinus stenting. Cases were reviewed for clinical data including initial presenting symptoms, devices used, other attempted IIH treatments, and follow up symptoms.
Results
Four female patients were identified and treated under general endotracheal anesthesia. Stenoses measured 72%, 78%, 67%, and 70% with MPGs across the transverse-sigmoid sinus of 19, 16, 9 and 13 mmHg, respectively. Post-stenting MPGs were 1, 0, 1 and 1 mmHg, respectively. Three patients had complete resolution of symptoms and 1 had partial resolution. No complications occurred.
Conclusions
This case series demonstrates successful transverse-sigmoid sinus stenting from the contralateral dural sinus and provides an alternative approach to DVSS in patients with IIH.
Keywords: Idiopathic intracranial hypertension, dural venous sinus stenting, endovascular procedure
Introduction
Endovascular dural venous sinus stenting (DVSS) has emerged as a safe and effective therapy for idiopathic intracranial hypertension (IIH) with an elevated mean pressure gradient (MPG) across the transverse-sigmoid sinus.1–3 IIH causes significant morbidity including disabling headache, permanent visual loss, and symptomatic pulsatile tinnitus. Currently the gold standard intervention for medically refractory patients remains CSF shunting and optic nerve sheath fenestration in patients with severe visual loss.
In some cases the retrograde approach, jugular to sigmoid to transverse, is not technically feasible due to the degree of stenosis or other anatomic factors. To this point, there has been no reported cases of DVSS from the contralateral sinus system. Herein we describe four cases of dural sinus stenting with a contralateral transverse to sigmoid sinus approach.
Methods
We identified patients with IIH presenting with transverse-sinus stenosis associated with a significant MPG. Patients were treated between March 2019 and January 2020. Variables collected included description of clinical presentation, other treatment modalities, devices used, degree of stenosis, pre- and post-stent MPGs, type of antiplatelet medications used, and presence or resolution of symptoms on follow up.
Endovascular technique
All cases were performed through the common femoral vein and under general endotracheal anesthesia. In three cases a 6 F 90 cm Shuttle sheath (Cook Medical, Bloomington, IN) was used as the main delivery system for the stent. In the fourth case an 8 F TracStar 95 cm sheath (Imperative Care, Campbell, CA) was used as the stent delivery system. In cases 1, 2, and 4, a Zilver Vascular stent (Cook Medical, Bloomington, IN) was used. In case 3, a Carotid Wallstent (Boston Scientific, Marlborough, MA) was used.
In all four cases the degree of high-grade stenosis and the vector at which the tip of the stent delivery catheter entered the transverse sinus using the retrograde approach resulted in multiple failed attempts to advance the stent delivery catheter despite using a variety of microwires and catheters. To prevent vascular injury and potential perforation, the decision was made to place the stent from the contralateral dural sinus system. In all four cases the contralateral dural sinus system was hypoplastic and had stenosis at the transverse-sigmoid sinus junction.
Under fluoroscopic guidance the sheath was advanced over a diagnostic catheter into the contralateral transverse sinus. The stent delivery catheter was then advanced over the microwire, across the torcula, and successfully deployed in the stenotic dural sinus. Balloon angioplasty, via the contralateral dural sinus system, was performed in all cases due to significant post deployment residual stenosis.
Post-stenting mean sinus pressures were transduced through the microcatheter along the sigmoid sinus transverse sinus, and torcula, demonstrating normalization of the MPG and resolution of the stenosis.
Results
Four female patients underwent contralateral transverse-sigmoid sinus stenting for treatment of IIH due to transverse-sinus stenosis associated with a significant MPG. Each patient initially presented with typical symptoms of IIH including headache, pulsatile tinnitus, and/or visual disturbances including diplopia, visual obscurations, or blurriness. Average age was 39.50 years (30-57). Each patient was initially treated with medical therapy with adequate doses of acetazolamide prior to stenting.
Patient 1 had left transverse-sigmoid sinus stenosis (TSSS) measuring 72% with an MPG of 19 mmHg and a post-stenting MPG of 1 mmHg (Figure 1). Patient 2 had right TSSS measuring 78%. The pre-stent MPG was 16 mmHg and was 0 mmHg following stent placement (Figure 2). Patient 3 had left TSSS measuring 67%. The pre-stent MPG was 9 mmHg and was 1 mmHg after stent placement (Figure 3). Patient 4 had right TSSS measuring 70%. The pre-stent MPG was 13 mmHg and was 1 mmHg following stent placement.
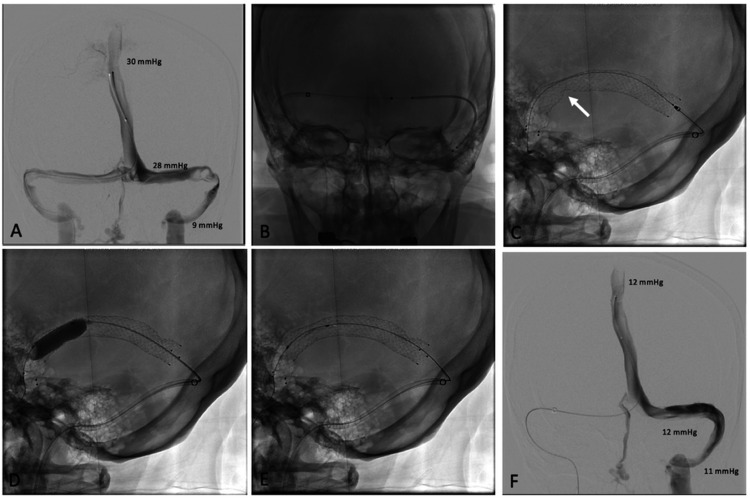
Figure 1.
(a) Cerebral venogram of patient 1 demonstrating left transverse-sigmoid sinus stenosis due to multiple enlarged arachnoid granulations. (b) Stent delivery catheter being advanced over the microwire, across the torcula, and successfully deployed into the stenotic dural sinus. (c) Stent deployment being performed via the contralateral dural sinus system. (d) Balloon angioplasty being performed via the contralateral dural sinus system. (e) Post-angioplasty. (f) Mean sinus pressures transduced through the microcatheter demonstrating normalization of the MPG.
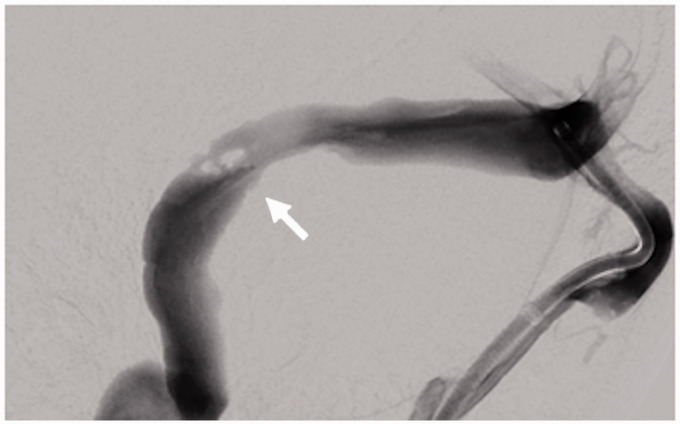
Figure 2.
Cerebral venogram lateral image of patient 2 demonstrating a dural sinus trabeculum (white arrow) in the stenotic segment. Due to the angle of entry into the transverse sinus, the stent delivery catheter repeatedly abutted the trabeculum and could not be redirected into the transverse sinus.
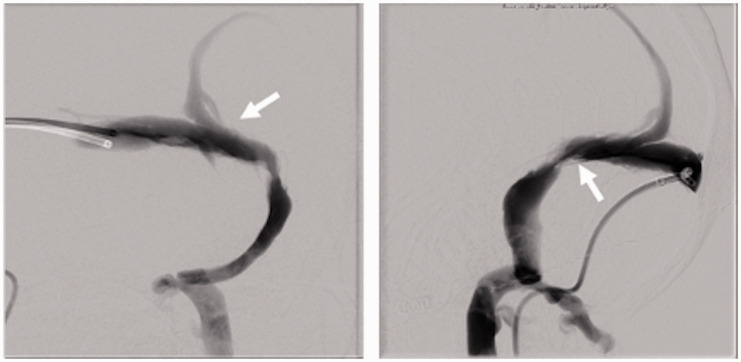
Figure 3.
Cerebral venogram AP and lateral images of patient 3 demonstrating enlarged cortical vein (white arrow) immediately above the stenotic segment. Due to the angle of entry into the transverse sinus, the stent delivery catheter repeatedly entered the cortical vein and could not be redirected into the transverse sinus.
No patients experienced procedural complications related to stent placement including any post-procedural worsening of symptoms, stroke, venous dissection, bleeding, or hematoma formation at the access site. Each patient was discharged on dual antiplatelet therapy for 6 months, with the exception of patient 4 was who had been on prior therapy with apixaban.
There were no delayed complications encountered on follow up. All patients noted improvement in their main symptom (headache) after dural venous sinus stenting. Patients 1 and 2 had complete resolution of their initial symptoms on follow up evaluation at 6 months. Patient 3 had complete resolution of tinnitus with some improvement in headache at time of follow up. Patient 4 reported some improvement in headache, but initial symptoms of tinnitus and blurry vision remained on follow up (Table 1).
Table 1.
Summary of patient characteristics, treatment, and outcome.
| Patient | Clinical presentation | Pre-stent treatment | LP opening pressure | Devices used | Stenosis | Pre-stent MPG | Post-stent MPG | Antiplatelet | Follow-up | Etiology of stenosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Headache Pulsatile Tinnitus Blurry Vision |
ACTZ 250mg BID |
34 cm H20 | Zilver 8 mm × 80 mm stent; Sterling 8 mm × 20 mm balloon | 72% | 19 | 1 | ASA Clopidogrel |
Resolution of Symptoms | Enlarged Arachnoid Granulations |
| 2 | Headache Tinnitus |
ACTZ 500mg BID |
11 cm H20 | Wallstent 8 mm × 36 mm stent; Sterling 7 mm × 40 mm balloon | 78% | 16 | 0 | ASA Prasugrel |
Resolution of Symptoms | Trabeculum |
| 3 | Headache Pulsatile Tinnitus |
ACTZ 1000mg BID |
28 cm H20 | Zilver 8 mm × 80 mm stent; Sterling 7 mm × 20 mm balloon | 67% | 9 | 1 | ASA Clopidogrel |
Resolution of pulsatile tinnitus | Cortical Vein Ostium |
| 4 | Headache Tinnitus Blurred Vision Papilledema Dural Sinus Thrombosis |
ACTZ 1000mg BID |
44 cm H20 | Zilver 8 mm × 80 mm stent; Sterling 6 mm × 20 mm balloon | 70% | 13 | 1 | Ticagrelor Apixaban |
Headache improvement, still with tinnitus and blurry vision | Septum and Trabeculae |
Discussion
Dural venous sinus stenting was first reported as a treatment by Higgins et al (2003).4 Since then it has become an increasingly performed procedure in patients with IIH who have a hemodynamically significant pressure gradient (typically ≥8 mmHg). These pressure gradients are often a result of high-grade stenoses within the dural sinus system. To date there have been 29 case series of DVSS for IIH reported in the literature.5,6 We describe the first series of patients who underwent DVSS via the contralateral dural sinus system
Multiple factors can prohibit stenting via the antegrade approach, in addition to the degree of stenosis. These include arachnoid granulations, fibrous trabeculae and septae, blind pouches, the angle of the stenotic segment, and the presence of a cortical vein draining into the dural sinus.7 Case 1 had multiple enlarged arachnoid granulations above the stenotic segment. Case 2 had a trabecula in the stenosis. Case 3 had a large cortical vein draining into the lateral transverse sinus, just above the stenotic segment. The sheath could not be advanced into the transverse sinus and the tip of the stent delivery catheter kept abutting the ostium of the cortical vein, despite using stiffer microwires to attempt to change the angle of entry into the transverse sinus. To avoid potential avulsion of the cortical vein, the contralateral approach was used. Case 4 initially had bilateral transverse and sigmoid sinus thromboses, then presented 18 months later with symptomatic pulsatile tinnitus due turbulent venous drainage from partial recanalization of her right transverse-sigmoid sinus junction. Catheter venography demonstrated a septum in the stenosis. In cases 1, 2, and 4, the tip of the stent delivery catheter kept abutting the physical obstruction. Similar to case 3, using stiffer microwires did not change the angle of entry into the transverse sinus. Delivering the stent delivery catheter from the contralateral dural sinus system changed the angle of entry, now into the sigmoid sinus, which resulted in the stent delivery catheter not abutting the structural obstruction or cortical vein.
A 90 cm Shuttle sheath was used in the first three cases and with the introduction of the 95 cm TracStar sheath, this was used in the fourth case. The additional five centimeters of length is helpful in both antegrade and retrograde approaches, as the 90 cm sheath may not be long enough to advance into the transverse sinus. Though the 90 cm sheath was used in the first 2 cases, enough length was lost crossing to the left internal jugular vein that it could not be advanced into the left transverse sinus. The 90 cm sheath was able to be advanced into the right transverse sinuses in these first two cases. In case 2, the Zilver stent delivery catheter could not be advanced across the stenosis; therefore, a Carotid Wallstent was advanced through a Benchmark 105 cm intracranial access catheter (Penumbra Inc, Alameda, CA) which was hubbed at the level of the torcula. In case 4, the TracStar sheath was able to be advanced into the torcula.
Contralateral dural venous sinus stenting is only possible with a patent contralateral sinus and torcula. In our patients the contralateral sinus was hypoplastic and had stenosis in the transverse-sigmoid sinus junction, which contributed to the elevated intracranial pressure (Figure 4). Though the contralateral dural sinus system was hypoplastic, it was sufficient in size to accommodate the 6 F delivery catheter. Aside from the typically encountered effort needed to traverse the transverse-sigmoid sinus junction turn, there were no other difficulties in this approach. In all cases the delivery catheter was advanced at least past the transverse-sigmoid sinus junction (Figure 5). It is assumed that without being able to advance the delivery catheter into the transverse sinus, the stent delivery catheter would be too stiff to make the turn from the sigmoid to the transverse sinus on its own. Additionally, a high bifurcation of the superior sagittal sinus will preclude use of the contralateral approach. Of note, we had a different case where the retrograde approach was not possible due to same reasons described above. Her contralateral, hypoplastic dural sinus was patent but the torcula was fenestrated and did not make a complete connection with the symptomatic transverse sinus; therefore, stenting was unable to be performed in this patient (Figure 6). A direct jugular venous approach was not considered as the problem was not stability of the catheter systems but rather the anatomy at the stenotic segment.
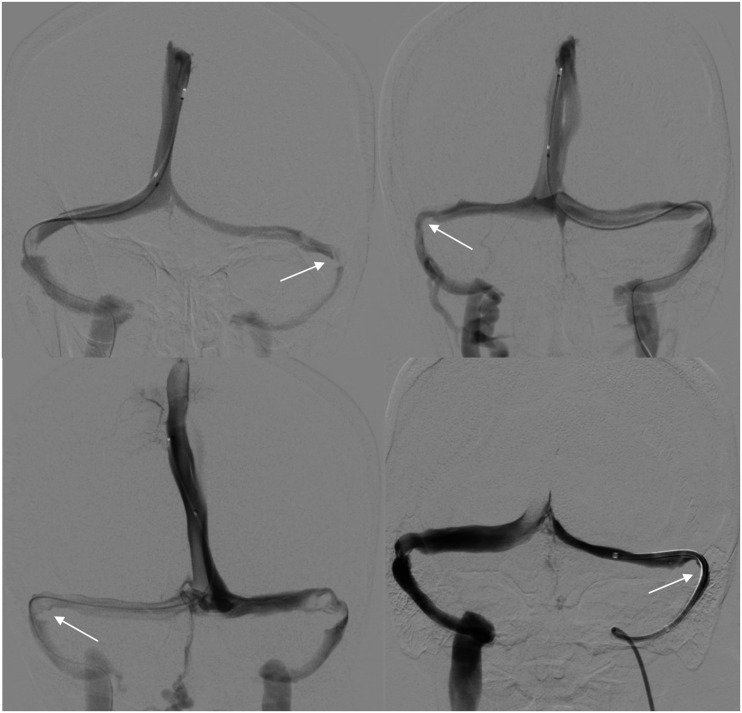
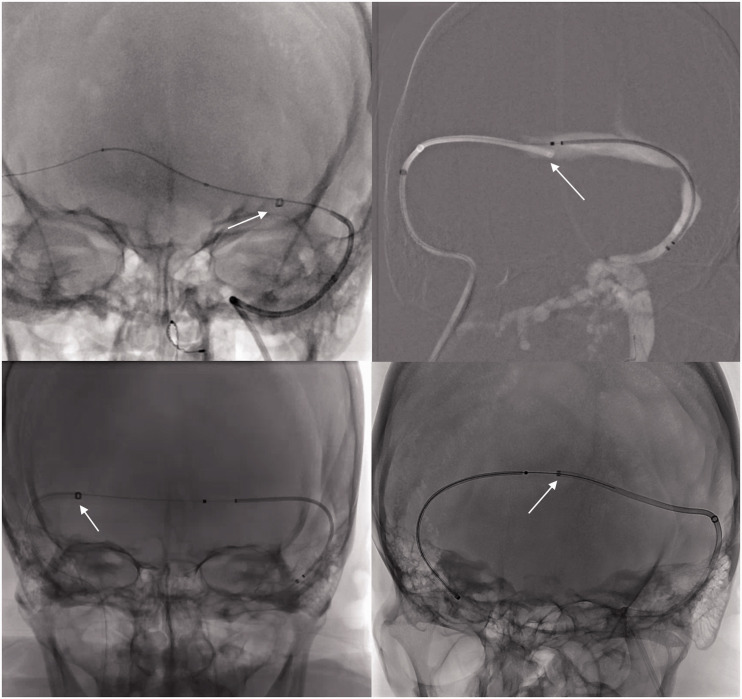
Figure 4.
AP views demonstrating the hypoplastic, patent dural sinus systems with stenosis (white arrows).
Figure 5.
AP views demonstrating the delivery catheters advanced past the contralateral transverse-sigmoid sinus junction stenosis.
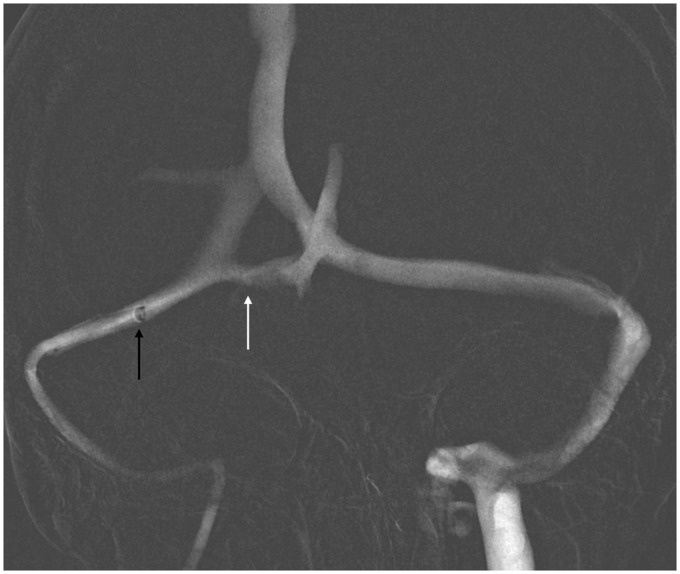
Figure 6.
6 F catheter in contralateral sinus (black arrow). Due to fenestrated torcula and incomplete connection with symptomatic transverse sinus (white arrow), the contralateral approach was not possible.
Stenting aims to normalize the intrasinus pressure and reduce the increased pressure proximal to the stenosis.8 Potential complications from venous stenting include sinus perforation or rupture, thromboembolism, and access site complications including hematoma formation or infection, which did not occur in our patients. Patients most likely to benefit from DVSS include stenosis of bilateral transverse-sigmoid sinuses, unilateral transverse-sigmoid sinus with contralateral hypoplasia or aplasia, or focal superior sagittal sinus stenosis.9
A recent review by Satti et al (2019) of 29 studies and 410 patients who underwent DVSS reported a high technical success of 99.5%, low repeat procedure rates (10%), and low complication rates (1.5%). In two studies the technical failures were described as stagnation of contrast concerning for dissection and tracking difficulty preventing stent placement. Importantly, there were no reports of failure to advance the microcatheter or deploy stent due to severity of stenosis or vessel tortuosity.10
Conclusion
Our case series demonstrates an alternative, safe, and successful approach to dural sinus stenting if the antegrade approach is not feasible.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Weston Gordon https://orcid.org/0000-0003-4008-3536
References
- 1.Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clin Neurol Neurosurg 2013; 115: 252–259. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002; 59: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 3.Smith KA, Peterson JC, Arnold PM, et al. A case series of dural venous sinus stenting in idiopathic intracranial hypertension: association of outcomes with optical coherence tomography. Int J Neuroscience 2017; 127: 145–153. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JNP, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003; 74: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satti SR, Leishangthem L, Spiotta A, et al. Dural venous sinus stenting for medically and surgically refractory idiopathic intracranial hypertension. Interv Neuroradiol 2017; 23: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson P, Brinjikji W, Radovanovic I, et al. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and Meta-analysis. J Neurointerv Surg 2019; 11: 380–385. [DOI] [PubMed] [Google Scholar]
- 7.McCormick MW, Bartels HG, Rodriguez A, et al. Anatomical variations of the transverse-sigmoid sinus junction: implications for endovascular treatment of idiopathic intracranial hypertension. Anat Rec (Hoboken) 2016; 299: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 8.Fields JD, Javedani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg 2013; 5: 62–68. [DOI] [PubMed] [Google Scholar]
- 9.Satti SR, Leishangthem L, Chaudry MI, et al. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2015; 36: 1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leishangthem L, SirDeshpande P, Dua D, et al. Dural venous sinus stenting for idiopathic intracranial hypertension: an updated review. J Neuroradiol 2019; 46: 148–154. [DOI] [PubMed] [Google Scholar]