Abstract
Purpose
Sigma 1 receptor is a novel therapeutic target for retinal disease. Its activation, using a high-affinity, high-specificity ligand (+)-pentazocine ((+)-PTZ), rescues photoreceptor cells in the rd10 mouse model of RP. Here, we asked whether the robust retinal neuroprotective properties of (+)-PTZ are generalizable to SA4503 and PRE084, two other high-affinity sigma 1 receptor ligands.
Methods
We treated 661W cells with SA4503 or PRE084. Cell viability, oxidative stress, and expression of Nrf2 and NRF2-regulated antioxidant genes (Nqo1, Cat, and Sod1) were assessed. Rd10 mice were administered SA4503 (1 mg/kg), PRE084 (0.5 mg/kg), or (+)-PTZ (0.5 mg/kg). Visual acuity, retinal architecture, and retinal electrophysiologic function were measured in vivo and retinal structure was assessed histologically.
Results
Similar to (+)-PTZ, SA4503 and PRE084 improved cell viability, attenuated oxidative stress, and increased Nrf2, Nqo1 and Cat expression. Although treatment of rd10 mice with (+)-PTZ improved visual acuity, increased outer retinal thickness, and improved photopic a- and b-wave responses compared with nontreated rd10 mice, treatment with SA4503 or PRE084 did not. The number of photoreceptor nuclei/100 µm retinal length in SA4503- and PRE084-treated rd10 mice (approximately 11/100) did not differ significantly from nontreated rd10 mice, whereas (+)-PTZ-treated mice had significantly more nuclei (approximately 22/100 µm).
Conclusions
Cell survival and gene regulation experiments yielded similar outcomes when SA4503, PRE084, or (+)-PTZ were conducted in vitro, however neither SA4503 or PRE084 afforded in vivo protection in the severe rd10 retinopathy model comparable to (+)-PTZ. Despite all three compounds demonstrating the potential to activate sigma 1 receptor, the retinal neuroprotective properties of the three ligands differ significantly.
Keywords: sigma 1 receptor, retina, photoreceptor, cutamesine, retinal degeneration
The underpinning of retinal degenerative diseases is the inability of the retina to detect and/or transmit light-triggered signals to the brain. These diseases, often characterized by photoreceptor cell (PRC) loss, are responsible for many cases of incurable blindness.1 Efforts to treat retinal degenerative diseases are under intensive investigation and include gene-specific therapies, transplantation of PRC precursors, induced pluripotent stem cell–derived organoid transplantation, visual prosthetics, and neuroprotection.2 Regarding neuroprotection, there is interest in compounds that can mitigate oxidative stress, which is known to underlie retinal degenerative diseases such as RP.3 A novel target for treatment of retinal degenerative disease is sigma nonopioid intracellular receptor 1 (sigma 1 receptor [Sig1R]).4 Activation of Sig1R attenuates oxidative stress and improves retinal cell viability in in vitro5–11 and in vivo12–17 models of retinal dystrophy.
Over the last several years, we have investigated Sig1R as a therapeutic target in retinal diseases by activating it using the classic prototypical ligand (+)-pentazocine ((+)-PTZ) (or 1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol lactate), which binds to the orthosteric Sig1R binding site (Ki = 1.62 nM). As new Sig1R-targeting drugs are developed, they are tested in binding assays for their ability to displace radiolabeled (+)-PTZ from Sig1R.18,19 This process has allowed major advancements in the Sig1R biology field, including distinctions of two sigma binding sites and identification of a minimal Sig1R pharmacore, featuring a single positively charged nitrogen flanked by two hydrophobic or aromatic moieties of 6 to 10 Å and 2.5 to 3.9 Å in length.20
Our studies using (+)-PTZ demonstrated marked attenuation of ganglion cell death in the Ins2Akita/+ mouse model of diabetic retinopathy17 and rescue of cone PRCs in the Pde6brd10/J (rd10) mouse model of RP.13 Owing to its devastating retinopathy,21 the promising findings in rd10 mice are particularly noteworthy. Rd10 mice lose PRCs rapidly over the first 6 weeks of life, with fewer than one row of cells present in the outer nuclear layer (ONL) by postnatal day 42 (P42). Manifestations of rd10 retinopathy include reduced scotopic and photopic ERG responses21 and markedly decreased visual acuity.22,23 When administered (+)-PTZ, rd10 mice showed significant preservation of cone function by ERG, an extension of visual acuity measured as the optokinetic response and improved retinal architecture assessed by spectral domain optical coherence tomography (SD-OCT).13,14
We know of no other studies of Sig1R activation in rd10 mice. However, improved function has been reported when the Sig1R ligand, SA4503, was administered in a light-induced retinal degeneration model. SA4503 (1-(3,4-dimethyoxyphenethyl)-4-(3-phenylpropyl) piperazine dihydrochloride) has a high affinity for Sig1R (Ki = 4.6 nm).24 Adult mice exposed to visible light (8000 lux) for 3 hours had reduced scotopic a- and b-waves and reduced ONL thickness.12 When SA4503 was injected intravitreally 1 hour before intense light exposure, the mice had significantly improved scotopic ERG responses and the ONL was thicker than nontreated mice. A second promising Sig1R compound for neurodegenerative diseases is PRE084. PRE084 (2-(4-morpholino)ethyl 1-phenylcyclo-hexane-1-carboxylate hydrochloride), derived from phencyclidine, shows a high affinity for Sig1R (Ki = 2.2 nM).25 PRE084 injected intravitreally in rats abrogated retinal toxicity induced by amyloid beta.15 Additionally, PRE084-induced Sig1R activation inhibited osmotic swelling of retinal Müller glial cells, suggesting that Sig1R may be useful in conditions of retinal edema.26
The chemical structures of (+)-PTZ, SA4503 and PRE084 (Fig. 1) reflect an intriguing aspect of Sig1R pharmacology, which is its ability to bind (with moderate to high affinity), a wide spectrum of known compounds of very different structural classes. Given that SA4503 and PRE084 exhibit a high affinity for Sig1R and provide neuroprotection in induced-retinal degeneration models, we investigated whether either of these compounds would afford benefit in severe, inherited retinal dystrophy similar to (+)-PTZ. Here, we compared the oxidative stress-reducing properties of SA4503 and PRE084 to (+)-PTZ in a cone PRC line followed by a comprehensive analysis of these Sig1R ligands administered systemically to rd10 mice.
Figure 1.
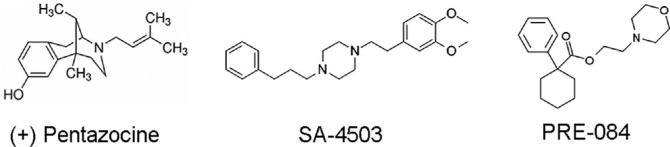
Chemical structure of three Sig1R ligands: (+)-PTZ, SA4503, and PRE084. (A) (+) pentazocine ((+)-PTZ) is a high affinity, highly selective Sig1R ligand (IC50 (nM) 2.34; Ki (nM) 1.62). It is frequently used in binding assays to establish whether other compounds can bind to Sig1R.19 (B) SA-4503, high affinity, selective Sig1R ligand (IC50 [nM] 6.67; Ki [nM] 4.6). (C) PRE084, high affinity, selective Sig1R ligand (Ki [nM] 2.2 nM; IC50 [nM] 44).
Methods
Cell Culture and Assessment of Cell Viability
We obtained 661W cells via a material transfer agreement with Dr. M. Al-Ubaidi (University of Houston). The cells express blue and green cone pigments, transducin, and cone arrestin, which are proteins expressed in cone photoreceptors.27 They were cultured in Dulbecco's modified Eagle's medium (ThermoFisher Scientific, Waltham, MA) containing 100 U/mL penicillin and 100 µg/mL streptomycin supplemented with 5% fetal bovine serum for the maintenance culture or 1% fetal bovine serum for the treatment studies. Cells were exposed to tert-butyl hydroperoxide (tBHP) to induce oxidative stress.28,29,42 The tBHP (5.5 M in decane; Sigma-Aldrich, St. Louis, MO) was dissolved in 0.01 M PBS. Cells were treated for 24 hours with SA4503, PRE084, or (+)-PTZ over a concentration range (3, 25, 50, and 100 µM) in the presence or absence of tBHP (55 µM). In addition, some samples were treated with tBHP, the Sig1R ligand plus BD1063, a Sig1R antagonist. SA4503 and PRE084 were purchased from Tocris Bioscience (Minneapolis, MN); (+)-PTZ and BD1063 were from Sigma-Aldrich. Cell viability was measured using Vybrant MTT Cell Proliferation Assay Kit (ThermoFisher), which measures reduction of yellow 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase. In metabolically active cells, MTT enters the cell and passes into mitochondria where it is reduced to formazan, an insoluble, dark purple product. Cells were solubilized in dimethylsulfoxide and released, solubilized formazan reagent was measured spectrophotometrically using a Synergy H1 Hybrid Multi-Mode plate reader (Winooski, VT) at 540 nm. Three independent experiments were performed with four repetitions per assay.
Assessment of Oxidative Stress In Vitro
The capacity of SA4503 and PRE084 to mitigate oxidative stress was compared with that of (+)-PTZ. The 661W cells were seeded on coverslips and exposed to tBHP (55 µM) for 2 hours in the presence or absence of SA4503 (3, 50, and 100 µM), PRE084 (3, 50, and 100 µM) or (+)-PTZ (3 and 50 µM). Control cells received no tBHP nor treatment with Sig1R ligands. To detect intracellular reactive oxygen species (ROS), cells were incubated with 5 µM CellROX Green Reagent (ThermoFisher). CellROX Green reagent is a cell-permeant dye that fluoresces weakly in a reduced state but brightly upon oxidation by ROS with an absorption and emission maxima of approximately 485 and 520 nm, respectively. It is used in living cells to detect hydroxyl, peroxyl, peroxynitrite, and hydroxyl radicals. DAPI was used to detect cell nuclei. Immunofluorescent detection was performed using a Zeiss Axio Imager D2 microscope (Carl Zeiss, Göttingen, Germany) and Zeiss Axiocam 305 camera equipped with ZenPro software. Fluorescence intensity was quantified using National Institutes of Health Image J 1.48v software (National Institutes of Health, Bethesda, MD). For this analysis, three independent experiments were performed with three repetitions per assay.
Assessment of Antioxidant Gene Expression In Vitro
Previous studies suggest that Sig1Rs regulate ROS by modulating NRF2.9,30,31 We investigated whether treatment of 661W cells with SA4503 or PRE084 increased expression of Nrf2 and genes whose transcription is regulated by NRF2 including Nqo1, Cat, and Hmox1 compared with cells treated with (+)-PTZ. Cells were treated for 8 hours in the presence or absence of SA4503 (3, 50, and 100 µM), PRE084 (3, 50, and 100 µM), or (+)-PTZ (3 and 50 µM). Expression levels of mRNA transcripts specific for Nrf2, Nqo1, Cat, and Hmox1 were determined by quantitative RT-PCR. Total RNA, purified from cells using TRIzol (Invitrogen, Carlsbad, CA), was reverse transcribed using the iScript Synthesis kit (Bio-Rad Hercules, CA). The cDNAs were amplified for 40 cycles using SsoAdvanced SYBR Green Supermix (Bio-Rad) and gene-specific primers (Table 1) in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Expression levels were calculated by comparing cycle threshold values.32 Two independent experiments were performed with three repetitions of each analysis.
Table 1.
Sequences of Primers Used for Real-Time Quantitative Reverse Transcription Plus the Polymerase Chain Reaction. Cat, Catalase; Nqo1, Nicotinamide Adenine Dinucleotide Phosphate (NAD(P)H)-Quinone Dehydrogenase 1, Hmox1, Heme Oxygenase 1; Nrf2, Nuclear Factor Erythroid 2-Related Factor 2; Gapdh, Glyceraldehyde 3-Phosphate Dehydrogenase
| Gene | NCBI Accession Number | Primer Sequence | Product Size (bp) | |
|---|---|---|---|---|
| Cat | NM_009804 | Forward | 5′-AGCGACCAGATGAAGCAGTG-3′ | |
| Reverse | 5′-TCCGCTCTCTGTCAAAGTGTG-3′ | 181 | ||
| Nqo1 | NM_008706 | Forward | 5′-AGGATGGGAGGTACTCGAATC-3′ | |
| Reverse | 5′-AGGCGTCCTTCCTTATATGCTA-3′ | 144 | ||
| Hmox1 | NM_010442 | Forward | 5′-AAGCCGAGAATGCTGAGTTCA-3′ | |
| Reverse | 5′-GCCGTGTAGATATGGTACAAGGA-3′ | 100 | ||
| Nrf2 | NM_010902 | Forward | 5′-TAGATGACCATGAGTCGCTTGC-3′ | |
| Reverse | 5′-GCCAAACTTGCTCCATGTCC-3′ | 153 | ||
| GAPDH | NM_008084 | Forward | 5′-AGGTCGGTGTGAACGGATTTG-3′ | |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | 123 |
Assessment of Sig1R Protein Levels In Vitro
We investigated whether treatment of cells with SA4503 and PRE084 altered levels of Sig1R as reported for (+)-PTZ.31 The 661W cells were treated for 24 hours with SA4503, PRE084 or (+)-PTZ (3 and 25 µM). Total protein was extracted, subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Immunoblotting was performed to assess Sig1R levels using an antibody generated and validated in our laboratory.33,34 Proteins were visualized using the SuperSignal West Pico Chemiluminescent Substrate detection system (Pierce Biotechnology/ThermoFisher). β-Actin served as the loading control. Band density was quantified using ImageJ (National Institutes of Health) and data are represented as a ratio of Sig1R/β-actin. Two independent experiments were performed with three repetitions of each analysis.
Animals and Administration of SA4503, PRE084, or (+)-PTZ
Breeding pairs of B6.CXBI-Pde6βrd10/J (rd10) mice (Jackson Laboratories, Bar Harbor, ME) were used to establish our colony; genotype was confirmed.13 Mice were negative for Crb1rd8/rd8.35 Teklad Irradiated Rodent diets 8904 and 2918 (Teklad, Madison, WI) were provided for breeding and maintenance, respectively. Mice were maintained on a standard 12-hour light:dark cycle. We adhered to institutional guidelines for humane treatment of animals and to the ARVO statement for Use of Animals in Ophthalmic and Vision Research. Four groups of mice were evaluated over a period of 42 days: rd10 mice (nontreated), rd10-SA4503, rd10-PRE084, and rd10 (+)-PTZ mice. Mice received an intraperitoneal (i.p.) injection on alternate days beginning at P14 of: SA4503 (1 mg/kg, dissolved in 0.01 M PBS), or PRE084 (0.5 mg/kg, dissolved in 0.01 M PBS), or (+)-PTZ, (0.5 mg/kg, dissolved in dimethylsulfoxide and 0.01 M PBS). The dosage of SA4503 was based on studies showing benefit against experimental stroke at a concentration that ranged from 0.3 to 1.0 mg/kg.36 The concentration of PRE084 was based on promising results in experimental models of neurodegenerative diseases including ALS and Parkinson's over a range from 0.25 to 1.0 mg/kg.37,38 The dosage of (+)-PTZ was based on our previously published reports.13,14 Table 2 provides information on the ages and numbers of mice used.
Table 2.
Number of Animals Used in the Study
| Mouse Group | N | Age (Postnatal Days) |
|---|---|---|
| ERG analysis of retinal function | ||
| WT | 6 | 35 |
| rd10 | 7 | 35 |
| rd10 (0.5 mg kg-1 (+)-PTZ) | 7 | 35 |
| rd10 (1 mg kg-1 SA4503) | 4 | 35 |
| rd10 (0.5 mg kg-1 PRE084) | 4 | 35 |
| OCT analysis of retinal structure | ||
| WT | 6 | 42 |
| rd10 | 7 | 42 |
| rd10 (0.5 mg kg-1 (+)-PTZ) | 7 | 42 |
| rd10 (1 mg kg-1 SA4503) | 4 | 42 |
| rd10 (0.5 mg kg-1 PRE084) | 4 | 42 |
| OptoMotry analysis of vision acuity | ||
| WT | 6 | 21 |
| rd10 | 7 | 21 |
| rd10 (0.5 mg kg-1 (+)-PTZ) | 7 | 21 |
| rd10 (1 mg kg-1 SA4503) | 4 | 21 |
| rd10 (0.5 mg kg-1 PRE084) | 4 | 21 |
| WT | 6 | 28 |
| rd10 | 7 | 28 |
| rd10 (0.5 mg kg-1 (+)-PTZ) | 7 | 28 |
| rd10 (1 mg kg-1 SA4503) | 4 | 28 |
| rd10 (0.5 mg kg-1 PRE084) | 4 | 28 |
| WT | 6 | 41 |
| rd10 | 7 | 41 |
| rd10 (0.5 mg kg-1 (+)-PTZ) | 7 | 41 |
| rd10 (1 mg kg-1 SA4503) | 4 | 41 |
| rd10 (0.5 mg kg-1 PRE084) | 4 | 41 |
Visual Acuity Assessment
Visual acuity was evaluated in rd10-nontreated, rd10-SA4503, rd10-PRE084, and rd10-(+)-PTZ mice ages P21, P28, P35, and P42. Wild-type (WT) (C57Bl/6J) mice (Jackson Laboratories), were included for comparison. Acuity was measured as described.39–41 The OptoMotry system (CerebralMechanics, Medicine Hat, Alberta, Canada) was used to measure spatial thresholds for optokinetic tracking of sine-wave gratings. Mice, situated on a pedestal within a four-sided chamber, were presented vertical sine-wave gratings moving at 12°/s or gray of the same mean luminance. Grating rotation elicited reflexive tracking, which was scored via live video using a method-of-limits procedure with a yes/no criterion. Spatial resolution was taken as the asymptote of a staircase procedure. The two eyes were tested in an interleaved fashion.
ERG
Rd10-non, rd10-SA4503, rd10-PRE084, and rd10-(+)-PTZ mice were dark adapted overnight. At P35, mice were anesthetized by i.p. injection of a ketamine/xylazine cocktail, pupils were dilated maximally, and mice were placed on a heated platform. Electrodes were placed on the cornea using 0.3% hypromellose solution; ERGs were obtained using the Celeris Ophthalmic Electrophysiology System (Diagnosys LLC, Lowell, MA) following a “touch/touch” protocol that stimulates one eye at a time and uses the fellow, unstimulated eye as reference. Dark-adapted rod function was assessed using a series of tests with flashes of increasing energy (0.001–1.000 cd.s/m2), followed by assessment of light-adapted cone function using flashes of 3 cd.s/m2 and 10 cd.s/m2.
SD-OCT
Rd10-non, rd10-SA4503, rd10-PRE084, and rd10-(+)-PTZ mice were anesthetized with ketamine/xylazine. The Bioptigen Spectral Domain Ophthalmic Imaging System (Bioptigen Envisu R2200, NC) was used to examine retinal architecture in mice at P42.39,40 Imaging included averaged volume intensity scans centered on the optic nerve head and single B scans. Owing to significant outer retinal disruption in rd10 mice, we used the manual caliper feature to measure total retinal thickness (TRT), the inner retina (from upper edge of inner limiting membrane to lower edge of inner nuclear layer [INL]), outer retina (from lower INL edge to inferior RPE boundary).
Morphometric Analysis of Retinas
Eyes were harvested from euthanized rd10-non, rd10-SA4503, rd10-PRE084, and rd10-(+)-PTZ mice (age P42). They were either processed for embedding in JB-4 methacrylate or flash frozen for cryosectioning as described.40 Sections were viewed using a Zeiss Axio Imager D2 microscope (Carl Zeiss) equipped with a high-resolution camera and processed using Zeiss Zen23pro software. Given extensive PRC loss in the rd10 mouse, we measured ONL thickness and also counted the total number of PRC nuclei along the full length of retina (from the temporal to nasal ora serrata) in three retinal sections per mouse. Nuclei counts were averaged per mouse and per treatment group. To evaluate whether treatment with the different Sig1R ligands was associated with decreased oxidation in retina, cryosections were incubated with hydroethidine as reported13 (dihydroethidium, Molecular Probes/ThermoFisher). Retinal cryosections were viewed by epifluorescence using the Zeiss Axio Imager D2 microscope. To evaluate whether cone PRCs were preserved, retinal cryosections were subjected to FITC-labeled peanut agglutinin (PNA) (Millipore/Sigma), which selectively binds cone inner/outer segments. Earlier reports showed increased PNA-labeling in (+)-PTZ-treated rd10 mice compared with nontreated mice.13
Data Analysis
Statistical analysis used GraphPad Prism Analytical Program (La Jolla, CA). Data were analyzed by one-way or two-way ANOVA; Tukey's test was used as the post hoc test and significance was set at a P value of less than 0.05.
Results
Cell Viability and Oxidative Stress
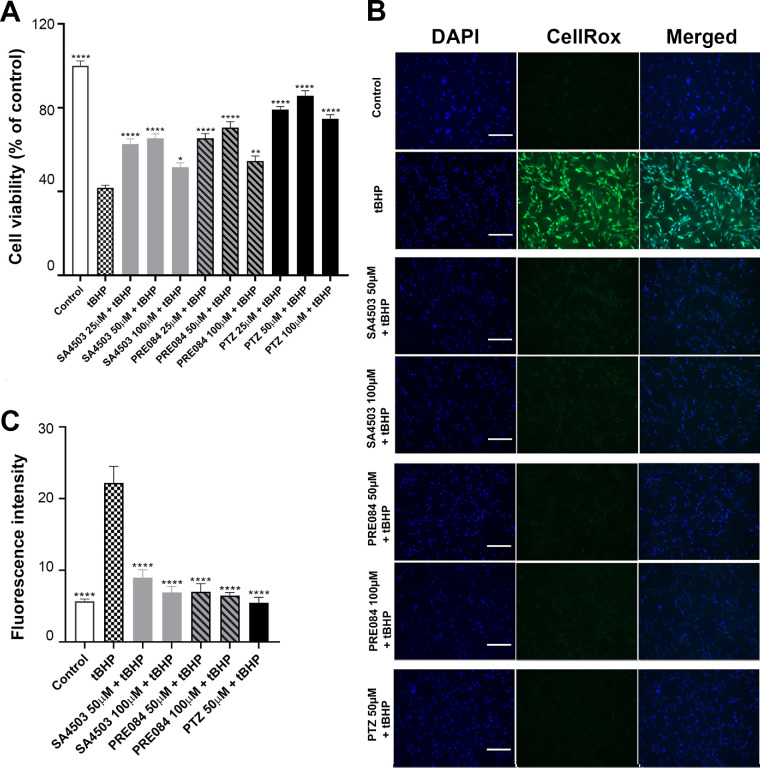
We recently reported that (+)-PTZ enhanced viability of 661W cells, which had been exposed to tBHP.31 To investigate whether SA4503 or PRE084 enhanced viability in a manner similar to (+)-PTZ, we treated 661W cells with SA4503 or PRE084 (25, 50, and 100 µM) concurrent with tBHP for 24 hours. Cell viability was measured using the MTT assay. tBHP exposure decreased cell survival (viability approximately 40%, Fig. 2A). Treatment of tBHP-stressed cells with SA4503 or PRE084 (25 or 50 µM) improved viability in a manner that approached that of (+)-PTZ (Fig. 2A). Treatment of tBHP-stressed cells with a lower concentration of the Sig1R ligands (3 µM) improved viability as well, although cotreatment with BD1063 abrogated the beneficial effects of the treatments (Supplementary Fig. 1A).
Figure 2.
Analysis of cell viability and oxidative stress following exposure to SA4503, PRE084, and (+)-PTZ. (A) These 661W cells were exposed to tBHP (55 µM) to induce oxidative stress in the presence/absence of Sig1R ligands SA4503 (25, 50, and 100 µM), PRE084 (25, 50, and 100 µM), or (+)-PTZ (25, 50, and 100 µM) for 24 hours. Cell viability was measured using the MTT assay. Independent experiments were performed three times with four repetitions per assay. (B) These 661W cells were seeded on coverslips for 18 hours, after which they were exposed 2 hours to tBHP (55 µM) in the presence or absence of SA4503 (50 and 100 µM), PRE084 (50 and 100 µM), or (+)-PTZ (50 µM). They were incubated with CellROX Green Reagent to detect ROS; green fluorescent signals indicating ROS were visualized by epifluorescence. DAPI was used to label nuclei (blue). Calibration bars = 100 µm. (C) Fluorescence intensity was quantified by ImageJ. The experiment was performed three times with three repetitions of the assay. One-way ANOVA, significance is indicated in reference to tBHP group: *P < 0.05; **P < 0.01; ****P < 0.0001.
We investigated whether oxidative stress would be attenuated by treatment with SA4503 or PRE084 as observed with (+)-PTZ.31 Oxidative stress induced by tBHP (55 µM), dramatically increased ROS (reflected as bright green fluorescence using the CellROX reagent compared with untreated cells) (Fig. 2B). Cotreatment with SA4503 (25, 50, and 100 µM] or PRE084 (25, 50, and 100 µM) significantly decreased ROS in a manner similar to (+)-PTZ (Fig. 2B). Quantification of fluorescence reflects this significant attenuation of oxidative stress using all three Sig1R ligands (Fig. 2C). Treatment of cells with SA4503, PRE084 or (+)-PTZ alone did not alter the fluorescence levels (data not shown). We asked whether the three Sig1R ligands would alter ROS levels when administered at a lower dosage (3 µM) and observed attenuated oxidative stress at this concentration as well (Supplementary Figs. 1B, 1C).
Gene Expression and Protein Levels
Sig1R-mediated retinal neuroprotection has been associated with NRF2 modulation. The studies utilized (+)-PTZ as the Sig1R ligand and experiments demonstrated increased expression of Nrf2 and genes whose transcription is regulated by NRF2.9,31 Given that SA4503 or PRE084 attenuated oxidative stress (Figs. 2B, 2C and Supplementary Fig. 1B, 1C), we investigated their effects on expression of Nrf2 and Nqo1, Cat, and Hmox1 compared with (+)-PTZ. We examined effects of higher dosages (50 and 100 µM) (Figs. 3A–D), as well as a lower dosage (3 µM) (Figs. 3E–H). Exposure to Sig1R ligands SA4503, PRE084 and (+)-PTZ resulted in significantly increased Nrf2 expression (Figs. 3A, 3E), at both higher and lower dosages. In general, there was an increase in expression levels of Nqo1 when cells were exposed to either of the three ligands (Figs. 3B, 3F), which was observed also with Cat expression levels (Figs. 3C, 3G). Expression of Hmox1 (Figs. 3D, 3H) increased in cells exposed to 3, 50, and 100 µM of PRE084 compared with (+)-PTZ, whereas only the highest dosage of SA4503 (100 µM) resulted in expression increase. Thus, the Sig1R ligands have a positive modulatory effects on expression of Nrf2 and some of its downstream targets.
Figure 3.
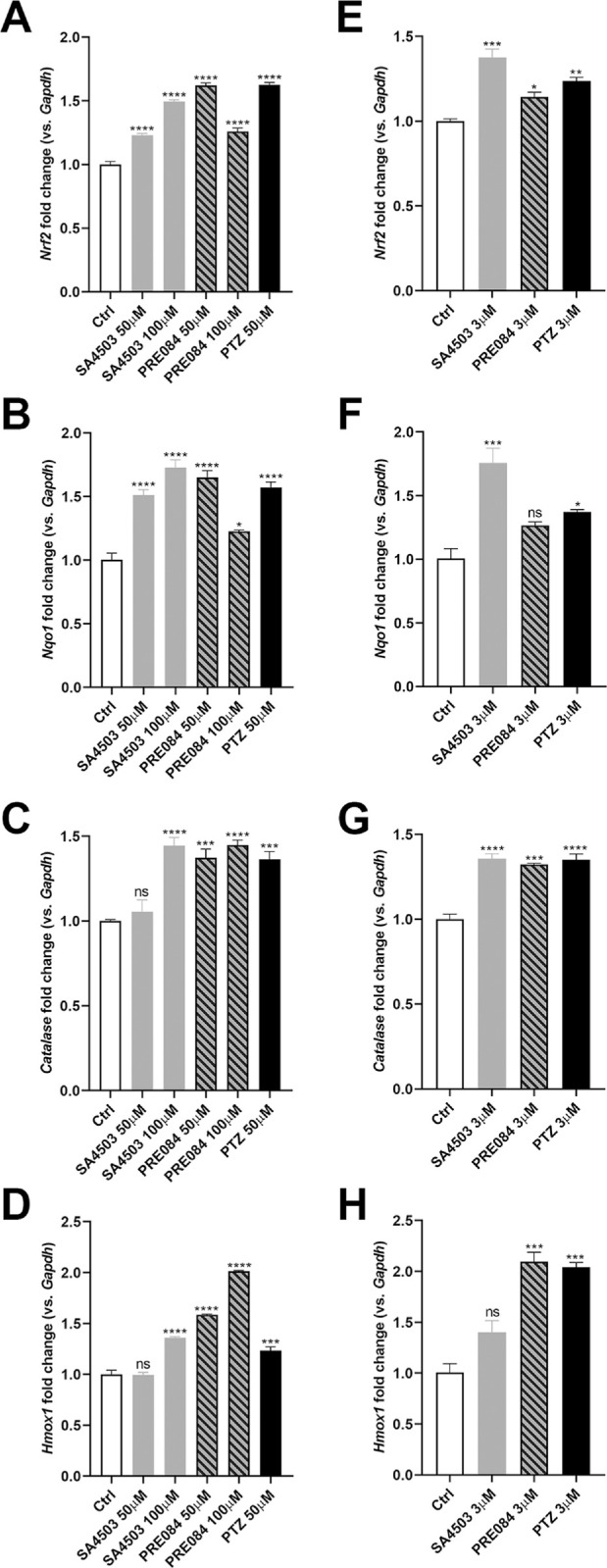
Analysis of expression of Nrf2 and NRF2-related genes after exposure to SA4503, PRE084 and (+)-PTZ. 661W cells were treated with SA4503 (50 and 100 µM), PRE084 (50 and 100 µM) or (+)-PTZ (50 µM) for 8 hours. In additional studies, cells were treated with a lower concentration of the Sig1R ligands (3 µM). RNA was isolated and subjected to quantitative RT-PCR to analyze the expression of (A, E) Nrf2, (B, F) Nqo1, (C, G) Cat, and (D, H) Hmox1. Data are mean ± SEM of two independent experiments with three repetitions per assay. One-way ANOVA, significant differences (*P < 0.05; **P < 0.01;***P < 0.001; ****P < 0.0001; ns, not significant) are in reference to control (nontreated) cells.
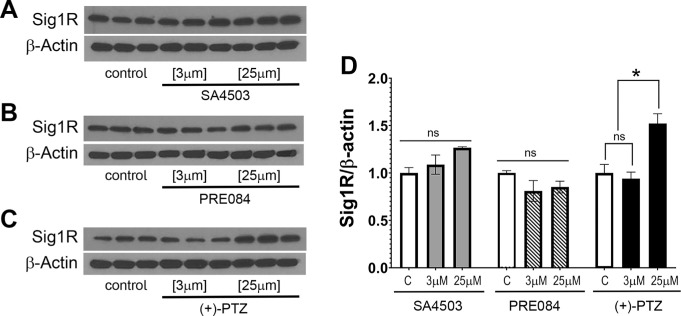
We examined whether exposure of 661W cells to SA4503 or PRE084 would alter levels of Sig1R protein as we have observed previously with (+)-PTZ treatment.31 The immunoblotting experiments showed that neither SA4503 nor PRE084 induced a significant increase in Sig1R protein levels at the concentrations tested (3 and 25 µM) (Figs. 4A and 4B), whereas there was a significant increase when cells were treated with (+)-PTZ (25 µM) (Fig. 4C). In keeping with earlier reports,31 exposure of 661W cells to 3 µM (+)-PTZ did not alter Sig1R protein levels compared with nontreated cells (Fig. 4C). Quantification of the ratio of Sig1R levels compared with levels of β-actin are shown (Fig. 4D).
Figure 4.
Analysis of Sig1R protein levels after exposure to SA4503, PRE084 and (+)-PTZ. These 661W cells were treated with SA4503 (3 and 25 µM), PRE084 (3 and 25 µM), or (+)-PTZ (3 and 25 µM) for 24 hours. Immunoblotting was performed to determine the level of Sig1R protein after treatment with Sig1R ligands compared with no treatment. β-Actin served as protein loading control. Data are mean ± SEM of two independent experiments with three repetitions per assay. Two-way ANOVA, significant differences (*P < 0.05; ns, not significant) are in reference to control (nontreated) cells.
Visual Acuity
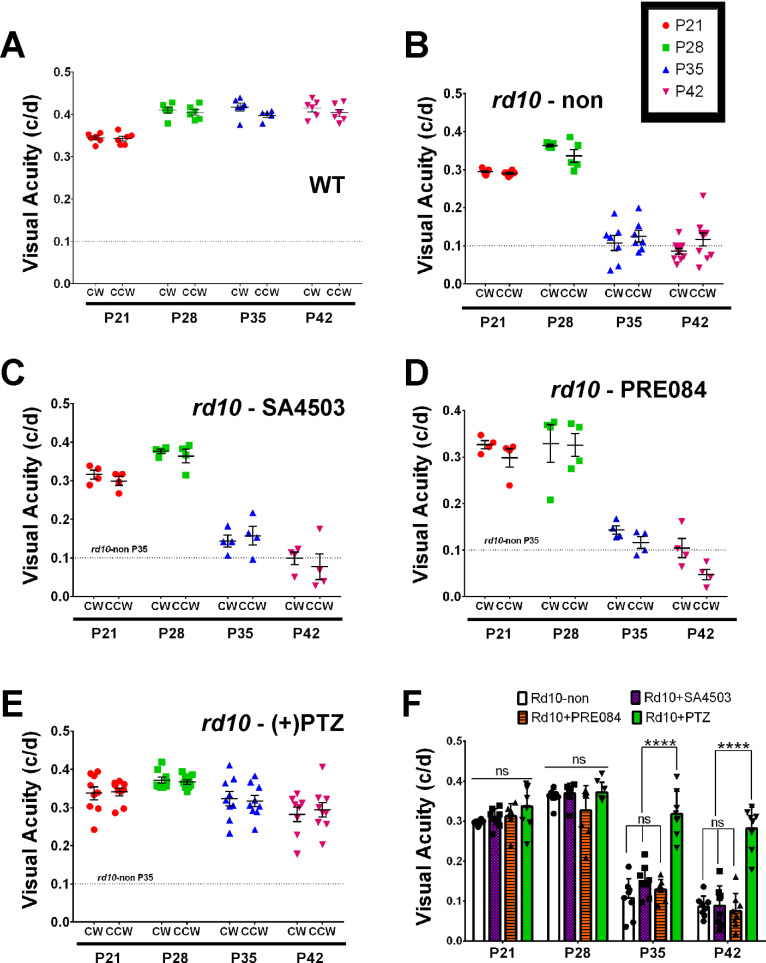
In WT mice, visual acuity registers approximately 0.35 c/d at P21 and, with age, plateaus to approximately 0.4 c/d. Our measurements at P28, P35, and P42 were consistent with this trend (Fig. 5A). In contrast, nontreated rd10 mice rapidly declined in visual acuity over the 6-week testing period. The acuity at P28 was approximately 0.36 c/d, it was decreased to approximately 0.1 c/d when tested at P35 and P42 (Fig. 5B). In rd10 mice treated with SA4503, the visual acuity was approximately 0.35 at P28; it decreased significantly to approximately 0.15 by P35 and approximately 0.1 by P42 (Fig. 5C), which was similar to nontreated rd10 mice. In rd10 mice administered PRE084, the visual acuity was approximately 0.32 at P28, and also decreased significantly to approximately 0.12 by P35 and approximately 0.08 by P42 (Fig. 5D). In contrast, (+)-PTZ–treated rd10 mice retained visual acuity through P42 (median visual acuity was approximately 0.35 c/d at P21, approximately 0.38 c/d at P28, approximately 0.34 c/d at P35, and approximately 0.31 c/d at P42) (Fig. 5E). The averaged values for visual acuity data are shown (Fig. 5F).
Figure 5.
Assessment of visual acuity in rd10 mice treated with SA4503, PRE084, or (+)-PTZ. The rd10 mice were administered SA4503, PRE-084, or (+)-PTZ every other day beginning at P14 and visual acuity was compared with WT and nontreated rd10 mice. The optokinetic tracking response (OKR) was recorded at P21, P28, P35, and P42. Data are expressed as cycles/degree (c/d) for (A) WT mice, (B) nontreated rd10 mice, (C) rd10-SA4503 mice, (D) rd10-PRE084 mice, and (E) rd10-(+)-PTZ mice. (F) Data from panels A–E are summarized to reflect statistical significance among treatment groups. Two-way ANOVA, significance is depicted as: ****P < 0.0001, ns = not significant. CW = left eye, CCW = right eye. As a reference, a feint dotted line has been inserted in panels A–E to reflect visual acuity of rd10 mice at P35.
ERG Assessment
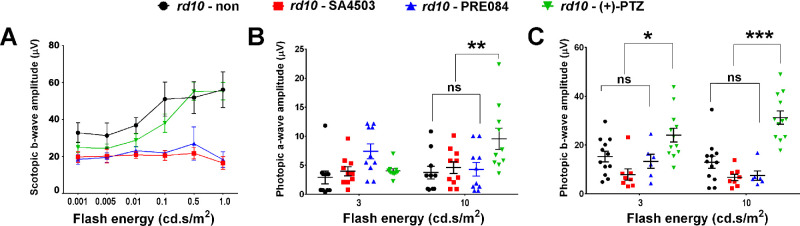
We assessed rod (scotopic) and cone (photopic) responses at P35 in rd10 mice administered SA4503, PRE084 or (+)-PTZ. Responses were compared with age-matched, rd10 nontreated mice. Regarding rod activity, there was minimal detection of function at low light intensities, but detectable responses, albeit low, at the highest intensities (Fig. 6A). The b-wave amplitude recorded for SA4503- or PRE084-treated mice was quite low at all intensities compared with nontreated and with (+)-PTZ–treated rd10 mice (Fig. 6A). These data are consistent with earlier reports that (+)-PTZ does not alter rod function significantly in rd10 mice.13 In contrast with rod function, treating rd10 mice with (+)-PTZ improved cone function,13 which was observed in our current assessments of both a- and b-wave amplitudes (Figs. 6B, 6C). Regarding the photopic a-wave, the amplitude was similar between rd10 nontreated, rd10-SA4503, and rd10-PRE084-treated mice at stimuli of 3 and 10 cd.s/m2. It was significantly greater in (+)-PTZ-treated rd10 mice at the stimuli of 10 cd.s/m2 (Fig. 6B). Regarding the photopic b-wave, the amplitude was similar between rd10 nontreated, rd10-SA4503, and rd10-PRE084–treated mice at both stimuli (3 and 10 cd.s/m2). It was significantly greater in (+)-PTZ–treated rd10 mice at both flash intensities (Fig. 6C). The data indicate that PRC electrophysiologic responses are more robust in (+)-PTZ–treated rd10 mice compared with SA4503- or PRE084-treated mice, as well as nontreated mice.
Figure 6.
Assessment of retinal function by scotopic and photopic ERG in rd10 mice treated with SA4503 and PRE084. Rd10 mice were administered SA4503, PRE084, or (+)-PTZ every other day beginning at P14 and electrophysiologic responses were compared with nontreated rd10 mice. Scotopic and photopic ERG responses were recorded from rd10-non, rd10-SA4503, rd10-PRE084, and rd10-(+)-PTZ mice at P35. (A) Averaged scotopic ERG b-wave amplitude responses to a series of intensities of flash energy (0.001, 0.005, 0.01, 0.1, 0.5, and 1.0 cd.s/m2). Averaged photopic a-wave amplitude (B) and b-wave amplitude (C) responses to a series of intensities of flash energy (3 and 10 cd.s/m2). Intensities are in units of candela-seconds per meter squared (cd.s/m2). Two-way ANOVA, significance is depicted as *P < 0.05; **P < 0.01, ***P < 0.001, ns = not significant. Data are the mean ± SEM of analyses in four to six mice per group.
SD-OCT Assessment
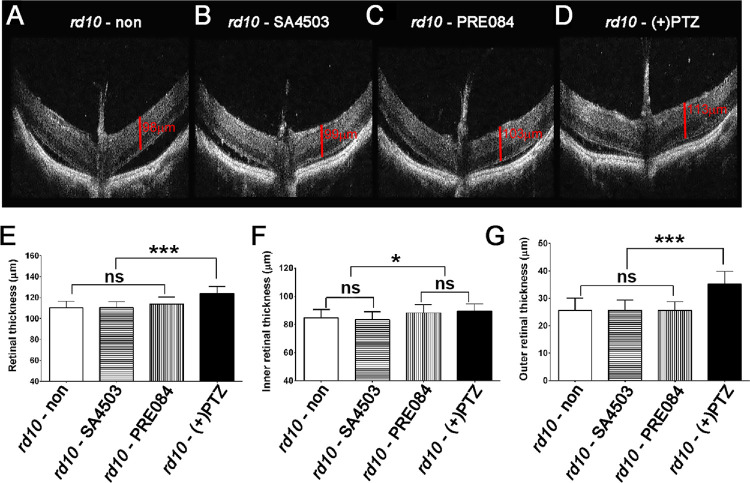
We visualized retinal structure in living mice using SD-OCT. Mice administered either SA4503, PRE084 or (+)-PTZ were subjected to SD-OCT at P42. Representative OCT images are provided (Figs. 7A–7D). We used the calipers feature of the OCT software to determine thickness of the entire retina (TRT), the inner and the outer retina. In WT mice TRT is approximately 225 to 250 µm with the contributions of inner and outer retina being nearly equivalent. In rd10 mice, the TRT averaged slightly more than 100 µm, which was similar to mice treated with SA4503 or PRE084, whereas TRT was significantly greater in (+)-PTZ–treated mice (approximately 124 µm) (Fig. 7E). Regarding the inner retina thickness, nontreated rd10 mice measured approximately 84 µm, which did not differ significantly from SA4503-treated rd10 mice (approximately 83 µm) and was only slightly less than PRE084-treated mice (approximately 88 µm) or (+)-PTZ (approximately 89 µm) (Fig. 7F). The portion of the retina most impacted by the loss of rods in the rd10 retina is the outer retina. Indeed, as early as P42, the average thickness is reduced to approximately 25 µm. When we measured outer retinal thickness of SA4503 or PRE084-treated rd10 mice, we observed no significant differences compared with nontreated rd10 mice, whereas (+)-PTZ–treated mice had significantly thicker outer retinas (approximately 35 µm) (Fig. 7G).
Figure 7.
Assessment of retinal structure in vivo using SD-OCT in rd10 mice treated with SA4503, PRE084, or (+)-PTZ. Rd10 mice were administered SA4503, PRE084, or (+)-PTZ every other day beginning at P14. The retinal architecture was visualized using SD-OCT at P42. Representative SD-OCT images of (A) nontreated rd10 mouse, (B) rd10 - PRE084, (C) rd10 - SA4503, and (D) rd10- (+)PTZ. The manual caliper feature of the Spectral Domain Ophthalmic Imaging System was used to measure (E) TRT, (F) inner retinal thickness, and (G) outer retinal thickness of nontreated rd10 mice compared with rd10 mice treated with SA4503, PRE084 or (+)-PTZ. Data are the mean ± SEM of analyses in four to six mice per group. One-way ANOVA, significance is depicted as *P < 0.05; ***P < 0.001, ns = not significant.
Histologic Assessment
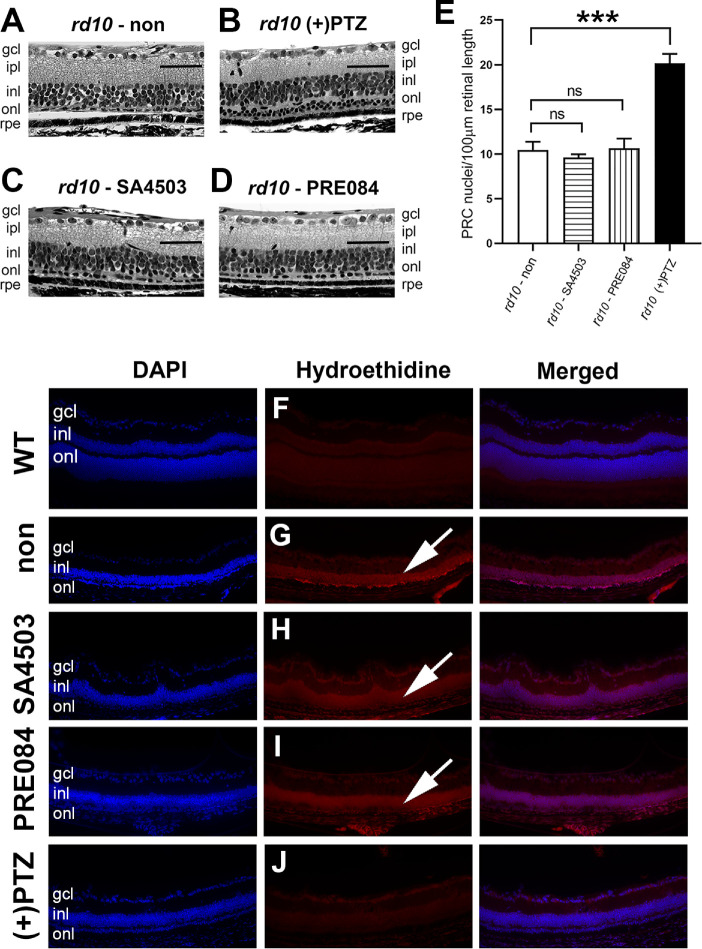
After in vivo analyses, eyes were harvested from rd10 mice treated with SA4503, PRE084, or (+)-PTZ. Representative sections of retinas from mice in each treatment group are provided (Fig. 8). The inner retina of untreated rd10 mice has a fairly normal appearance at P42 with many cells in the ganglion cell layer, a well-formed inner plexiform layer and a multi-layered INL (Fig. 8A). The outer retina of untreated rd10 mice has an abnormal appearance. The outer plexiform layer is difficult to discern reflecting the markedly decreased synaptic input from PRCs. By P42, there are a few PRCs remaining in the ONL; the layer is approximately 1 cell thick (Fig. 8A, arrow). In contrast, (+)-PTZ–treated rd10 mice frequently had two (and sometimes three) rows of cells within the ONL (Fig. 8B, arrow). The retinas of rd10 mice treated with SA4503 had inner retinal features similar to (+)-PTZ–treated mice; however, the ONL was not multilayered. Rather, it was similar to nontreated rd10 mice with approximately one row of cells detectable (Fig. 8C, arrow). A similar phenotype was observed in PRE084-treated mice (Fig. 8D). The number of PRC nuclei in rd10 nontreated retinas was approximately 10 nuclei per 100 µm length, which was similar to SA4503- and PRE084-treated animals. In contrast (+)-PTZ–treated rd10 mice had significantly more nuclei (approximately 20 nuclei per 100 µm) (Fig. 8E). The data suggest more robust PRC rescue in the (+)-PTZ–treated mice compared with mice treated with SA4503 or PRE084. Evaluation of histologic sections using PNA-FITC, a marker for cones, showed robust labeling in WT mice (Supplementary Fig. 2A) and virtually no labeling in nontreated rd10 mice (Supplementary Fig. 2B). There was considerably more labeling of cones in the (+)-PTZ–treated mice (Supplementary Fig. 2E) versus those treated with the other Sig1R ligands (Supplementary Figs. 2C and 2D).
Figure 8.
Assessment of retinal histologic structure in rd10 mice treated with SA4503, PRE084, or (+)-PTZ. Rd10 mice were administered SA4503, PRE084, or (+)-PTZ every other day beginning at P14. Mice were euthanized at P42 and eyes prepared for histology. Representative photomicrographs of hematoxylin-eosin stained retinal sections are provided for (A) rd10 (nontreated), (B) rd10+PTZ, (C) rd10 - SA4503, and (D) rd10 - PRE084. (E) The number of PRC nuclei within the ONL were counted and data expressed per 100 µm retinal length. One-way ANOVA, significance is depicted as ***P < 0.001, ns = not significant. Data are the mean ± SEM of analyses in four to six mice per group. (Arrows in A–D point to row[s] of PRC nuclei.) (F–J) Immunodetection of hydroethidine, which fluoresces red upon reaction with superoxide species in retinal cryosections of (F) WT, (G) rd10-non, (four to six) rd10-SA4503, (I) rd10-PRE084, and (J) rd10-(+)-PTZ. (Arrows in G–I point to red fluorescence.) gcl, ganglion cell layer; ipl, inner plexiform layer. Calibration bars (A–D) = 50 µm; (F–J) = 100 µm.
We investigated whether treatment with SA4503 or PRE084 was associated with decreased oxidation as has been reported previously in (+)-PTZ–treated rd10 mice.13 We subjected retinal cryosections to hydroethidine, which emits red fluorescence when it reacts with superoxide radicals. Red fluorescence was minimal in retinal cryosections of WT mice (Fig. 8F). It was, however, quite notable in retinas of rd10 mice; oxidative stress was detected in the severely decrease the ONL and the INL (Fig. 8G, arrow). There was considerable red fluorescence indicative of oxidative stress detected in retinas of rd10 mice treated with SA4503 (Fig. 8H) or PRE084 (Fig. 8I), whereas red fluorescence was decreased in retinas of PTZ-treated rd10 mice (Fig. 8J) compared with the nontreated rd10 mic, consistent with earlier observations.13
Discussion
We investigated whether the robust retinal neuroprotective properties of (+)-PTZ are generalizable to SA4503 and PRE084, two other high-affinity Sig1R ligands. Although similar characteristics were observed for the three compounds in vitro, there were noticeable differences in vivo. Here, we discuss in vitro versus in vivo findings and the implications for targeting Sig1R in severe retinal disease.
Treatment with either SA4503 or PRE084 improved 661W cell viability and decreased oxidative stress similar to (+)-PTZ, consistent with reports that SA4503 and PRE084 have positive, retinal cell survival promoting properties.6,43,44 Treatment of cells with SA4503 or PRE084 altered the expression of Nrf2 and genes that are under transcriptional control by NRF2. NRF2 (nuclear factor erythroid 2-related factor 2) plays a key role in regulating the oxidative stress response by binding antioxidant response elements of more than 500 antioxidant and cytoprotective genes.45 Elsewhere, we reported that treatment of 661W cells with (+)-PTZ induced dose-dependent increases in NRF2–antioxidant response elements binding activity and NRF2 gene/protein expression.31 Others reported that SA4503 attenuated lipopolysaccharide-induced inflammatory reactions and oxidative stress accompanied by increased Nrf2 and Hmox1 expression.31 The current studies showed that SA4503 and PRE084 upregulated Nrf2, Nqo1 and Cat similar to (+)-PTZ. PRE084 treatment resulted in robust upregulation of Hmox1 compared with the other Sig1R ligands. Although there are slight differences between the ligands with respect to regulation of these antioxidant genes, all three promoted an antioxidant response. Interestingly, treatment of the cells with the three ligands did not yield a similar effect on Sig1R protein levels. Neither SA4503 or PRE084 altered the level of Sig1R protein, whereas treatment with (+)-PTZ (at 25 µM) resulted in an increase in protein level. This observation may be relevant to our differential findings regarding neuroprotective effects in the rd10 mice.
The in vivo studies did not yield comparable results when rd10 mice were administered SA4503 or PRE084 compared with (+)-PTZ. Assessment of visual acuity using the virtual optomotor task confirmed that rd10 mice typically experience a sharp decline between P28 and P35.23,40,14 Treatment of rd10 mice with (+)-PTZ extended visual acuity significantly through P42, the age when the experiment was terminated in the present work. Earlier studies evaluating effects of (+)-PTZ over an extended period of time showed robust acuity through at least 56 days, which is extremely encouraging for the treatment of retinal disease.14 In the current study, we anticipated improvement in the visual acuity in rd10 mice treated with SA4503 or PRE084; however, we did not observe significant differences compared with nontreated mice. We conducted additional analyses in rd10 mice, including ERG and SD-OCT assessments. As with the visual acuity testing, we did not observe improved PRC electrophysiologic activity (by ERG) or improved thickness of the outer retina (by OCT) in SA4503- or PRE084-treated mice. Histologic evaluation of the retina showed twice the number of PRC nuclei in (+)-PTZ–treated rd10 mice versus nontreated mutants, which was not observed in the SA4503 or PRE084-treated mice. The PRCs were primarily cones as demonstrated by PNA labeling. There were some PNA-positive cells detected in SA4503- and PRE084-treated mice, although the labeling was not nearly as robust as in the (+)-PTZ–treated group (Supplementary Fig. 2).
Although Sig1R ligands SA4503 and PRE084 are considered potent Sig1R ligands, they did not yield the retinal neuroprotective properties in vivo (in this study) that have been reported for (+)-PTZ. This finding was unexpected, because both compounds have proven beneficial in other models of neurodegenerative disease. Regarding the administration of SA4503, improved cognitive function was reported in a model of ATR-X syndrome, a genetically linked intellectual disability disease.46 In that study, mutant AtrxΔE2 mice were administered SA4503 via an i.p. route (1 mg/kg) and performed significantly better on a maze test and an object recognition test than nontreated mice. In addition, SA4503 administered to mice in a noise-induced hearing loss model protected hearing function against intense noise.47 A possible explanation for the beneficial effects in these disease models is that SA4503 was administered daily, whereas we administered per the regimen that was in place for (+)-PTZ (every other day). In the case of the noise-induced hearing loss model, SA4503 treatment was actually initiated 10 days before noise exposure and continued daily throughout the study, which may have influenced the positive outcome. Even more relevant to the present study was the study by Hara's group in which SA4503 was used in a mouse model of light-induced retinopathy.12 Initial studies were performed in 661W cells using light-induced cell death and SA4503 (10 µM) prevented caspase 3/7 activation and disruption of mitochondrial membrane potential. Subsequent in vivo studies in mice showed improved ERG and histology. It is important to note, however, that SA4503 was injected before the light damage, thus constituting a pretreatment paradigm. Moreover SA4503 was injected into the vitreous rather than i.p. Because the rd10 mouse is a chronic, genetically induced retinopathy rather than an acute, induced model, we opted to use an i.p. administrative route in our protocol. Our goal was to deliver the ligands over the course of several weeks and it is not practical to administer compounds repeatedly using an intravitreal route, because the mouse eyeball will collapse. The ability to administer the compound systemically and derive benefit is critical to the comparison in a chronic retinal degenerative model.
Regarding administration of PRE084, this Sig1R ligand has also proven useful for treating in vivo neurodegenerative disease models. For example, in two different models of Parkinson's disease, administration of PRE084 (1 mg/kg, i.p.) normalized motor dysfunction and attenuated loss of dopamine neurons in the striatum.48,49 Zhao et al.50 used PRE084 in an ischemia/reperfusion injury model and found that the number of apoptotic cells was decreased and cortical neurons were preserved. A similar finding was reported by Liu et al.51 In their study, daily administration of PRE084 (1 mg/kg i.p.) improved neurobehavioral performance and attenuated neuronal damage and white matter lesions in the brain.51 Daily administration of PRE084 (0.25 mg/kg i.p. for 8 weeks) in the SOD1 (G93A) mouse model of amyotrophic lateral sclerosis maintained the amplitude of muscle action potentials and locomotor behavior and preserved neuromuscular connections in the spinal cord.38 In contrast to the aforementioned promising results is a report of a mouse model of spinal muscular atrophy, a muscle denervation disease that can be severely debilitating. Using the Smn2B/− mouse model of spinal muscular atrophy, which suffers gradual, prominent α-motor neuron degeneration in the lumbar spinal cord, PRE084 was administered (0.25 mg/kg i.p.) over an 8-week period. PRE084 attenuated reactive gliosis, mitigated M1 (harmful) and M2 (beneficial) microglial imbalance, and prevented α-motor neuron deafferentation in Smn2B/− mice. These effects were also observed in a severe spinal muscular atrophy model, the SMNΔ7 mouse. Nevertheless, PRE084 did not diminish the pathologic changes present in neuromuscular junctions nor improve motor function in the mutant models.52
Neurodegenerative diseases of the central nervous system share many pathologic mechanisms with retinal degenerative diseases including oxidative stress, endoplasmic reticulum stress, inflammation, aberrant calcium signaling, and so on. Sig1Rs are recognized targets for therapeutic intervention in these diseases because of their demonstrated properties in mitigating these pathologic phenomena.53 If a given Sig1R ligand shows efficacy in a neurodegenerative model, it is worthwhile to explore its efficacy in retinal degenerative disease. That idea was the basis of the current study comparing two well-established neuroprotective Sig1R ligands, SA4503 and PRE084 with (+)-PTZ, the prototypical ligand. Our study did not yield functional improvements comparable to (+)-PTZ as anticipated. The findings may be a reflection of differences in the structures of the three compounds and their interactions with the complex Sig1R trimeric complex. Of course, the dosing regimen (route of administration, concentration, and frequency of administration) used in the present work may not have been sufficient to afford beneficial effects in rd10 mice, although similar dosing schemes of these compounds were beneficial in other degenerative disease models. The rd10 model is said to have a catastrophic retinopathy and it is conceivable that other, less severe but still chronic, genetic models of retinal disease54,55 might be amenable to treatment with SA4503 or PRE084. The (+)-PTZ–induced activation of Sig1R is associated with modulation of NRF2, as previously reported.31 It is possible, and indeed likely, that (+)-PTZ–induced Sig1R activation mediates other downstream, beneficial pathways that have not yet been uncovered. This is a promising area for future research and determining the extent to which other compounds (such as SA4503 and PRE084) have similar properties would be informative. Another explanation for the differences between (+)-PTZ and the other two Sig1R ligands may be related to compound stability. Although each compound was prepared fresh before administration, it is possible that the stability of the compounds may differ in vivo.
Despite the minimal beneficial effects of SA44503 or PRE084 in attenuating retinal dysfunction in the rd10 mouse, Sig1R remains an important target for neuroprotection in retinal disease. The robust neuroprotective effects of (+)-PTZ provide impetus to continue to test compounds that target Sig1R. Studies have shown that rd10/Sig1R−/− mice are not afforded PRC rescue when treated with (+)-PTZ indicating that (+)-PTZ acts through Sig1R to mediate neuroprotective effects.13 An additional study that could be performed relevant to this observation would be to administer a Sig1R antagonist (such as BD1063 or NE100) in conjunction with (+)-PTZ in rd10 mice and evaluate whether neuroprotective effects are blocked.
Although (+)-PTZ has proven to be a powerful tool to explore Sig1R as a promising target for treating retinopathies, its usefulness in the clinical arena is less certain. (+)-PTZ is approved by the US Food and Drug Administration and controlled by the US Drug Enforcement Agency (as a schedule IV substance) used in the treatment of pain. It was developed in the late 1960s as an analgesic with minimal abuse potential. It is now recognized that patients may develop a sense of euphoria when taking (+)-PTZ and some may experience side effects (respiratory depression, increased heart rate and blood pressure, and skin ulcerations).56 For this reason, it is now marketed as Talwin, because its oral formulation combines it with naloxone, which blocks the euphoric effects. Whether Talwin would have the same retinal neuroprotective effects as the low dosage benefits of (+)-PTZ remains to be evaluated. Despite its significant value to the field of Sig1R biology and its clear neuroprotective properties under highly controlled experimental conditions, (+)-PTZ as a therapeutic strategy may be complicated. Studies are underway by pharmaceutical companies to develop compounds with the specificity and potency of (+)-PTZ without untoward consequences. Our group has been involved in synthesizing new compounds that might mimic (+)-PTZ in efficacy. For example, through a tandem Winstein rearrangement Friedel–Crafts alkylation that enabled the synthesis of differentially functionalized heterocyclic azides, heterocyclic products were converted to primary amine or pyrrolidine motifs. These were investigated for affinity to G-protein coupled receptors including Sig1R and a new lead compound (4J-MRP) was identified as a high-affinity Sig1R ligand.57 This compound improved cell viability and attenuated oxidative stress induced by tBHP. Future studies will investigate its potential in improving retinal function in vivo.
In summary, Sig1R has significant potential as a therapeutic target in retinal disease, although not all ligands are equivalent in their capacity to enhance retinal function. A critical area of future research is the development of compounds with high affinity and selectivity for Sig1R that are also functionally neuroprotective in retina, nontoxic and well-tolerated by human patients.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (R01EY028103) and the Foundation Fighting Blindness (TA-NMT-0617-0721-AUG), an National Eye Institute P30 Center Core Grant for Vision Research (P30 EY031631). We thank Penny Roon for excellent technical support in preparing retinal histology sections. The 661W cells were obtained via a material transfer agreement executed with M. Al-Ubaidi (University of Houston).
Disclosure: J. Wang, None; H. Xiao, None; S.R. Barwick, None; S.B. Smith, None
References
- 1. Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis Model Mech. 2015; 8(2): 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duncan JL, Pierce EA, Laster AM, et al;. and the Foundation Fighting Blindness Scientific Advisory Board. Inherited retinal degenerations: current landscape and knowledge gaps. Transl Vis Sci Technol. 2018; 7(4): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campochiaro PA, Strauss RW, Lu L, et al. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid Redox Signal. 2015; 23(7): 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith SB, Wang J, Cui X, Mysona BA, Zhao J, Bollinger KE. Sigma 1 receptor: a novel therapeutic target in retinal disease. Prog Retin Eye Res. 2018; 67: 130–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellis DZ, Li L, Park Y, He S, Mueller B, Yorio T. Sigma-1 receptor regulates mitochondrial function in glucose- and oxygen-deprived retinal ganglion cells. Invest Ophthalmol Vis Sci. 2017; 58(5): 2755–2764. [DOI] [PubMed] [Google Scholar]
- 6. Bucolo C, Drago F, Lin LR, Reddy VN. Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport. 2006; 17(3): 287–291. [DOI] [PubMed] [Google Scholar]
- 7. Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007; 48(10): 4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res. 2004; 123(1-2): 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Shanmugam A, Markand S, Zorrilla E, Ganapathy V, Smith SB. Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc(−), the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med. 2015; 86: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanmugam A, Wang J, Markand S, et al.. Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) by retinal Müller glial cells. J Neurochem. 2015; 132(5): 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mueller BH 2nd, Y Park, Ma HY, et al.. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp Eye Res. 2014; 128: 156–169. [DOI] [PubMed] [Google Scholar]
- 12. Shimazawa M, Sugitani S, Inoue Y, Tsuruma K, Hara H. Effect of a sigma-1 receptor agonist, cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Exp Eye Res. 2015; 132: 64–72. [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Saul A, Roon P, Smith SB. Activation of the molecular chaperone, sigma 1 receptor, preserves cone function in a murine model of inherited retinal degeneration. Proc Natl Acad Sci U S A. 2016; 113: E3764–E3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Saul A, Smith SB. Activation of sigma 1 receptor extends survival of cones and improves visual acuity in a murine model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2019; 60(13): 4397‐4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantarella G, Bucolo C, Di Benedetto G, et al.. Protective effects of the sigma agonist Pre-084 in the rat retina. Br J Ophthalmol. 2007; 91(10): 1382–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao L, Chen G, Li J, et al.. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J Control Release. 2017; 247: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith SB, Duplantier J, Dun Y, et al.. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008; 49(9): 4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Costa BR, Bowen WD, Hellewell SB, et al.. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989; 251(1-2): 53–58. [DOI] [PubMed] [Google Scholar]
- 19. Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006; 59(6): 350‐358. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt HR, Kruse AC.. The molecular function of σ receptors: past, present, and future. Trends Pharmacol Sci. 2019; 40(9): 636–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007; 500: 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Delgado AB, Valdés-Sánchez L, Calado SM, Diaz-Corrales FJ, Bhattacharya SS. Rasagiline delays retinal degeneration in a mouse model of retinitis pigmentosa via modulation of Bax/Bcl-2 expression. CNS Neurosci Ther. 2018; 24: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanif AM, Lawson EC, Prunty M, et al.. Neuroprotective effects of voluntary exercise in an inherited retinal degeneration mouse model. Invest Ophthalmol Vis Sci. 2015; 56: 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S. Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur J Pharmacol. 1996; 306(1-3): 271–279. [DOI] [PubMed] [Google Scholar]
- 25. Su TP, Wu XZ, Cone EJ, et al.. Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. J Pharmacol Exp Ther. 1991; 259(2): 543–550. [PubMed] [Google Scholar]
- 26. Vogler S, Winters H, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Müller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci Lett. 2016; 610: 13–18. [DOI] [PubMed] [Google Scholar]
- 27. Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004; 45: 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanus J, Kolkin A, Chimienti J, Botsay S, Wang S. 4-Acetoxyphenol prevents RPE oxidative stress-induced necrosis by functioning as an NRF2 stabilizer. Invest Ophthalmol Vis Sci. 2015; 56(9): 5048‐5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Ye XL, Liu R, et al.. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact. 2010; 184(3): 328‐337. [DOI] [PubMed] [Google Scholar]
- 30. Bai T, Lei P, Zhou H, et al.. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med. 2019; 23: 7349–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Zhao J, Cui X, et al.. The molecular chaperone sigma 1 receptor mediates rescue of retinal cone photoreceptor cells via modulation of NRF2. Free Radic Biol Med. 2019; 134: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmittgen TD, Livak KJ.. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 33. Ola MS, Moore P, Maddox D, et al.. Analysis of sigma receptor (sigmaR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res. 2002; 107: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ha Y, Saul A, Tawfik A, et al.. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1). Invest Ophthalmol Vis Sci. 2011; 52: 7749–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang B, Hurd R, Wang J, Nishina P. Survey of common eye diseases in laboratory mouse strains. Invest Ophthalmol Vis Sci. 2013,54: 4974–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruscher K, Shamloo M, Rickhag M, et al.. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011; 134(Pt 3): 732–746. [DOI] [PubMed] [Google Scholar]
- 37. Francardo V, Bez F, Wieloch T, Nissbrandt H, Ruscher K, Cenci MA. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014; 137(Pt 7): 1998–2014. [DOI] [PubMed] [Google Scholar]
- 38. Mancuso R, Oliván S, Rando A, Casas C, Osta R, Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics. 2012; 9(4): 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Navneet S, Zhao J, Wang J, et al.. Hyperhomocysteinemia-induced death of retinal ganglion cells: the role of Müller glial cells and NRF2. Redox Biol. 2019,24: 101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao H, Wang J, Saul A, Smith SB. Comparison of neuroprotective effects of monomethylfumarate to the sigma 1 receptor ligand (+)-pentazocine in a murine model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2020; 61(3): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004,45: 4611–4616. [DOI] [PubMed] [Google Scholar]
- 42. Chang E. 1,25-dihydroxyvitamin D decreases tertiary butyl-hydrogen peroxide-induced oxidative stress and increases AMPK/SIRT1 activation in C2C12 muscle cells. Molecules. 2019; 24(21): 3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Senda T, Mita S, Kaneda K, Kikuchi M, Akaike A. Effect of SA4503, a novel sigma1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur J Pharmacol. 1998; 342(1): 105–111. [DOI] [PubMed] [Google Scholar]
- 44. Zhang XJ, Liu LL, Jiang SX, Zhong YM, Yang XL. Activation of the sigma receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience. 2011; 177: 12–22. [DOI] [PubMed] [Google Scholar]
- 45. Yamamoto M., Kensler TW, Motohashi H.. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 2018,98: 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamaguchi K, Shioda N, Yabuki Y, Zhang C, Han F, Fukunaga K. SA4503, a potent sigma-1 receptor ligand, ameliorates synaptic abnormalities and cognitive dysfunction in a mouse model of ATR-X syndrome. Int J Mol Sci. 2018; 19(9): 2811. Published 2018 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamashita D, Sun GW, Cui Y, et al.. Neuroprotective effects of cutamesine, a ligand of the sigma-1 receptor chaperone, against noise-induced hearing loss. J Neurosci Res. 2015; 93(5): 788–795. [DOI] [PubMed] [Google Scholar]
- 48. Voronin MV, Kadnikov IA, Voronkov DN, Seredenin SB. Chaperone sigma1R mediates the neuroprotective action of afobazole in the 6-OHDA model of Parkinson's disease. Sci Rep. 2019; 9(1): 17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo CH, Cao T, Zheng LT, Waddington JL, Zhen XC. Development and characterization of an inducible Dicer conditional knockout mouse model of Parkinson's disease: validation of the antiparkinsonian effects of a sigma-1 receptor agonist and dihydromyricetin. Acta Pharmacol Sin. 2020; 41(4): 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao X, Zhu L, Liu D, et al.. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis. 2019; 24(1-2): 157–167. [DOI] [PubMed] [Google Scholar]
- 51. Liu DY, Chi TY, Ji XF, et al.. Sigma-1 receptor activation alleviates blood-brain barrier dysfunction in vascular dementia mice. Exp Neurol. 2018; 308: 90–99. [DOI] [PubMed] [Google Scholar]
- 52. Cerveró C, Blasco A, Tarabal O, et al.. Glial activation and central synapse loss, but not motoneuron degeneration, are prevented by the sigma-1 receptor agonist PRE-084 in the Smn2B/- mouse model of spinal muscular atrophy. J Neuropathol Exp Neurol. 2018; 77(7): 577–597. [DOI] [PubMed] [Google Scholar]
- 53. Nguyen L, Lucke-Wold BP, Mookerjee S, Kaushal N, Matsumoto RR. Sigma-1 receptors and neurodegenerative diseases: towards a hypothesis of sigma-1 receptors as amplifiers of neurodegeneration and neuroprotection. Adv Exp Med Biol. 2017; 964: 133–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Collin GB, Gogna N, Chang B, et al.. Mouse models of inherited retinal degeneration with photoreceptor cell loss. Cells. 2020; 9(4): 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gorbatyuk MS, Starr CR, Gorbatyuk OS. Endoplasmic reticulum stress: new insights into the pathogenesis and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2020;100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prasad HR, Khaitan BK, Ramam M, et al.. Diagnostic clinical features of pentazocine-induced ulcers. Int J Dermatol. 2005; 44(11): 910–915. [DOI] [PubMed] [Google Scholar]
- 57. Porter MR, Xiao H, Wang J, Smith SB, Topczewski JJ. 3-Amino-chromanes and tetrahydroquinolines as selective 5-HT2B, 5-HT7, or σ1 receptor ligands. ACS Med Chem Lett. 2019; 10(10): 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.