Abstract
Purpose
To determine whether aging modifies the effect of intraocular pressure (IOP) on progressive glaucomatous retinal nerve fiber layer (RNFL) thinning over time.
Methods
This was a retrospective cohort study involving patients with glaucoma or suspected of having glaucoma who were followed over time from the Duke Glaucoma Registry. Rates of RNFL loss from spectral-domain optical coherence tomography (SD-OCT) were used to assess disease progression. Generalized estimating equations with robust sandwich variance estimators were used to investigate the effects of the interaction of age at baseline and mean IOP on rates of RNFL loss over time. Models were adjusted for gender, race, diagnosis, central corneal thickness, follow-up time, and baseline disease severity.
Results
The study included 85,475 IOP measurements and 60,026 SD-OCT tests of 14,739 eyes of 7814 patients. Eyes had a mean follow-up time of 3.5 ± 1.9 years. The average rate of change in RNFL thickness was –0.70 µm/year (95% confidence interval, –0.72 to –0.67). There was a significant interaction between age and mean IOP and the rate of RNFL loss (P = 0.001), with older eyes having significantly faster rates of RNFL loss than younger ones for the same level of IOP. The effect of IOP on rates of change was greater in the inferior and superior regions of the optic disc.
Conclusions
Age is a significant modifier of the relationship between IOP and glaucomatous loss in RNFL thickness over time. Older patients may be more susceptible to glaucomatous progression than younger patients at the same level of IOP.
Keywords: glaucoma, optical coherence tomography, age, intraocular pressure, retinal nerve fiber layer
Glaucoma is the leading cause of irreversible blindness and is estimated to affect approximately 80 million people worldwide.1 Although reducing intraocular pressure (IOP) helps to decrease the risk of the onset and progression of glaucomatous disease,2–6 many eyes with relatively low IOP continue to progress while others with high IOP levels never develop damage.7–11 Aging plays an important role in glaucoma, as the prevalence of glaucoma increases exponentially with age.12,13 However, the pathophysiology underlying increasing age as a risk factor for glaucoma is not well understood. Interestingly, the increased prevalence of glaucoma in older individuals does not seem to be explained solely by an increased prevalence of high IOP with aging.6,14,15 This suggests that aging may increase the vulnerability of the optic nerve to IOP-related damage, ultimately resulting in loss of retinal ganglion cells (RGCs). This age-related increased vulnerability to neural injury has also been observed in other neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease, and may be related to mitochondrial dysfunction and impaired capacity to handle oxidative stress, among other factors.16–21
If aging increases susceptibility to glaucoma damage as shown in experimental studies, then one would expect that the relationship between RGC loss and IOP would be greater for older patients compared to younger ones. However, clinical studies to date have not adequately investigated the interaction between age and IOP and its effect on glaucoma progression, probably because producing reliable estimates for such an interaction requires a large quantity of data from a heterogeneous population with a wide range of ages. Such data are not typically available from clinical trials, which have limited sample sizes with specific inclusion and exclusion criteria that can be quite restrictive.
In the present study, we investigated the hypothesis that older age is associated with an increased susceptibility to IOP damage by evaluating the interaction effects between age and IOP on the rate of retinal nerve fiber layer (RNFL) thinning. We used a large cohort of glaucoma and suspected glaucoma patients extracted from an electronic health record (EHR) database in order to obtain precise estimates of the interaction effects.
Methods
This was a retrospective cohort study of patients from the Duke Glaucoma Registry, an EHR database developed by the Vision, Imaging, and Performance Laboratory.22 The database contained clinical information from baseline and follow-up visits, including patient diagnostic and procedure codes, medical history, best-corrected visual acuity, slit-lamp biomicroscopy, IOP measurement using Goldmann applanation tonometry (GAT; Haag-Streit, Konig, Switzerland), central corneal thickness (CCT), gonioscopy, ophthalmoscopy examination, stereoscopic optic disc photographs, and the results of all Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) scans from adults 18 years or older with glaucoma or suspected glaucoma diagnoses who were evaluated at the Duke Eye Center or its satellite clinics between January 2009 and September 2019. The Duke University Institutional Review Board approved this study with a waiver of informed consent due to the retrospective nature of this work. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and were conducted in accordance with regulations of the Health Insurance Portability and Accountability Act.
Participant Selection
Patients were included in the study if they had glaucoma or suspicion of glaucoma was suspected based on International Classification of Diseases (ICD) codes at baseline. All tests and visits from all available subjects in the Duke Glaucoma Registry at the time of the analysis were used for this study. Data for eyes were censored after the first occurrence of any diagnosis of retinal detachment, retinal or malignant choroidal tumors, non-glaucomatous disorders of the optic nerve and visual pathways, uveitis, and venous or arterial retinal occlusion according to ICD codes. In addition, tests performed after treatment with panretinal photocoagulation according to Current Procedural Terminology (CPT) codes were also excluded. Tests were further censored after any filtration procedure (i.e., trabeculectomy or tube shunt surgery) that occurred during follow-up. ICD and CPT codes used for inclusion and exclusion in the study have been described in detail previously.11 Finally, subjects were required to have at least two valid SD-OCT scans (see below) and two IOP measures with GAT on different days over a minimum follow-up period of 6 months.
Assessment of Glaucoma Progression
Rates of glaucoma progression were evaluated by changes in global and sectorial average SD-OCT RNFL thicknesses over time. We opted to use a structural metric in this work, as attempts to study this relationship with perimetry, an indirect measurement of neural damage, could be confounded by the subjective nature of the test and by nonlinearities in translating RGC loss to visual sensitivity thresholds (see Discussion section).23–26
Peripapillary RNFL thickness measurements were obtained from 12° (for single circle scans) or comparable 3.45-mm-diameter circle scans (for scans from the Heidelberg Engineering Glaucoma Module Premium Edition) acquired using the Spectralis SD-OCT, as described in detail previously.27 The global average was calculated as the average thickness of all 768 points distributed equidistantly around the optic nerve head. Sectorial RNFL thicknesses were calculated using the averages of the points in each quadrant (i.e., superior, inferior, temporal, and nasal quadrants, as provided by the built-in software). We also calculated the average RNFL thickness within smaller sectors, representing each clock hour around the optic disc (i.e., 12 sectors of 30° each). Tests were acquired using the latest available software version at the time of the scan and exported using the latest available version at the time of the analysis (Heidelberg Engineering Heyex, software version 6.8).
Only good-quality scans were included in the analyses. A good-quality scan was defined as a test with a quality score of 15 or greater. Furthermore, because a manual review of all tests was impractical, scans that had average global RNFL thickness measurements with implausible values (i.e., lower than 20 and greater than 150 µm) were considered to be of low quality and were excluded. Those cutoffs represent measurements above the higher range of reported RNFL thicknesses for normal controls and below the lower range for glaucoma subjects28–30 and may indicate the presence of acquisition or segmentation errors in the presence of otherwise good-quality scores.31 From the total of 136,322 eligible circle scans from the database (i.e., after exclusions for ICD and CPT codes), 6337 tests (4.6%) were excluded due to low-quality scores, and 1678 tests (1.2%) were further excluded due to implausible average RNFL thickness values. When more than one good-quality test was available for the same date, the mean global RNFL thickness of all tests from that date was used in the analysis. Remaining tests were excluded when eyes had less than 6 months of follow-up or due to the unavailability of complete IOP or CCT data. The baseline characteristics and demographics were drawn from the date when the first valid SD-OCT test for each eye was performed.
Data Analyses
Generalized estimating equation (GEE) models were used to estimate the effect of age and IOP, as well as their interaction, on rates of RNFL thickness change over time. For each eye, IOP was summarized as the average IOP across follow-up visits. Multivariable models were adjusted for gender, race, glaucoma diagnosis, CCT, follow-up time, and baseline RNFL thickness. The GEEs assumed a Gaussian variance and identity link, with a robust sandwich variance estimator and an exchangeable correlation to account for repeated measures at the eye level. The GEE models properly account for longitudinal correlation (i.e., dependencies within eyes) and yield unbiased population estimates.32,33
To summarize the impact of age and IOP on rates of RNFL change, predicted RNFL trajectories were presented across levels of IOP (between 6 and 30 mm Hg) and age (40, 60, and 80 years old) using the multivariable GEE. To calculate the trajectories, mean values were assumed for the remaining clinical characteristics. Trajectories are presented with 95% confidence intervals (CIs) using the robust covariance estimate. This visualization technique allows for the nonlinear interaction between age and IOP to be easily interpreted. We also present rates of RNFL change across age and IOP levels. Again, these were determined using the multivariable GEE and fixing the remaining clinical characteristics at their mean values. To interpret the rates at different levels of IOP and age, we used contour plots, which allow for the nonlinear relationship to be visualized. The average rate of RNFL thickness loss was subtracted from the predicted rate of change for each given age and mean IOP, so that results can be interpreted as either protective (green color, or slower rates of change) or harmful (warmer colors, or faster rates of change). To account for variability, each contour plot has a corresponding plot of standard errors, obtained from the robust covariance estimate.
Finally, we present polar plots for the visualization of the rates of change in RNFL thickness in each sector around the optic disc (i.e., clock hour). The average rates of change for each sector were similarly derived from multivariable GEE models, as described above, using longitudinal RNFL thickness in each clock hour as our outcome.
All statistical analyses were completed in Stata 16 (StataCorp LLC, College Station, TX) within the Protected Analytics Computing Environment, a highly protected virtual network space developed by Duke University for analysis of identifiable protected health information.
Results
This study included 14,739 eyes of 7814 patients and a total of 60,026 SD-OCT tests acquired over 55,969 SD-OCT visits. The average age ± SD of subjects at baseline was 65.3 ± 13.1 years (range, 18–98 years) and eyes had a mean ± SD follow-up time of 3.5 ± 1.9 years (range, 0.5–9.5 years), with a mean number of 3.8 ± 1.7 SD-OCT visits, ranging from 2 to 14. The dataset had a total of 85,475 valid visits where IOP was measured with GAT, with an average of 5.8 ± 3.6 visits per patient. Of these patients, 4482 were female (57.4%) and 2230 were self-identified as black or African American (28.6%). According to ICD codes from the baseline visit, 47.4% of the eyes were classified as glaucoma suspect, 30.5% as primary open-angle glaucoma, and 22.1% as “other” glaucoma types. The unadjusted mean rate of change for global RNFL thickness in the overall population was –0.70 µm/year (95% CI, –0.72 to –0.67). Table 1 details the demographic and clinical characteristics of the eyes included in the study according to their baseline diagnoses.
Table 1.
Demographics and Clinical Characteristics of Subjects Included in the Study
| Characteristic | Overall |
|---|---|
| Subject-specific | |
| Number of patients, n | 7814 |
| Age, y | |
| Mean ± SD | 65.3 ± 13.1 |
| Median (IQR) | 66.6 (57.8–74.1) |
| Sex, female, n (%) | 4482 (57.4) |
| Race, n (%) | |
| White or Caucasian | 4802 (61.5) |
| Black or African American | 2230 (28.5) |
| Other | 782 (10.0) |
| Eye-specific | |
| Number of eyes, n | 14,739 |
| Years of follow-up, mean ± SD | 3.5 ± 1.9 |
| CCT, µm, mean ± SD | 549.5 ± 41.8 |
| Diagnosis at baseline, n (%) | |
| Glaucoma suspect | 6986 (47.4) |
| Primary open-angle glaucoma | 4492 (30.5) |
| Other | 3261 (22.1) |
| SD-OCT (60,026 tests over 55,969 visits) | |
| Number of visits | 55,969 |
| Number of visits per eye, mean ± SD (range) | 3.8 ± 1.7 (2–14) |
| Baseline global RNFL thickness, µm | |
| Mean ± SD | 82.4 ± 17.0 |
| Median (IQR) | 84.0 (72.0–94.0) |
| Baseline mean SD-OCT quality | |
| Mean ± SD | 24.6 ± 4.3 |
| Median (IQR) | 25.0 (22.0–28.0) |
| IOP | |
| Number of visits | 85,475 |
| Number of visits per eye, mean ± SD (range) | 5.8 ± 3.6 (2–34) |
| During follow-up, mm Hg | |
| Average, mean ± SD | 16.1 ± 3.5 |
| Peak, mean ± SD | 19.4 ± 5.5 |
IQR, interquartile range.
Table 2 shows the results of the GEE model investigation of the effect of each potential predictive factor on rates of global RNFL thickness change. In univariable analyses, both higher mean IOP during follow-up and older age were significantly associated with faster rates of global RNFL loss. However, an important interaction effect was seen between IOP and age, as shown in the multivariable model. For older eyes, the impact of higher IOP was significantly greater on rates of change than in younger eyes (β = –0.010 µm/year per each 1 mm Hg higher IOP and 10 years older at baseline; 95% CI, –0.018 to –0.005; P < 0.001), even after adjusting for other variables. Thicker baseline RNFL, diagnosis at baseline, and longer follow-up time were also significantly associated with rates of global RNFL thickness change.
Table 2.
Individual and Multivariable Models of the Effect of Each Clinical Characteristic on the Rate of Change of SD-OCT RNFL Thickness Over Time
| Individual Effects Over Time | ||||
|---|---|---|---|---|
| Univariable Models | Multivariable Model | |||
| Characteristic | Coefficient | P | Coefficient | P |
| Diagnosis | ||||
| Glaucoma suspect | 0 (base) | – | 0 (base) | – |
| Primary open-angle glaucoma | –0.109 | <0.001 | –0.266 | <0.001 |
| Other | –0.270 | <0.001 | –0.377 | <0.001 |
| Age at baseline, per 10 y older | –0.025 | 0.025 | 0.111 | 0.025 |
| Sex, female | 0.040 | 0.132 | 0.080 | 0.003 |
| Race, black or African American | –0.005 | 0.862 | –0.024 | 0.442 |
| Years of follow-up | –0.020 | 0.003 | –0.022 | 0.001 |
| Baseline mean global RNFL thickness, per 10 µm thicker | –0.071 | <0.001 | –0.091 | <0.001 |
| CCT, per SD (40 µm) thinner | 0.008 | 0.604 | 0.038 | 0.006 |
| Average IOP during follow-up, per 1 mm Hg higher | –0.049 | <0.001 | 0.013 | 0.519 |
| Interaction term (age at baseline and average IOP during follow-up) | – | – | –0.010 | 0.001 |
Boldface indicates statistical significance (P < 0.05).
Trajectories of global RNFL thickness across time are presented across levels of average IOP and age in Figure 1. For these predictions, all other independent variables in the model were set to their means in the sample (e.g., 82 µm of RNFL at baseline and mean CCT value of 550 µm). It can be seen that older eyes had significantly faster rates of change than younger eyes for the same level of IOP. If sharing the same clinical characteristics, an 80-year-old patient with an average IOP of 18 mm Hg would have a significantly faster rate of RNFL loss than a 40-year-old patient (–0.92 vs. –0.61 µm/year, respectively; 95% CI of difference, –0.43 to –0.20). This effect increased constantly with higher values of IOP.
Figure 1.
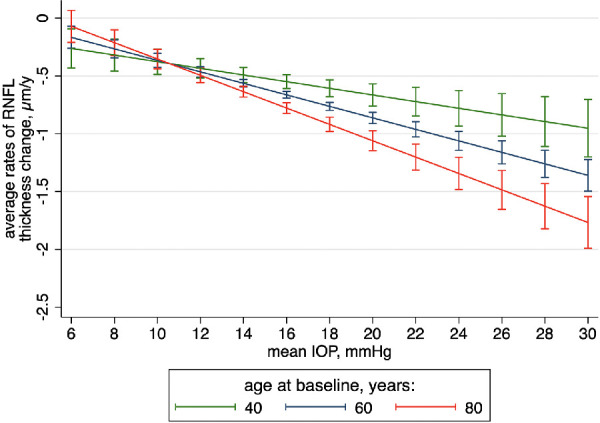
Trajectories of changes in RNFL thickness over time across levels of IOP for 40-, 60-, and 80-year-old average subjects from the whole sample. Other covariates were set to their mean values. Capped spikes indicate 95% CIs.
Figure 2 shows a contour plot illustrating the interaction effects between mean IOP and baseline age on the rate of global RNFL thickness change. Readers are encouraged to examine the x-axis values for age at baseline to estimate the influence of average IOP (y-axis) on the rates of RNFL thickness change. Warmer colors represent faster rates of global RNFL loss over time (in µm/year). The predictions have also been adjusted for the confounding factors described in the previous paragraph, and all covariates were set to their means (see above). One can note that, with increasing age, contours become progressively thinner; therefore, for an older individual 80 years of age at baseline, small increases in mean IOP during follow-up can lead to a fast shift toward warmer contours (i.e., a faster rate of RNFL loss). In contrast, younger patients may be able to withstand wider ranges of IOP while maintaining similar rates (i.e., same contours) of RNFL loss. The corresponding standard error plot (Supplementary Fig. S1) indicates a minimal amount of uncertainty across the whole region. Supplementary Figure S2 shows similar contour plots for subjects divided according to baseline diagnoses. The overall interaction between age and IOP can still be seen in each plot.
Figure 2.
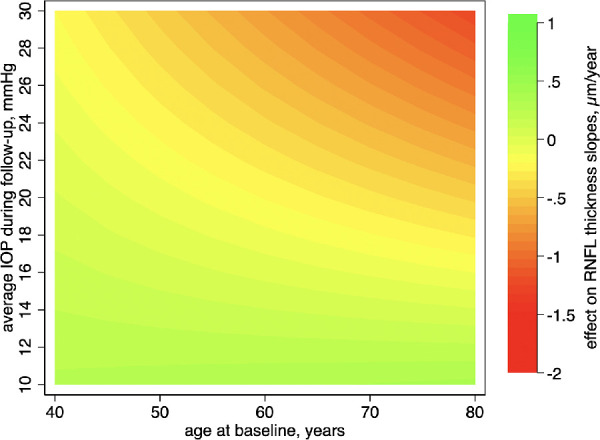
Contour plot showing the effect of the interaction between mean IOP and age on rates of RNFL thickness change over time. Readers can examine the x-axis values to estimate the influence of average IOP (y-axis) on the rates of RNFL thickness change over different age groups. The average rate of RNFL thickness loss (–0.70 µm/year) was subtracted from the predicted rate of change for each given age and mean IOP, so the results can be interpreted as either protective (green, or slower rates of change) or harmful (warmer colors, or faster rates of change) in relation to the average rate of change of the sample.
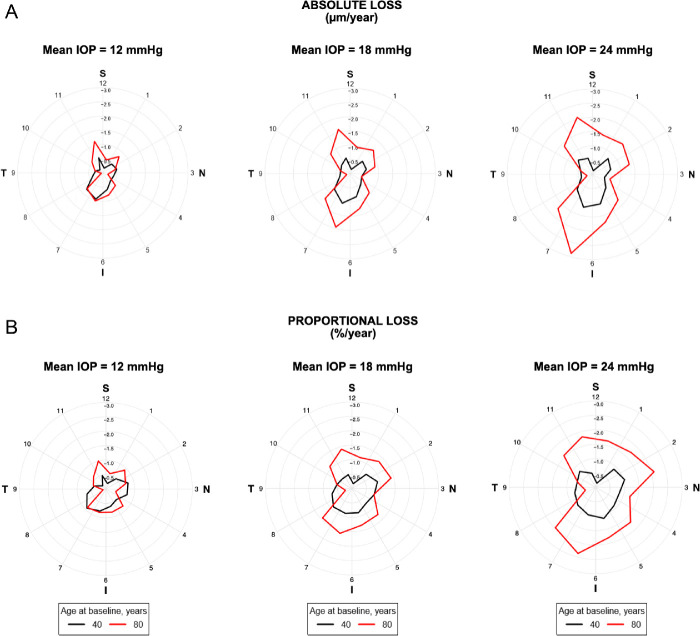
Similar multivariable GEE models were derived for RNFL thickness in each 30° sector around the optic disc. The effects of the interaction of age at baseline and mean IOP during follow-up on the rates of RNFL change for each sector are presented in Figure 3. The resulting rates of change are represented in Figure 4 as polar plots, with clock hours 3, 6, 9, and 12 representing the nasal, inferior, temporal, and superior cardinal points, respectively. Points farther from the center represent faster rates of RNFL loss on that sector. It can be seen that the impact of IOP was significantly greater in older eyes compared to younger ones and that the effect was markedly greater in the inferior and superior sectors of the optic nerve. Additional contour plots for the interaction effects of mean IOP and age on the rate of RNFL thickness change in each quadrant are shown in Supplementary Figure S3.
Figure 3.
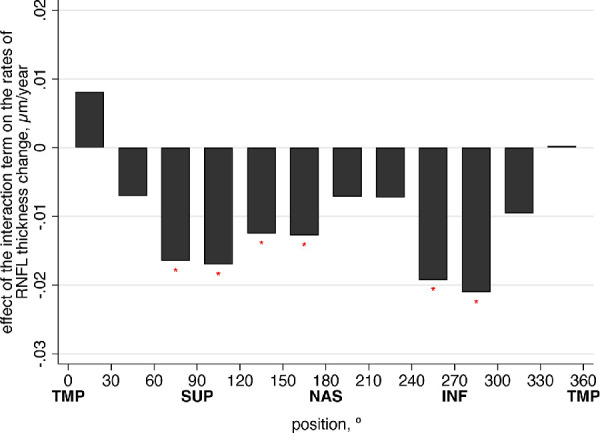
Effect of the interaction term (baseline age and average IOP during follow-up) on the rates of peripapillary RNFL thickness change for each sector. Coefficients were derived from multivariable models adjusted for gender, race, glaucoma diagnosis, CCT, follow-up time, and baseline RNFL thickness in each sector. Asterisk indicates statistical significance (P < 0.05). Sectors start at the temporal cardinal point as 0° (clock hour 9), proceeding clockwise around the optic disc in 30° steps, correlating to the report of the Spectralis SD-OCT. INF, inferior; NAS, nasal; SUP, superior; TMP, temporal.
Figure 4.
Polar plots illustrating the estimated rates of change in RNFL thickness according to the sectors around the optic disc for subjects 40 and 80 years of age and different levels of average IOP during follow-up. It can be seen that the impact of IOP was significantly greater in older eyes compared to younger ones and that the effect was markedly greater in the inferior and superior sectors of the optic nerve, in terms of both absolute loss (µm/year) (A) and percentage of loss from the baseline thickness (%/year) (B) for each sector.
Discussion
In this study, we used a large cohort of eyes undergoing routine clinical care to investigate the hypothesis that age would act as a modifier of the impact of IOP on glaucoma progression. Our study included subjects between the ages of 18 and 98 years at baseline who were followed for up to 9.5 years. We observed that, at similar IOP levels, older eyes showed faster rates of RNFL loss than younger eyes. These findings corroborate previous experimental work suggesting that aging may increase the vulnerability of the optic nerve to IOP-related damage.34 They also suggest that older patients may require tighter levels of IOP control to prevent disease progression.
Increasing age and elevated IOP are the most well-characterized risk factors for glaucoma development and progression, but thus far they have mostly been analyzed independently, with the effect of their interaction on glaucoma progression not being fully explored. In this study, we used statistical models to allow an interaction term between age and IOP and demonstrated that subjects with older age had increased susceptibility to progressive glaucomatous RNFL thinning at the same level of IOP compared to younger subjects with otherwise similar characteristics. The nonlinear effects of this interaction were visualized in the contour plots of Figure 2 and Supplementary Figure S2. Take, for example, a 40-year old “average” glaucoma patient from the sample with a mean IOP of 12 mm Hg during follow-up. The expected rate of RNFL thickness change for this subject would be –0.43 µm/year (95% CI, –0.52 to –0.35), which would be similar to that of an 80-year-old patient with similar clinical characteristics (–0.49 µm/year; 95% CI, –0.56 to –0.43). However, for an IOP of 24 mm Hg, the 40-year-old patient would have a rate of RNFL loss of –0.78 µm/year, but the 80-year-old patient would progress at a rate of –1.34 µm/year, or 72% faster. These differences would, of course, accumulate over time if the levels of IOP were to persist.
The increased susceptibility to IOP effects with aging, as found in our study, helps to explain several clinical and experimental observations. For example, it is noteworthy that cases of “normal tension glaucoma” seem less frequent in younger age groups, whereas glaucoma is more commonly seen with relatively lower pressure in older individuals compared to younger ones.35,36 Epidemiological studies have also suggested that the increased prevalence of glaucoma with aging cannot be fully explained by an increased prevalence of high IOP in older age, suggesting that an increased susceptibility to glaucoma damage must somehow occur with aging.12,13,37 Experimental models have corroborated this. Steinhart and colleagues34 observed that older mice are significantly more likely to lose RGCs in experimental glaucoma than younger mice with similar IOP levels. Our findings support these observations by showing that older eyes present significantly faster rates of RNFL thickness loss for a given level of IOP compared to younger ones and hence would be at increased risk for the development and progression of glaucoma. Glaucoma also shares several characteristics in the pathogenesis of the disease that are common to other neurodegenerative disorders of aging,38 such as selective loss of a single neuronal cell population, similar mechanisms of neuronal cell death,39 and mitochondrial dysfunction.40,41 It should be noted that our work was not able to address or isolate other potential explanations for an increased susceptibility to optic nerve damage with aging, such as vascular effects or changes in the biomechanics of the optic nerve head and adjacent tissues, for example.19,42–44 In fact, the age-related susceptibility of the optic nerve to glaucoma damage is likely to be multifactorial.
We also investigated the interaction between IOP and age and the loss of RNFL thickness at different sectors around the optic disc. The impact of IOP on both absolute and percentual rates of RNFL loss was greater on the inferior and superior sectors, which is in agreement with the expected pattern of RNFL and neuroretinal rim loss in glaucoma.45 In fact, Quigley et al.16 observed regional differences in the structure of the lamina cribrosa in the superior and inferior poles compared to the nasal and temporal poles using electron microscopy. In particular, the superior and inferior parts of the lamina have a combination of larger pore openings—through which RNFL bundles and blood vessels pass—and thinner supporting sheets of connective tissue. In the situation of increased IOP, pores in those regions would be more easily distorted and structures passing through could be more susceptible to damage. Although it is possible that the faster rates of change in the superior and inferior regions may reflect a better capacity of SD-OCT to detect damage at these locations, such a simplistic explanation does not seem to fully account for the different patterns of RNFL rates of change across age and IOP observed in this study.
It could be argued that some eyes in our cohort may not have had enough tests to assess progression over time; however, excluding eyes with fewer tests could potentially bias the results by removing eyes that may have had fast progression over a short period of time. As we were interested in population effects, rather than individual estimates of rates of change, including all eyes with available follow-up data improved the accuracy and precision of the estimates and avoided such unwarranted biases. To demonstrate this, we repeated the analysis in a subsample of eyes with five or more SD-OCT visits during follow-up (Supplementary Fig. S4). The overall predictions of the effect of the interaction of age and IOP on the rates of change remained essentially unchanged, but the uncertainty in the predictions was actually higher.
In this study, RNFL thickness was used as a surrogate measure for RGC loss in glaucoma. Assessment of RNFL thinning with SD-OCT represents an objective metric of neural loss that is widely used in clinical practice, and it has been shown to be predictive of future visual field loss and decline in quality of life.46–50 It is likely, however, that such a surrogate is not perfect. In fact, animal models have shown that RGC loss may precede RNFL thinning, so that RNFL thinning may actually represent a late stage of the RGC degeneration process.51 There may also be additional effects of age and IOP on other constituent tissues of the optic nerve head and adjacent areas that may influence the rate of RGC loss in glaucoma. It is also not clear whether analyses with other structural parameters, such as neuroretinal rim, could show different results, and this deserves further investigation.52,53 Importantly, the alternative use of functional metrics such as SAP would provide additional challenges to the evaluation of the relationship of interest in our study. Although it is clear that preservation of visual function is the primary goal of glaucoma management, assessment of how IOP impacts neural loss in glaucoma using SAP may be confounded by the subjective nature of perimetry, as well as by nonlinearities in translating RGC loss to visual sensitivity thresholds.23–26 In that regard, structural measurements from SD-OCT seem a more suitable tool for studying the relationship among IOP, age, and progressive neural loss in glaucoma.
Due to the large sample size of this study, we were able to develop precise estimates of the interaction between age and IOP and the progression of glaucoma; however, this study is not without limitations. IOP was considered to be the sole mediator of the effect of different treatments on the rate of progressive RNFL loss, but it is possible that different forms of treatment may affect rates of progression by additional mechanisms. Addressing the effect of each individual treatment on progression and its interaction with age would be impossible given the retrospective nature of our data and limitations of EHR data, along with the impossibility of assessing patient adherence. In addition, certain forms of treatment, such as topical prostaglandin analogs, have been shown to affect corneal biomechanics which may artifactually affect IOP measurement, although this effect seems to be of small clinical relevance.54 As such, our findings should be interpreted based on the variables that were ultimately used in the analyses, using caution to avoid unwarranted generalizations.
It should also be noted that given that the EHR dataset was drawn from a single institution and a majority of the patients self-identified as Caucasian, the results might not be generalizable to different populations. It is possible that the interaction of age and IOP may differ, for example, in African Americans, as they have been shown to develop glaucoma earlier and have more aggressive disease.55,56 Of note, African American race was not significantly associated with faster rates of change in either the univariable or multivariable models in our sample. Also, the three-way interaction term among race, IOP, and age was not statistically significant (P = 0.177). Future studies with more racially diverse populations should provide better estimates for other specific groups. Finally, because coding for glaucoma diagnosis was done by the attending physicians without following prespecified guidelines, it is possible that eyes classified as suspects may have had glaucomatous damage and eyes with glaucoma may have received this diagnosis based on previous history, without necessarily having confirmed optic nerve damage.
In conclusion, our study found that age was a significant effect modifier of the relationship between IOP and glaucomatous loss in RNFL thickness over time, suggesting that older patients may be more susceptible to glaucomatous progression than younger patients at the same level of IOP.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Eye Institute, National Institutes of Health (EY029885 and EY031898; FAM). The funding organization had no role in the design or conduct of this research.
Disclosure: A.A. Jammal, None; S.I. Berchuck, None; A.C. Thompson, None; V.P. Costa, Aeri Pharmaceuticals (C), Alcon (C, F), Allergan (C, F), Novartis (C, F), Iridex (F), Carl Zeiss Meditec (F); F.A. Medeiros, Aeri Pharmaceuticals (C), Allergan (C, F), Annexon (C), Biogen (C), Carl Zeiss Meditec (C, F), Galimedix (C), Google (F), Heidelberg Engineering (F), IDx (C), NGoggle Diagnostics (P), Novartis (F), Stealth Biotherapeutics (C), Reichert (C, F)
References
- 1. Mariotti SP. Global Data on Vision Impairments 2010. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130(4): 429–440. [DOI] [PubMed] [Google Scholar]
- 3. Garway-Heath DF, Crabb DP, Bunce C, et al.. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015; 385(9975): 1295–1304. [DOI] [PubMed] [Google Scholar]
- 4. Heijl A, Leske MC, Bengtsson B, et al.. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120(10): 1268–1279. [DOI] [PubMed] [Google Scholar]
- 5. Lichter PR, Musch DC, Gillespie BW, et al.. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001; 108(11): 1943–1953. [DOI] [PubMed] [Google Scholar]
- 6. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126(4): 487–497. [DOI] [PubMed] [Google Scholar]
- 7. Leske MC, Heijl A, Hyman L, et al.. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007; 114(11): 1965–1972. [DOI] [PubMed] [Google Scholar]
- 8. Gordon MO, Beiser JA, Brandt JD, et al.. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120(6): 714–720, discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 9. European Glaucoma Prevention Study Group, Miglior S, Pfeiffer N, et al.. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007; 114(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 10. Susanna BN, Ogata NG, Jammal AA, Susanna CN, Berchuck SI, Medeiros FA. Corneal biomechanics and visual field progression in eyes with seemingly well-controlled intraocular pressure. Ophthalmology. 2019; 126(12): 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jammal AA, Thompson AC, Mariottoni EB, et al.. Impact of intraocular pressure control on rates of retinal nerve fiber layer loss in a large clinical population. Ophthalmology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996; 103(10): 1661–1669. [DOI] [PubMed] [Google Scholar]
- 13. Varma R, Ying-Lai M, Francis BA, et al.. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004; 111(8): 1439–1448. [DOI] [PubMed] [Google Scholar]
- 14. Leske MC, Connell AM, Wu SY, Hyman L, Schachat AP. Distribution of intraocular pressure. The Barbados Eye Study. Arch Ophthalmol. 1997; 115(8): 1051–1057. [DOI] [PubMed] [Google Scholar]
- 15. Rochtchina E, Mitchell P, Wang JJ. Relationship between age and intraocular pressure: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2002; 30(3): 173–175. [DOI] [PubMed] [Google Scholar]
- 16. Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981; 99(1): 137–143. [DOI] [PubMed] [Google Scholar]
- 17. Friberg TR, Lace JW. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988; 47(3): 429–436. [DOI] [PubMed] [Google Scholar]
- 18. Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35(6): 2857–2864. [PubMed] [Google Scholar]
- 19. Flammer J, Orgul S, Costa VP, et al.. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002; 21(4): 359–393. [DOI] [PubMed] [Google Scholar]
- 20. Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24(1): 39–73. [DOI] [PubMed] [Google Scholar]
- 21. Grossniklaus HE, Nickerson JM, Edelhauser HF, Bergman LA, Berglin L. Anatomic alterations in aging and age-related diseases of the eye. Invest Ophthalmol Vis Sci. 2013; 54(14): ORSF23–ORSF27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jammal AA, Thompson AC, Mariottoni EB, et al.. Rates of glaucomatous structural and functional change from a large clinical population: the Duke Glaucoma Registry Study. Am J Ophthalmol. 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000; 41(7): 1774–1782. [PubMed] [Google Scholar]
- 24. Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004; 45(2): 466–472. [DOI] [PubMed] [Google Scholar]
- 25. Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006; 124(6): 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medeiros FA, Lisboa R, Weinreb RN, Girkin CA, Liebmann JM, Zangwill LM. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. 2012; 130(9): 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011; 118(7): 1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal Latinos. Invest Ophthalmol Vis Sci. 2003; 44(8): 3369–3373. [DOI] [PubMed] [Google Scholar]
- 29. Patel NB, Lim M, Gajjar A, Evans KB, Harwerth RS. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci. 2014; 55(8): 5134–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. Am J Ophthalmol. 2017; 175: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014; 132(4): 396–402. [DOI] [PubMed] [Google Scholar]
- 32. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986; 42(1): 121–130. [PubMed] [Google Scholar]
- 33. Hubbard AE, Ahern J, Fleischer NL, et al.. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010; 21(4): 467–474. [DOI] [PubMed] [Google Scholar]
- 34. Steinhart MR, Cone-Kimball E, Nguyen C, et al.. Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res. 2014; 119: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamal D, Hitchings R. Normal tension glaucoma—a practical approach. Br J Ophthalmol. 1998; 82(7): 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klein BE, Klein R, Sponsel WE, et al.. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992; 99(10): 1499–1504. [DOI] [PubMed] [Google Scholar]
- 37. Klein B, Klein R, Pedula K. IOP in an American community - the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992; 33(7): 2224–2228. [PubMed] [Google Scholar]
- 38. Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer's disease: a systematic review of the evidence. Neurotoxicology. 2017; 61: 143–187. [DOI] [PubMed] [Google Scholar]
- 39. Nucci C, Martucci A, Cesareo M, et al.. Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res. 2015; 221: 49–65. [DOI] [PubMed] [Google Scholar]
- 40. Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009; 18(2): 93–100. [DOI] [PubMed] [Google Scholar]
- 41. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006; 443(7113): 787–795. [DOI] [PubMed] [Google Scholar]
- 42. Topouzis F, Wilson MR, Harris A, et al.. Association of open-angle glaucoma with perfusion pressure status in the Thessaloniki Eye Study. Am J Ophthalmol. 2013; 155(5): 843–851. [DOI] [PubMed] [Google Scholar]
- 43. Giarelli L, Grandi G, Delendi M, Falconieri G. The pathology of optic nerve aging. Metab Pediatr Syst Ophthalmol ( 1985). 1989; 12(1–3): 61–63. [PubMed] [Google Scholar]
- 44. Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012; 53(4): 1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999; 43(4): 293–320. [DOI] [PubMed] [Google Scholar]
- 46. Leung CK, Cheung CY, Weinreb RN, et al.. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009; 116(7): 1257–1263, 1263.e1–2. [DOI] [PubMed] [Google Scholar]
- 47. Yu M, Lin C, Weinreb RN, Lai G, Chiu V, Leung CK. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmology. 2016; 123(6): 1201–1210. [DOI] [PubMed] [Google Scholar]
- 48. Zhang X, Dastiridou A, Francis BA, et al.. Comparison of glaucoma progression detection by optical coherence tomography and visual field. Am J Ophthalmol. 2017; 184: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuang TM, Zhang C, Zangwill LM, Weinreb RN, Medeiros FA. Estimating lead time gained by optical coherence tomography in detecting glaucoma before development of visual field defects. Ophthalmology. 2015; 122(10): 2002–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gracitelli CP, Abe RY, Tatham AJ, et al.. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol. 2015; 133(4): 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rovere G, Nadal-Nicolas FM, Agudo-Barriuso M, et al.. Comparison of retinal nerve fiber layer thinning and retinal ganglion cell loss after optic nerve transection in adult albino rats. Invest Ophthalmol Vis Sci. 2015; 56(8): 4487–4498. [DOI] [PubMed] [Google Scholar]
- 52. Chauhan BC, O'Leary N, AlMobarak FA, et al.. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013; 120(3): 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu Z, Lin C, Crowther M, Mak H, Yu M, Leung CK. Impact of rates of change of lamina cribrosa and optic nerve head surface depths on visual field progression in glaucoma. Invest Ophthalmol Vis Sci. 2017; 58(3): 1825–1833. [DOI] [PubMed] [Google Scholar]
- 54. Wu N, Chen Y, Yu X, Li M, Wen W, Sun X. Changes in corneal biomechanical properties after long-term topical prostaglandin therapy. PLoS One. 2016; 11(5): e0155527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991; 266(3): 369–374. [PubMed] [Google Scholar]
- 56. Bowd C, Zangwill LM, Weinreb RN, et al.. Racial differences in rate of change of spectral-domain optical coherence tomography-measured minimum rim width and retinal nerve fiber layer thickness. Am J Ophthalmol. 2018; 196: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.