Abstract
Background
Endovascular therapy with liquid embolic agents (LEAs) is the gold standard for the treatment of cerebral dural arteriovenous fistulas (cDAVFs). The aim of the study is to retrospectively evaluate effectiveness, safety, and midterm follow-up results of endovascular treatment of cDAVFs using SQUID 12.
Methods
Between June 2017 and January 2020 the authors retrospectively reviewed clinical, demographic and embolization data of 19 consecutive patients with cDAVF who underwent embolization using SQUID 12. The number of arteries catheterized for each procedure, the total amount of embolic agent, the occlusion rate, the injection time, any technical and/or clinical complications were recorded. Mid-term follow-up with DSA was reviewed.
Results
20 procedures were performed in 19 patients. A transarterial approach was accomplished in 19 procedure; a combined transvenous-transarterial approach was realized in 1 treatment. The average time of injection was 33 minutes (2–82 minutes), and the average amount of SQUID 12 was 2.8 mL (0.5–6 mL). Complete angiographic cure at the end of the procedure was achieved in 17 patients. No major periprocedural adverse events were recorded. Mid-term follow-up was achieved in 15 out of 19 patients and confirmed complete occlusion of the cDAVFs in 13/15 patients (87%); in 2 of the initially cured patients a small relapse was detected.
Conclusions
The treatment of the cDAVFs using SQUID 12 was effective and safe. The lower viscosity seems to allow an easier penetration of the agent with a high rate of complete occlusion of the cDAVFs.
Keywords: SQUID, cerebral dural arteriovenous fistula, endovascular embolization, embolic agent, EVOH
Introduction
Cerebral dural arteriovenous fistulas (cDAVFs) are acquired abnormal arteriovenous connections between an arterial feeder and a dural venous sinus or leptomeningeal vein that account for 10%–15% of all intracranial vascular malformations.1 The origin of these malformations is not entirely understood but they are supposed to be secondary to several conditions including venous thrombosis, intracranial surgery, tumors, puerperium, and trauma.2 There are different classification systems regarding DAVFs. Borden3 and Cognard4 have proposed the two most practical classifications.
cDAVFs may be asymptomatic or present with symptoms such as pulsatile tinnitus (related to increased dural venous drainage), neurologic deficits or intracranial hemorrhage.5 Adverse clinical events are strongly related to retrograde leptomeningeal or cortical venous drainage, presentation modality, and venous ectasia.6
The treatment options include endovascular embolization, surgery, and radiosurgery, or a combination of these modalities2; however, endovascular therapy with liquid embolic agents (LEAs) is currently the gold standard for the treatment of these acquired vascular malformations.7
SQUID (Emboflu, Gland, Switzerland) is an ethylene vinyl alcohol (EVOH) copolymer that has become recently available. It is composed of EVOH copolymer with suspended micronized tantalum powder for radiopacity and DMSO (dimethyl sulfoxide) solvent. There are 4 different formulations depending on viscosity (18–standard viscosity and 12–low viscosity) and the percentage of dissolved tantalum powder (standard and LD – low density). While SQUID 18 has similar characteristics to other LEAs, SQUID 12 is innovative because of its lower viscosity.8
We review our preliminary experience in endovascular treatment of cDAVFs with SQUID 12 with the aim of evaluating its safety, effectiveness, and midterm follow-up results.
Methods
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Study design and data collection
This was a retrospective observational study on cDAVFs treated with Squid 12.
Between June 2017 and January 2020 the authors retrospectively reviewed clinical, demographic and embolization data of 19 consecutive patients (7 F, 12 M; median age 59 years-old, range 37-80 yrs) with cDAVF who underwent embolization using SQUID 12. The main criterion for the endovascular treatment was the presence of a cDAVF type IIb or higher according to the Cognard classification.
The number of arteries catheterized for embolization, the total amount of embolic agent, the rate of occlusion, the time of the procedure, any technical or clinical complications were recorded.
After discharge, the systematic follow-up included MRI at 3-6 months, cerebral DSA at 9-12 months and MRI at 18-24 months. Mid-term follow-up with DSA is reviewed.
Clinical follow-up with mRS at 3 months was collected.
Results
Patients characteristics
According to Cognard classification, 4 cases were type IIb cDAVF, 8 cases were type III and 7 cases were type IV. Fistulas were located in the transverse sinus (9 cases), straight sinus (3 cases), anterior cranial fossa/crista galli (2 cases), vein of Galen (1 case), occipital sinus (1 case), sphenoparietal sinus (1 case), vein of Labbé (1 case), torcula (1 case). Five patients were symptomatic for headache, 2 patients presented with vertigo, 2 patients with limb tremor, 1 patient with tinnitus, 1 patient with facial nerve palsy, 1 patient with seizure. Intracranial hemorrhage was the sudden onset in 3 patients, admitted for aphasia (2 cases) and headache (1 case). Four patients were asymptomatic and the cDAVF was discovered incidentally.
Procedure (Table 1)
Table 1.
Endovascular procedure.
|
DAVF |
Procedure |
||||||
|---|---|---|---|---|---|---|---|
| Patient | Cognard classification | Location | Amount of Squid 12 (ml) | Feeders embolized | Time of injection (min) | Reflux (mm) | Adjunctive treatment tools |
| 1 | IV | Left transverse sinus | 4 | MMA | 82 | 19 | – |
| 2 | IIb | Left transverse sinus | 2 | MMA | 15 | 6 | Transvenous + Transarterial approach |
| 3a | IIb | Right transverse sinus | 2 | MMA, OA | 15 + 10 | 13 + 2 | Pressure cooker |
| 2 | Neuromeningeal trunk of APhA | 15 | 11 | – | |||
| 4 | IV | Left transverse sinus | 2,3 | MMA | 40 | 5 | – |
| 5 | IV | Vein of Galen | 6 | MMA | 30 | 30 | Pressure cooker |
| 6 | IIb | Left transverse sinus | 6 | OA, posterior auricolar artery | 15 + 15 | 22 + 40 | Pressure cooker |
| 7 | IIb | Occipital sinus | 2 | PICA | 60 | 20 | Pressure cooker |
| 8 | III | Straight sinus | 1,2 | Posterior meningeal artery | 21 | 40 | – |
| 9 | III | Crista galli | 4,5 | MMA | 25 | 13 | – |
| 10 | IV | Straight sinus | 1 | MMA | 20 | 4 | – |
| 11 | III | Left transverse sinus | 0,5 | MMA | 15 | 5 | – |
| 12 | III | Crista galli | 5 | MMA | 55 | 30 | – |
| 13 | IV | Left sphenoparietal sinus | 3 | MMA | 80 | 5 | – |
| 14 | IV | Left transverse sinus | 4 | MMA | 82 | 3 | – |
| 15 | III | Vein of Labbe | 1 | MMA | 3 | 0 | – |
| 16 | III | Straight sinus | 4 | MMA | 25 | 30 | – |
| 17 | III | Right transverse sinus | 2 | MMA | 2 | 1 | Glubran by Magic 1.2 |
| 18 | III | Left transverse sinus | 1 | MMA | 4 | 1 | – |
| 19 | IV | Torcula | 1,7 | Posterior meningeal artery | 21 | 28 | – |
aThe patient underwent a two-stage treatment.
A total of 20 procedures were performed in 19 patients. A transarterial approach was accomplished in 19 procedures; a combined transvenous-transarterial approach was realized in 1 treatment.
All the treatments were performed with DMSO compatible microcatheters. The microcatheter Headway DUO (Microvention, California, USA) was used in 15 out of 20 procedures. In the remaining 5 cases we used the detachable tip microcatheter Apollo Onyx Delivery Microcatheter (Medtronic, Dublin, Ireland): in 2 out of 5 cases the microcatheter was pulled out intact, in 2 out of 5 the detachable tip detached during the retraction of the microcatheter at the end of the procedure and in one case the tip detached prematurely during the SQUID 12 injection.
A “pressure-cooker” technique with coiling of the feeder was performed in 4 cases in order to reduce the reflux and facilitate the distal penetration of the liquid embolic agent: coils were placed in the occipital artery in 2 cases, in the posterior inferior cerebellar artery (PICA) and in a branch of the middle meningeal artery (MMA) in 1 case each.
A single arterial feeder was embolized in 17 patients, 2 feeders in 1 patient, 3 feeders in 1 patient. MMA was used to reach the cDAVF in 15 procedures, occipital artery in 2 cases, meningeal branches of the vertebral artery in 2 cases, the neuromeningeal trunk of the ascending pharyngeal artery in 1 case, PICA in 1 case, posterior auricular artery in 1 case.
The average time of injection was 33 minutes (median 21 minutes, range: 2–82 minutes), and the average amount of SQUID 12 was 2.8 mL (median 2 mL, range: 0.5 – 6 mL). The extent of reflux along the catheter tip was <1 cm in 9/20 procedures, between 1 and 2 cm in 4/20 procedures, and >2 cm in 7/20 procedures (median 12 cm; range: 0–4 cm; average: 1.6 cm).
Efficacy (Table 2)
Table 2.
Efficacy and Safety.
|
DAVF |
Efficacy |
Complications |
|||||
|---|---|---|---|---|---|---|---|
| Patient | Cognard classification | Location | Immediate posttreatment angiographic result | DSA FUP (9–12 months) | Intraprocedural | Periprocedural (1–30 days) | Delayed (>30 days) |
| 1 | IV | Left transverse sinus | Complete occlusion | Complete occlusion | No | Seizure | . |
| 2 | IIb | Left transverse sinus | Complete occlusion | Complete occlusion | No | No | No |
| 3a | IIb | Right transverse sinus | Partial occlusion | Complete occlusion | No | No | No |
| Complete occlusion | No | No | No | ||||
| 4 | IV | Left transverse sinus | Complete occlusion | Complete occlusion | No | No | No |
| 5 | IV | Vein of Galen | Complete occlusion | Complete occlusion | No | No | No |
| 6 | IIb | Left transverse sinus | Complete occlusion | Complete occlusion | No | No | No |
| 7 | IIb | Occipital sinus | Partial occlusion | Complete occlusion | No | No | No |
| 8 | III | Straight sinus | Complete occlusion | Complete occlusion | No | No | No |
| 9 | III | Crista galli | Complete occlusion | Complete occlusion | No | No | No |
| 10 | IV | Straight sinus | Complete occlusion | Relapse | No | No | No |
| 11 | III | Left transverse sinus | Complete occlusion | Complete occlusion | No | Transient facial palsy | No |
| 12 | III | Crista galli | Complete occlusion | N/A | No | No | No |
| 13 | IV | Left sphenoparietal sinus | Complete occlusion | N/A | No | No | No |
| 14 | IV | Left transverse sinus | Complete occlusion | Complete occlusion | No | No | No |
| 15 | III | Vein of Labbe | Complete occlusion | Complete occlusion | No | No | No |
| 16 | III | Straight sinus | Complete occlusion | Complete occlusion | No | No | No |
| 17 | III | Right transverse sinus | Complete occlusion | Relapse | No | No | No |
| 18 | III | Left transverse sinus | Complete occlusion | N/A | No | No | No |
| 19 | IV | Torcula | Complete occlusion | N/A | No | No | No |
aThe patient underwent a two-stage treatment.
Complete angiographic cure on immediate posttreatment angiography was achieved in 17/19 procedures (occlusion rate: 89%).
In one patient (Patient Nr 7) with a type IIb cDAVF of the occipital sinus, a minimal residual was recorded at the end of the embolization.
In one patient (Patient Nr 3) consistent residual was left at the end of the first embolization: it was a type IIb cDAVF of the right transverse sinus fed by multiples branches from MMA, the occipital artery and the neuromeningeal trunk. In the first procedure, the detachable tip broke during the initial injections in the MMA, then an embolization through the occipital artery was performed but failed. After 3 months, a second endovascular attempt was performed through the neuromeningeal trunk of the ascending pharyngeal artery, achieving a total exclusion of the shunt.
Mid-term angiographic follow-up was achieved in 15 patients and showed complete occlusion of the cDAVF in 13/15 patients (87%), including the one (Patient Nr 3) who had a minimal angiographic residual at the end of the embolization. Otherwise, a small relapse was detected in 2 of the initially cured patients, one with a type 4 cDAVF of the straight sinus (Patient Nr 10), the other with a type 3 cDAVF of the transverse sinus (Patient Nr 17).
Safety (Table 2)
A total of 2 patients (10%) had transient postoperative neurological complications. One patient experienced a single episode of seizure during the periprocedural period (Patient Nr 1). Another patient with a type III cDAVF of the traverse sinus region experienced a facial palsy, resolved after 2 weeks of corticosteroids (Patient Nr 11). There was 1 intra-procedural technical adverse event without clinical complication, that was the rupture of the detachable tip of the microcatheter at the site of the detachment during SQUID injection. No permanent complications nor mortality were recorded.
Clinical follow up
Clinical outcome through mRS score was evaluated for 15/19 (79%) patients at 3 months. Twelve of 15 patients had not experienced a change in the mRS score since the pre-treatment visit, while 3 patients reported an improvement in the mRS score from grade 1 to 0.
Discussion
Endovascular treatment is the actual first option for most of the cDAVFs. Conservative treatment is generally indicated in patients with low-grade fistulas (Borden I; Cognard I, IIa), except for cases with severe debilitating symptoms resulting in poor quality of life. High-grade lesions should be treated as soon as feasible, to avoid the risks of hemorrhage and neurological deficits.9 The goal of endovascular therapy is to occlude the shunt point by a transarterial approach, a transvenous approach or a combination of both.10
Over the years, different embolic agents have been used for the endovascular approach, including polyvinyl alcohol particles, platinum micro-coils and n–butyl cyanoacrylate (NBCA). In recent years, nonadhesive LEAs have become the gold standard for endovascular embolization of cDAVF, because of the slower precipitation time that allows for deeper penetration and more controlled injection of the agent.7
Onyx (Medtronic, Irvine, California) was the first commercially available EVOH polymer, as such, it has been the most widely used and studied.2
PHIL (MicroVention, Tustin, California) is a newer nonadhesive LEA, available in 4 different formulations depending on viscosity: 35%, 30%, 25% and 25% low viscosity (LV). PHIL differs from Onyx and SQUID because it is not an EVOH polymer; furthermore, its radiopacity is not due to tantalum powder but to a iodine component covalently bonded to the copolymer.11 Compared to Onyx, it seems to have a restrained reflux and fewer artifacts on CT.12
SQUID is an EVOH copolymer that has become recently available in 4 different formulations, of which the most innovative is SQUID 12 because of its lower viscosity.8 Currently, PHIL 25% LV is the only commercially available version of nonadhesive LEAs comparable to SQUID 12.13 About this, Vollherbst et al. reported not significantly differences between these 2 embolic agents in an in vivo endovascular embolization model, in particular in terms of embolization extent and the amount of reflux.14
Theoretically, the lower viscosity should aid distal migration of the agent with the possibility to inject far from the point of fistula. However, this reduced viscosity could affect the occlusion’s capability of the agent and thus have less efficacy in term of occlusion of the shunt or an increased rate of relapse of the cDAVF. Furthermore, even agent’s tendency to fragment and migrate to the venous side and proximal reflux could be enhanced by its lower viscosity.
Few cases of cDAVFs embolization with SQUID are reported in the literature.10,15
In our series, we performed embolization of cDAVFs using SQUID 12 and, in our opinion, its fluidity resulted in a more straightforward penetration into the fistulas (Figure 1) than Onyx. The same finding was noted by other authors: despite the few cases of cDAVFs treated with SQUID 12 (6 cases), Akmangit et al.15 observed the same effectiveness and safety as other nonadhesive LEAs and emphasized easier penetration of SQUID 12 compared to SQUID 18 or Onyx.
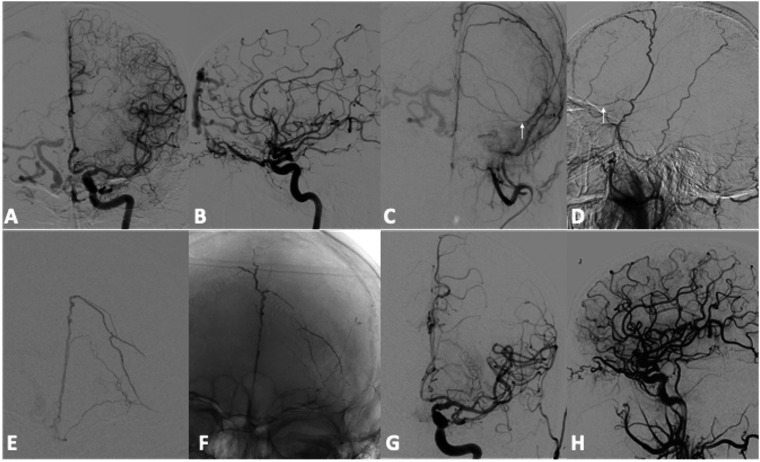
Figure 1.
Patient Nr 9. Preliminary DSA during selective injection of left internal and external carotid arteries (a, b, c, d) shows a DAVF of the Crista Galli with pial reflux in right cerebral hemisphere. Left MMA was the selected way for the embolization but the microcatheter stopped in the basal frontal branch (arrows). Frontal angiogram (e) during superselective injection through the microcatheter in the basal branch of the left MMA. Unsubtracted image (f) shows SQUID 12 cast at the end of the endovascular treatment. Despite the distance between the microcatheter and the DAVF, injection time was 25 minutes and the amount of embolic agent was 4,5 ml. Final angiograms of the left CCA (g, h) demonstrates the complete occlusion of the cDAVF.
We also noticed a satisfying visualization of the microcatheter tip within the embolic cast during the embolization. Gioppo et al.10 described the same advantageous feature in the treatment of a medial tentorial DAVF with SQUID 12, also highlighting an excellent visibility of the cast progression, relatively good injection control, durable angiographic result and optimal post-operative outcome. This feature may be the result of the smaller amount of tantalum inside SQUID compared to Onyx (30% less than Onyx).8
In our cases, SQUID 12 demonstrated faster penetration toward the fistula, ease of plug formation and no entrapment of the microcatheter. We achieved cDAVF cure on immediate post-treatment angiography in 17 out of 19 patients with a complete occlusion rate of 89%. This result in terms of complete occlusion is similar, or slightly higher, than other nonadhesive LEAs as Onyx or PHIL. In relation to Onyx, in two of the largest studies on embolization of cDAVF with this EVOH agent, Gross et al.16 and Vollherbst et al.17 reported an occlusion rate on the immediate posttreatment angiographic control of 80% and 78% respectively; in addition, a meta-analysis involving 19 studies with a total of 425 patients showed an initial complete occlusion rate of 82%.18
Compared to PHIL, Lamin et al.11 performed a retrospective analysis of 26 consecutive patients with cDAVFs who were treated by embolization with PHIL achieving an immediate and complete angiographic occlusion in 20 patients (77%).
After discharge, our systematic follow-up consisted of MRI at 3-6 months, cerebral DSA at 9-12 months and again MRI at 18-24 months. Mid-term angiographic follow-up was achieved in 15/19 patients (79%) and showed complete occlusion of the cDAVF in 13/15 patients (87%) (Figure 2). The recurrence rate in our series was 13%, similar to Onyx (11% at 4 months) in the work of Nogueira et al.2 and PHIL (18% at 3 months) in the work of Lamin et al.11 A small relapse was detected in 2 of the initially cured patients in the short-term follow up, one with a type IV cDAVF of the straight sinus, the other with a type III cDAVF of the transverse sinus. However, these results should be evaluated considering several factors such as the grade of the cDAVFs, their location and the complexity of the procedures.
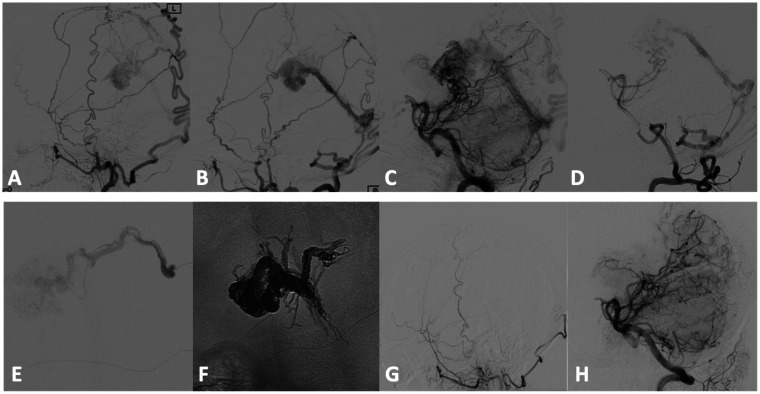
Figure 2.
Patient Nr 5. Preliminary DSA during selective injection of both external carotid arteries (a, b) and vertebral arteries (c, d) shows a high flow DAVF of the vein of Galen causing a major venous outflow impairment of the cerebral circulation (not shown but evident during ICA selective injection). SQUID 12 embolic agent was injected through the distal left MMA (e) after trapping detachable distal tip of the microcatheter with coils (“pressure cooker” technique), in order to reduce proximal reflux. Unsubtracted image (f) shows SQUID 12 cast at the end of the endovascular treatment. Injection time was 30 minutes and the amount of embolic agent was 6 ml. 9–months follow-up (g, h) confirms the complete occlusion of the cDAVF.
We performed 20 embolization procedures in 19 patients. The transarterial approach was chosen in 19 treatments; the remaining treatment was performed by a combined approach. In 15 out of 20 embolizations, MMA was used to access the cDAVF. The MMA generally represents the best choice because it is often much easier to catheterize than other arteries, the long course of its branches increases the tolerance for reflux, and it is well anchored to the dura so this reduces the risks of arterial rupture during the retrieval of the microcatheter.7
Out of 20 procedures, we experienced the rupture of the detachable tip of the microcatheter during one SQUID injection. In this case, we were forced to leave the tip of the microcatheter in place and choose another feeder to complete the treatment. We did not have other common technical complications, such as arterial vasospasm, dissection or microcatheter occlusion/entrapment.
We recorded a rate of adverse clinical events of 11% (2/19), similar to PHIL11 and Onyx series.19 Common clinical complications associated with arterial embolization include cranial nerve deficits, venous occlusion, brain infarction, reflexive bradycardia.20 In our series, one patient experienced a single episode of seizure the day after the procedure. The patient had a type IV cDAVF of the transverse sinus that had presented a few days before with bleeding; the seizure was likely related to the previous intraparenchymal hemorrhage than the treatment itself. Another patient with a type III cDAVF of the traverse sinus region experienced a facial palsy after the treatment, resolved after 2 weeks of corticosteroids. Cranial nerve palsy is not uncommon after the embolization of cDAVF (2%).18 Despite the lower viscosity (i.e. deeper penetration) of SQUID 12, we recorded a rate of cranial nerve palsy (5%) very similar to Onyx series: Cognard et al.21 reported 1/30 case of third and fourth cranial nerve palsy (3%), Rabinov et al.22 2/35 cases of facial nerve palsy (6%).
Despite the lower viscosity of SQUID 12, we did not experience significant technical difficulty in terms of reflux control. Reflux may be very dangerous, especially when operating close to the skull base due to the risk of cranial nerve injury and accidental embolization through anastomoses into cerebral arteries. In these cases, we performed technical measures such as the “pressure cooker” to reduce the risk of complications (4/20 procedures). When reflux in the feeding pedicle was noticed, the injection was stopped for 1–2 minutes to allow SQUID to precipitate. The average reflux was 1.6 cm but even in the cases with a consistent reflux >2 cm (7/20, 35%), no technical or permanent clinical complications were reported.
The duration of the injection ranged from 2 to 82 minutes, with an average of 33 minutes. The volume of SQUID 12 injection ranged from 0.5 to 6 mL, with an average of 2.8 mL per procedure. These records are comparable to the other EVOH series: in particular, Cognard et al.21 performed transarterial embolization with Onyx of 30 cDAVFs, recording an injection mean duration of 45 minutes (range 7 – 100 minutes) and an average of 2.5 mL of Onyx per patient (range 0.5 - 12.2 mL).
Limitations of the study
It is a retrospective observational study conducted on a small population. These limitations may affect efficacy and safety profile. Moreover, its observational nature does not allow a comparative evaluation with other LEAs. Further investigations (prospective comparative study) are needed to confirm our results.
Conclusions
Embolization with SQUID 12 of cDAVFs seems to be feasible, effective and safe, with a low recurrence rate in the mid-term follow-up. In our series, the lower viscosity allowed an easy penetration of the agent and a high rate of complete occlusion of the cDAVFs with a low risk of complications.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Emilio Lozupone https://orcid.org/0000-0003-3992-9071
Pietro Trombatore https://orcid.org/0000-0003-0749-0084
Iacopo Valente https://orcid.org/0000-0002-0451-2105
References
- 1.Natarajan SK, Ghodke B, Kim LJ, et al. Multimodality treatment of intracranial dural arteriovenous fistulas in the onyx era: a single center experience. World Neurosurg 2010; 73: 365–379. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Dabus G, Rabinov JD, et al. Preliminary experience with onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol 2008. Jan; 29: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995; 82: 166–179. [DOI] [PubMed] [Google Scholar]
- 4.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995; 194: 671–680. [DOI] [PubMed] [Google Scholar]
- 5.Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg 1999; 90: 78–84. [DOI] [PubMed] [Google Scholar]
- 6.Gross BA, Albuquerque FC, McDougall CG, et al. A multi-institutional analysis of the untreated course of cerebral dural arteriovenous fistulas. J Neurosurg 2018; 129: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 7.Hu YC, Newman CB, Dashti SR, et al. Cranial dural arteriovenous fistula: transarterial onyx embolization experience and technical nuances. J Neurointerv Surg 2011; 3: 5–13. [DOI] [PubMed] [Google Scholar]
- 8.Pop R, Mertz L, Ilyes A, et al. Beam hardening artifacts of liquid embolic agents: comparison between squid and onyx. J Neurointerv Surg 2019; 11: 706–709. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi D, Chen J, Pearl M, et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol 2012; 33: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gioppo A, Faragò G, Caldiera V, et al. Medial tentorial dural arteriovenous fistula embolization: single experience with embolic liquid polymer SQUID and review of the literature. World Neurosurg 2017; 107: 1050.e1–e7. [DOI] [PubMed] [Google Scholar]
- 11.Lamin S, Chew HS, Chavda S, et al. Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: a multicenter study. AJNR Am J Neuroradiol 2017; 38: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollherbst DF, Otto R, Do T, et al. Imaging artifacts of onyx and PHIL on conventional CT, cone-beam CT and MRI in an animal model. Interv Neuroradiol 2018; 24: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samaniego EA, Derdeyn CP, Hayakawa M, et al. In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model. Interv Neuroradiol 2018; 24: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollherbst DF, Otto R, Hantz M, et al. Investigation of a new version of the liquid embolic agent PHIL with extra-low-viscosity in an endovascular embolization model. AJNR Am J Neuroradiol 2018; 39: 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akmangit I, Daglioglu E, Kaya T, et al. Preliminary experience with squid: a new liquid embolizing agent for AVM, AV fistulas and tumors. Turk Neurosurg 2014; 24: 565–570. [DOI] [PubMed] [Google Scholar]
- 16.Gross BA, Albuquerque FC, Moon K, et al. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. JNS 2016; 126: 1884–1893. [DOI] [PubMed] [Google Scholar]
- 17.Vollherbst DF, Herweh C, Schönenberger S, et al. The influence of angioarchitectural features on the success of endovascular embolization of cranial dural arteriovenous fistulas with onyx. AJNR Am J Neuroradiol 2019; 40: 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeh-Gonike U, Magand N, Armoiry X, et al. Transarterial onyx embolization of intracranial dural fistulas: a prospective cohort, systematic review, and meta-analysis. Neurosurgery 2018; 82: 854–863. [DOI] [PubMed] [Google Scholar]
- 19.Lv X, Jiang C, Li Y, et al. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using onyx-18. J Neurosurg 2008; 109: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Li X, Sun S, et al. Transarterial ONYX embolization of intracranial dural arteriovenous fistulas in adults. Turk Neurosurg 2016; 26: 518–524. [DOI] [PubMed] [Google Scholar]
- 21.Cognard C, Januel AC, Silva NA, Jr, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using onyx. AJNR Am J Neuroradiol 2008; 29: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinov JD, Yoo AJ, Ogilvy CS, et al. ONYX versus n-BCA for embolization of cranial dural arteriovenous fistulas. J Neurointerv Surg 2013; 5: 306–310. [DOI] [PubMed] [Google Scholar]