Abstract
Background
The use of flow diverter stent (FDS) has limitations in cases of subarachnoid haemorrhage caused by ruptured aneurysm, due to the need for double antiplatelet therapy and the delay in the aneurysm occluding. The p48 MW and the p64 MW (Phenox) are available with Hydrophilic Polymer Coating (HPC), that reduces the risk of thrombus formation. Purpose of this study is to evaluate the safety and efficacy of p48 and p64 MW HPC with single antiplatelet therapy for the acute treatment of ruptured aneurysm.
Methods
We retrospectively evaluated all patients treated for acutely ruptured aneurysms with a p48 MW HPC or p64 MW HPC from October 2019 to April 2020 using single antiplatelet therapy. For each patient, we considered demographic and aneurysm-related data, clinical presentation, size and location of the implanted flow diverter stent, intra- and post-procedural complications, aneurysm occlusion.
Results
Seven patients were included. The ruptured aneurysms were four saccular, two blister-like and one dissecting, six in the anterior and one in posterior circulation. No intraprocedural stent thrombosis and rebleeding was observed. In two cases the aneurysm is completely excluded, in one patient it was found only neck perfusion, in three cases there were mild reduction of the sac and in one case there was a persistent perfusion. No patients needed retreatment in this series.
Conclusion
In our experience, FDS HPC appears a potential treatment option in selected cases. Our study is limited by small population and short-term follow-up. We report our preliminary data, but further investigations are necessary.
Keywords: Intracranial aneurysm, flow diverter, hydrophilic polymer coating, single antiplatelet therapy
Introduction
The use of flow diverter stents (FDSs) in endovascular treatment of unruptured intracranial aneurysms is increasingly widespread.1 However, the efficacy of these devices is hampered in patients presenting with subarachnoid hemorrhages caused by ruptured aneurysms. These patients are in fact treated with double antiplatelet therapy (DAPT) prior to device placement, which increases the risk of delayed occlusion of the aneurysm sac.
The p48 MW and p64 MW flow modulation devices with hydrophilic polymer coating (HPC) (Phenox, Bochum, Germany) are newly designed FDSs for the treatment of intracranial aneurysms, a process favored by the antithrombogenic activity conferred by their coating, as judged by in vitro experiments.2 In this regard, two recent studies, one of which involving a patient affected by subarachnoid hemorrhage, reported good efficacy of the p48 MW HPC device after single antiplatelet therapy (SAPT).3,4 However, to date there are no studies in the literature on the use of p64 MW HPC FDS for the treatment of acutely ruptured intracranial aneurysms.
In this monocentric observational retrospective study, we describe our preliminary experience with p48 MW HPC or—the first ever in Italy—p64 MW HPC FDS devices implanted in SAPT-treated patients presenting with subarachnoid hemorrhage due to acutely ruptured aneurysms.
Methods
Device description
The p48 MW flow modulation device consists of 48 drawn filled tubing (DFT) wires forming a braided mesh composed of a platinum core and a Nitinol outer tube. This device, compatible with a 0.021-inch microcatheter, is designed for the treatment of vessel diameters from 1.75 mm to 3 mm.
The p64 MW flow modulation device is made of a 64 Nitinol wire braid, with visualization achieved by eight proximal markers and two helical strands. It is compatible with a 0.027-inch microcatheter and designed for treating distal anatomies with vessel diameters ranging from 2.5 mm to 5 mm.
Both FDSs are coated with a hydrophilic polymer, which simulates the glycocalyx of the vessel wall, thereby reducing the risk of thrombus formation.
Patient characteristics, treatments and procedures
We retrospectively evaluated all patients from October 2019 to April 2020 due to acutely ruptured aneurysms treated with SAPT and implantation of a p48 MW HPC or p64 MW HPC device.
Each patient was classified according to demographic data and clinical presentation using Fisher Grade, Hunt and Hess Scale and Glasgow Coma Scale (GCS). We also recorded the aneurysm size—with regard to the two patients that had been already treated, we could only measure the size of the residual sac—, morphology and location as well as the size and location of the implanted FDS and any intra- and post-procedural complications.
All procedures were performed under general anaesthesia in the angiographic suite by means of a monoplane digital subtraction angiography system (Azurion, Philips, Amsterdam, NL), using a 6 F or an 8 F right femoral artery approach. During the procedure, all patients were heparinized with a bolus of 50 IU/kg unfractionated heparin IV and received a loading dose of acetylsalicylic acid (ASA, 500 mg) at the beginning of the procedure.
Platelet inhibition was assessed with light transmission aggregometry (LTA), performed in the biochemistry laboratory, 30 min after the loading dose. As all ASA-treated patients displayed platelet inhibition, we chose not to use P2Y12 platelet receptor antagonists (i.e., clopidogrel, prasugrel and ticagrelor).
During the procedure, we performed angiographic controls at 5 and 15 min after FDS implantation to detect any early thrombotic formation. In case of platelet aggregation, patients were treated with tirofiban (Aggrastat®).
After treatment, all patients started SAPT with twice-daily doses of 300 mg ASA for 1 week, which was then reduced to twice-daily doses of 100 mg at 1-month angiographic evaluation, and subsequently reduced to a daily dose of 100 mg. Angiographic follow-up was performed during the first week after the treatment and again at one month, unless different clinical conditions required otherwise.
The O'Kelly-Marotta (OKM) grading scale was used to assess the degree of angiographic filling and contrast stasis of the aneurysm sac.5
Statistical analysis
Descriptive statistics are reported as mean ± standard deviation (range) and frequency.
Results
In our analysis, we examined 7 patients (2 men and 5 women) treated for acutely ruptured intracranial aneurysm. The decision to implant an FDS was made jointly with a senior neurosurgeon, taking into account the patients' clinical conditions and the anatomical features of the aneurysm.
In two cases, the decision for flow diversion deployment was made to complete a previous endovascular treatment. Specifically, patient #1 had been treated due to reperfusion of the sac 18 days after coiling, and patient #2 had been treated following rebleeding 2 days after intrasaccular deployment of a Woven Endobridge® (WEB) device.
The mean age of aneurysm presentation in our patients was 51 ± 13 years. The ruptured aneurysms were classified as follows: saccular (n = 4; 57%), blister-like (n = 2; 29%) and dissecting (n = 1; 14%). Six (86%) aneurysms were in the anterior circulation, with 3 of them in the anterior communicating artery (AcomA, 43%) and the remainder 3 in the internal carotid artery (ICA, 43%). One case (patient #3; 14%) had a dissecting aneurysm of the right vertebral artery (Figure 1). The mean maximum diameter of the saccular aneurysms was 5 ± 2.2 mm (range: 2-9 mm).
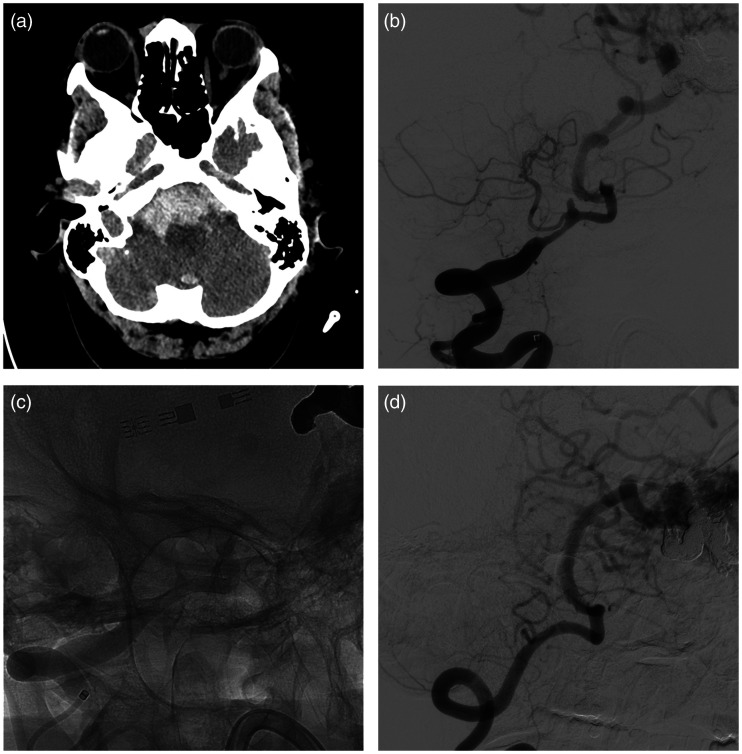
Figure 1.
Patient #3. Patient with AVM and subarachnoid haemorrhage mainly distributed in basal cisterns at CT scans (a). At digital subtracted angiography was found a dissecting aneurysm of the right vertebral artery (b). After collective discussion with a senior neurosurgeon, we decided to treat the aneurysm with implantation of a p48 MW HPC flow diverter stent (c). Delayed angiography showed complete occlusion of the aneurysm and patency of the posteroinferior cerebellar artery (d).
Demographic data, aneurysm characteristics and clinical condition at presentation are summarized in Table 1.
Table 1.
Demographic data, aneurysm characteristics and clinical presentation.
| N | Age (years) | Gender | Location | Morphology | Size (mm) | Fisher grade | Hunt and Hess scale | GCS |
|---|---|---|---|---|---|---|---|---|
| 1 | 51 | M | AcomA | Saccular | 4,5 × 3,8 | IV | V | 7 |
| 2 | 65 | M | AcomA | Saccular | 4,3 × 3 | I | II | 15 |
| 3 | 36 | F | V4 (right) | Dissecting | 5 × 2,5 | III | II | 12 |
| 4 | 34 | F | ICA (right) | Saccular | 6,5 × 4,5 | IV | V | 3 |
| 5 | 47 | F | ICA (right) | Blister | 2 × 1,2 | IV | III | 12 |
| 6 | 56 | F | AcomA | Saccular | 9 × 7 | IV | IV | 8 |
| 7 | 69 | F | ICA (left) | Blister | 4 × 3 | IV | III | 8 |
M = male; F = female; AcomA= anterior communicating artery; V4 = fourth segment (intracranial) of vertebral artery; ICA = internal carotid artery; GCS = Glasgow Coma Scale.
In all cases, a single FDS was used: five (71%) p48 MW HPC and two (29%) p64 MW HPC devices. In two cases (patients #2 and #6), an intrasaccular coiling was performed during treatment (Figure 2). Furthermore, an external ventricular drain was placed in four (57%) patients.
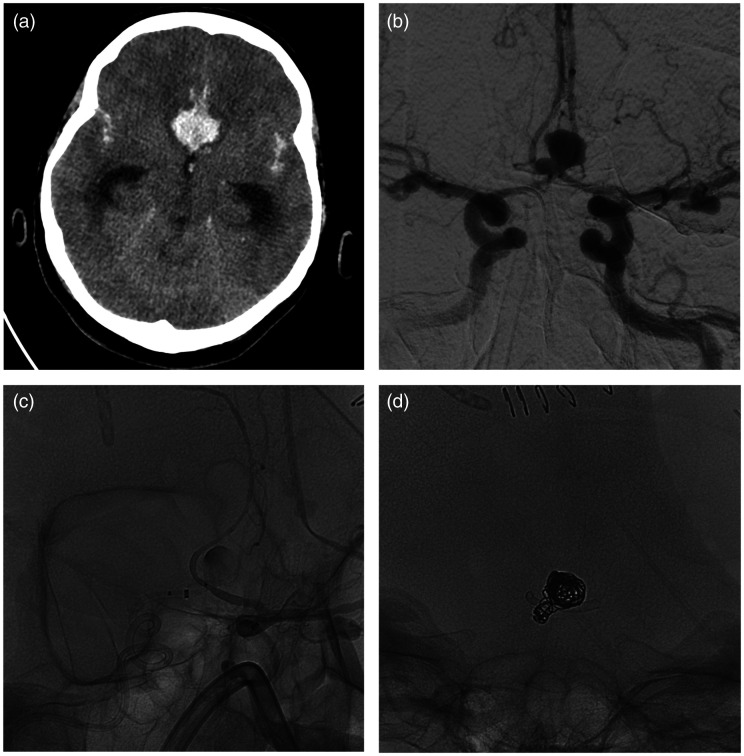
Figure 2.
Patient #6. Patient with intraparenchymal hematoma and subarachnoid haemorrhage at CT scans (a). At digital subtracted angiography were found multiple aneurysms, in particular a saccular aneurysm of anterior communicating artery (b). After collective discussion with a senior neurosurgeon, we decided to treat the aneurysm with implantation of a p48 MW HPC flow diverter stent from right A2 tract and left A1 tract (c) and additional coiling (d).
No intraprocedural thrombus formation was observed in 6 (86%) cases. In patient #2 (14%), occlusion of an angular branch of the right middle cerebral artery occurred at the end of the procedure, which required immediate endovascular thrombectomy, achieving partial revascularization of the occluded vessel (TICI 2B), with no neurological deficit.
In all ASA-treated patients, after FDS deployment, we did not record intrastent thrombus formation. Had we observed platelet aggregation under these conditions, we would have administered tirofiban (Aggrastat®).
Additionally, patients #4, #5 and #7 presented with cerebral vasospasm requiring, respectively, one, seven and two days of treatment with intra-arterial infusion of nimodipine (flow: 0.1 mg/min; max 6 mg).
Lastly, two intraprocedural device-related events were observed. Patient #5 needed an FDS replacement due to a technical problem during re-sheathing, with no clinical repercussions. Patient #7 required intrastent angioplasty to open the central portion of the stent, with complete resolution of the problem.
In order to evaluate stent patency and aneurysm occlusion, we performed complete angiographic follow-up for patients #1 to #5, while patients #6 and #7, because of their recent procedures, only received early angiographic follow-up (median 7 days). We were able to confirm FDS patency in all patients with the exception of one (patient #2, 14%) where we found minimal neointimal hyperplasia inside the stent. In all cases, no intraprocedural rebleeding occurred, and no patient died during the procedure or at follow-up.
Follow-up angiographic imaging revealed complete occlusion of the aneurysm (OKM D) in two (29%) patients, mild reduction of the sac (OKM B) in three (43%) cases, neck remnants (OKM C) in one (14%) patient and persistent perfusion (OKM A) in one (14%) patient. In this latter patient (#2), we also detected thrombocytopenia. None of our patients needed retreatment.
Stent characteristics, microcatheters used, complications and angiographic outcome are summarized in Table 2.
Table 2.
Stent characteristics, microcatheters used, complications and angiographic outcome.
| N | FDS type | FDS size (mm) | FDS position | Microcatheter | Coils | Intraprocedural complications | Postprocedural complications | OKM scale |
|---|---|---|---|---|---|---|---|---|
| 1 | p48 | 3 × 12 | A2/A1 (left) | Headway 21a | No | None | None | B1 |
| 2 | p48 | 3 × 12 | A2/A1 (right) | Headway 21a | Yes | Minor stroke | None | A2 |
| 3 | p48 | 3 × 18 | V4 (right) | Headway 21a | No | None | None | D |
| 4 | p48 | 3 × 12 | ICA (right) | Headway 21a | No | None | Severe vasospasm | B3 |
| 5 | p64 | 4 × 18 | ICA (right) | Rebar-18b | No | None | Mild vasospasm | D |
| 6 | p48 | 2 × 15 | A2 (right)/A1 (left) | Rebar-18b | Yes | None | None | C1 |
| 7 | p64 | 4.5 × 18 | M1/ICA (left) | ExcelsiorXT-27c | No | None | Severe vasospasm | B1 |
FDS = flow-diverter stent; OKM = O'Kelly-Marotta; A2 = second segment (postcommunicating) of the anterior cerebral artery; A1 = first segment (precommunicating) of the anterior cerebral artery; V4 = fourth segment (intracranial) of vertebral artery; ICA = internal carotid artery; M1 = first segment (sphenoidal) of the middle cerebral artery.
Microvention Terumo, Hatagaya, Tokyo, Japan.
Medtronic, Minneapolis, USA.
Stryker Corporation, Kalamazoo, USA.
Case presentations
Patient #1: A 51-year-old male with intraparenchymal hemorrhage due to rupture of an anterior communicating artery aneurysm, treated with coiling and displaying incomplete filling of the sac. Eighteen days after procedure, we decided to complete the treatment by implanting a p48 MW HPC device (3×12 mm from the left A2 segment to the left A1 segment), achieving no periprocedural complications.
Patient #2: A 65-year-old male suffering from thrombocytopenia with subarachnoid hemorrhage caused by rupture of an anterior communicating artery aneurysm treated by deploying a WEB device. Since the patient rebled two days after the first endovascular procedure, he underwent retreatment with a p48 MW HPC device (3×12 mm from right A2 segment to left A1 segment and additional coiling). At angiographic controls, we detected occlusion of the angular branch of the M2 tract of the right middle cerebral artery, which required immediate endovascular thrombectomy, achieving partial revascularization of the occluded vessel (TICI 2B). The patient did not display any neurological deficit upon wakening.
Patient #3: A 36-year-old female with subarachnoid hemorrhage—on CT scans, mainly distributed across the basal cisterns—resulting from a dissecting aneurysm of the right vertebral artery and arteriovenous malformation (AVM) with pial arteriovenous high-flow fistula. According to blood distribution, we treated the aneurysm with a p48 MW HPC device (3×18 mm), with no periprocedural complications.
Patient #4: A 34-year-old female with subarachnoid hemorrhage due to two saccular aneurysms of the right internal carotid artery, with the posterior communicating artery originating from the larger one. She was treated by deploying a p48 MW HPC device (3×12 mm) in the supraclinoid segment. Nine days after treatment, she developed severe vasospasm treated with intra-arterial infusion of nimodipine for 7 days.
Patient #5: A 47-year-old female with subarachnoid hemorrhage caused by a ruptured blister-like aneurysm of the right internal carotid artery, treated with a p64 MW HPC device (4×18 mm). During the procedure, we had to replace the FDS due to a technical problem arising while re-sheathing, with no clinical repercussions for the patient. She developed mild vasospasm 9 days after the procedure, requiring a single intra-arterial nimodipine treatment.
Patient #6: A 56-year-old female with subarachnoid and intraparenchymal hemorrhage due to the rupture of an anterior communicating artery aneurysm. She underwent deployment of a p48 MW HPC device (2 × 15 mm from right A2 segment to left A1 segment and additional coiling), with no periprocedural complications.
Patient #7: A 69-year-old female with subarachnoid hemorrhage caused by a blister-like left internal carotid artery aneurysm, treated using a p64 MW HPC device (4.5×18 mm). We initially failed to open the central portion of the stent by applying a curvilinear stretching force. However, this issue was successfully resolved by means of intrastent angioplasty.
Discussion
In recent years, the role of FDSs in treating ruptured intracranial aneurysms has become increasingly prevalent, especially in technically challenging cases.6,7 These devices, in fact, allow treating aneurysms with complex anatomy, do not require the insertion of guides and microcatheters into the aneurysmal sac, and generally ensure durability of the aneurysmal occlusion. However, the use of FDS devices present some limitations when dealing with cases of subarachnoid hemorrhages because bleeding patients require DAPT prior to device deployment, which inevitably delays the occlusion of the aneurysm.8 In addition, even though FDS implantation enables a high rate of long-term angiographic occlusion with a relatively low rate of aneurysm rebleeding, it has a complication rate of 18%.9
Recent advances in polymer coating technology have made it possible to manufacture FDS with reduced thrombogenicity. In particular, pHPC is a glycan-based hydrophilic multilayer polymer coating which can be applied to Nitinol stents. This new technology, based on glycocalyx simulation of the coating, makes the stent surface more hydrophilic and, therefore, less thrombogenic without evidence of acute inflammatory response.2,3 Thus, widespread adoption of FDS with HPC could represent a breakthrough in the treatment of ruptured intracranial aneurysms in selected patients, as it would expose patients undergoing antiplatelet therapy to a lower risk of rebleeding.
In support of this hypothesis, initial in vitro experiments by Lenz-Habijan et al.2 showed a significant reduction of adherent CD61+ platelets on pHPC nickel titanium surfaces compared to uncoated ones, thus paving the way for the development of antithrombogenic endovascular devices. Also, Bhogal et al.10 reported a reduction in thrombogenicity using a p48 HPC FDS in an in vitro model of human thrombin generation. On the other hand, a second investigation by Lenz-Habijan et al.,11 evaluating the effects of implantation of HPC FDS vs. uncoated FDS in rabbit carotid arteries, did not observe significant differences between the two devices with regard to implantation, foreign body response and endothelialization.
Preliminary in vivo canine experiments were also conducted using the p64 MW HPC device, which appeared to be biocompatible and unable to trigger acute inflammation.12 Consistent with a protective role of HPC against inflammation, other in vivo experiments using pCONUS HPC stents showed the absence of any acute or chronic inflammatory response, with no significant difference in tissue response compared to its bare Nitinol counterparts.13 Fittingly, pCONUS HPC stent and coils deployment in a patient with subarachnoid hemorrhage caused by a ruptured middle cerebral artery aneurysm led to the occlusion of the aneurysm and patency of the middle cerebral artery.14 Furthermore, in a pilot study in human subjects, Bhogal et al.3 confirmed that no thromboembolic complications occurred in SAPT-treated patients implanted with p48 MW HPC devices.
Another surface modified FDS is the PED-Shield® manufactured by Medtronic Inc (Dublin, Ireland). This device was implanted by Manning and co-workers in fourteen patients with acute ruptured intracranial aneurysms who had undergone SAPT, achieving complete or near complete aneurysm occlusion (Raymond-Roy <3) in 85.7% of patients at the end of early-acute follow-up. Permanent, treatment-related morbidity occurred in 7.1% of patients, as well as treatment-related death.15 By contrast, in our study, we obtained complete or near complete aneurysm occlusion (OKM C or D) in 43% of patients, but we did not detect any permanent treatment-related morbidity or death.
All patients analyzed in this study were subjected to SAPT with ASA as this treatment results in a lower rate of hemorrhagic complications compared to DAPT.16 Nevertheless, we routinely assessed the patients’ rate of platelet inhibition by LTA to check for intrastent thrombus formation. In case of poor or no efficacy of ASA, we would have switched to P2Y12 platelet receptor blockers (i.e., clopidogrel, prasugrel, ticagrelor).
Very recently, Aguilar-Perez et al. have examined the use of p48 MW HPC for the treatment of acutely ruptured aneurysms in patients undergoing SAPT, recording transient thrombus formation in half of cases, which required switching three patients to DAPT. These authors did not report any case of aneurysm rebleeding after treatment. At early angiographic follow-up, they obtained complete aneurysm occlusion in 37.5% of the patients (vs 29%) and repeated the FDS treatment in 37.5% of cases (vs 0%).4
Our study has several limitations such as its retrospective design, the small population size and the short-term follow-up. However, it is the first study assessing patients with ruptured aneurysms undergoing SAPT and then implanted with a p64 MW HPC device.
Conclusions
In this pilot study, we have used p64 and p48 MW HPC devices to treat acutely ruptured aneurysm patients following SAPT. Since we did not record any thrombotic complications in the treated patients, except for a minor stroke in a different artery from the treated one, we conclude that these HP-coated devices are a potentially effective solution to treat selected cases of ruptured aneurysm.
Being preliminary data, future studies are required to confirm these findings on a large sample size.
Research ethics approval: Human participants
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards
Consent for publication
Consent for publication was obtained for every individual person’s data included in the study.
Informed consent
Informed Consent Informed consent was obtained from all individual participants (or their legal representatives) included in the study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Giuseppe Guzzardi https://orcid.org/0000-0002-0979-4519
Andrea Galbiati https://orcid.org/0000-0002-5583-9052
References
- 1.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenz-Habijan T, Bhogal P, Marcus Peters M, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41: 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhogal P, Bleise C, Chudyk J, et al. The p48_HPC antithrombogenic flow diverter: initial human experience using single antiplatelet therapy. J Int Med Res 2020; 48(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar-Perez M, Hellstern V, AlMatter M, et al. The p48 flow modulation device with hydrophilic polymer coating (HPC) for the treatment of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. Cardiovasc Intervent Radiol 2020; 43(5): 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozupone E, Piano M, Valvassori L, et al. Flow diverter devices in ruptured intracranial aneurysms: a single-center experience. J Neurosurg 2018; 128: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 7.AlMatter M, Aguilar Pérez M, Hellstern V, et al. Flow diversion for treatment of acutely ruptured intracranial aneurysms: a single center experience from 45 consecutive cases. Clin Neuroradiol. Epub ahead of print 4 November 2019. DOI: 10.1007/s00062-019-00846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzzardi G, Stanca C, Cerini P, et al. Long-term follow-up in the endovascular treatment of intracranial aneurysms with flow-diverter stents: update of a single-Centre experience. Radiol Med 2018; 123: 449–455. [DOI] [PubMed] [Google Scholar]
- 9.Cagnazzo F, di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018; 39: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. Thrombogenicity of the p48 and antithrombogenic p48 hydrophilic polymer coating low-profile flow diverters in an in vitro human thrombin generation model. Interv Neuroradiol. Epub ahead of print 4 May 2020. DOI: 10.1177/1591019920923817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenz-Habijan T, Bhogal P, Bannewitz C, et al. Prospective study to assess the tissue response to HPC-coated p48 flow diverter stents compared to uncoated devices in the rabbit carotid artery model. Eur Radiol Exp 2019; 3: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez Moreno R, Bhogal P, Lenz-Habijan T, et al. In vivo canine study of three different coatings applied to p64 flow-diverter stents: initial biocompatibility study. Eur Radiol Exp 2019; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019; 42: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henkes H, Bhogal P, Aguilar Pérez M, et al. Anti-thrombogenic coatings for devices in neurointerventional surgery: case report and review of the literature. Interv Neuroradiol 2019; 25: 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J NeuroIntervent Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ries T, Buhk JH, Kucinski T, et al. Intravenous administration of acetylsalicylic acid during endovascular treatment of cerebral aneurysms reduces the rate of thromboembolic events. Stroke 2006; 37: 1816–1821. [DOI] [PubMed] [Google Scholar]