Mitochondrial homeostasis is necessary for the maintenance of cellular function and neuronal survival. Mitochondrial quality is tightly regulated by mitophagy, in which defective/superfluous mitochondria are degraded and recycled. Here, Hara et al demonstrate that induction of mitophagy via iron depletion suppresses the development of hepatocellular carcinoma (HCC). This work suggests turning up mitophagy as a potential therapeutic strategy against liver cancer.
Subject Categories: Autophagy & Cell Death, Molecular Biology of Disease,
A study in this issue proposes turning up mitophagy via iron depletion as a potential therapeutic strategy against liver cancer.

Mitochondrial dysfunction is considered as a hallmark of ageing and is implicated in a spectrum of diseases. Mitochondrial dysfunction and impairment of mitochondrial‐specific autophagy, namely mitophagy, have emerged as important components of the cellular processes underlying ageing and age‐predisposed conditions, such as neurodegenerative diseases and cancer (Palikaras, Lionaki et al, 2018). In particular, mutations of nuclear‐ or mitochondria‐encoded mitochondrial proteins cause rare mitochondrial disorders (Scheibye‐Knudsen, Fang et al, 2015), whilst mitochondria‐mediated ATP deprivation, oxidative stress and impaired cell signalling have been associated with various diseases and premature ageing (Fang, 2019; Fang, Hou et al, 2019). Moreover, impairment of lysosome targeting and recycling mechanisms have been highlighted as causative for the accumulation of damaged mitochondria, which consequently leads to cellular dysfunction and/or death (Lou et al, 2019), altogether implicating that mitochondrial maintenance is critical for health due to their necessity in coordinating multiple cellular processes.
Mitochondrial homeostasis is necessary for the maintenance of cellular function and survival. Mitochondrial quality is tightly regulated by mitophagy, in which defective/superfluous mitochondria are degraded and recycled. Several different mitophagy pathways are known, and many are conserved from C. elegans to rodents and humans (Aman, Frank et al, 2020). Interestingly, depletion of iron has been demonstrated to disrupt mitochondrial homeostasis and trigger mitophagy in C. elegans and mammalian cells (Allen, Toth et al, 2013; Kirienko, Ausubel et al, 2015; Schiavi, Maglioni et al, 2015). Coupled to the mitophagy‐inducing ability, iron chelators, such as deferoxamine (DFO), have been used as therapeutic agents against hepatocellular carcinoma (HCC; Yamasaki, Terai et al, 2011). However, the mechanism underlying iron depletion in the induction of mitophagy and suppression of HCC remains elusive.
In this issue of the EMBO Reports, Hara, Yanatori et al (2020) screened three iron chelators, namely DFO, deferiprone (DFP) and deferasirox (DFX), in human liver cells in order to evaluate the potential of mitophagy induction. Amongst the iron chelators examined, DFP emerged as the most potent mitophagy inducer as demonstrated by greatest abundance of mito‐autophagosome‐like structures. In addition, DFP‐induced induction of mitophagy was shown to be independent of PINK1/Parkin pathway, a finding in line with previous report by Allen et al (Allen et al, 2013). Using ferrozine‐based assay, Hara et al (2020) measured cytoplasmic and mitochondrial chelatable iron content and found DFP to reduce both the aforementioned components in comparison with DFX and DFO that were only able to decrease cytoplasmic iron content. Within the mitochondrial‐associated regulatory proteins examined, Hara et al (2020) identified an increase in expression of mitochondrial ferritin (FTMT) via the hypoxia‐inducible factor 1α (HIF1α)‐specific protein 1 (SP1) axis. Selective knockdown of FTMT using small interfering RNA (siRNA) diminished the DFP‐induced mitophagy and rescued the reactive oxygen species (ROS) production. In consequence, these data suggest that FTMT is required for DFP‐induced mitophagy that contributes to the suppression of cellular oxidative stress.
Following the identification that FTMT is important in DFP‐induced mitophagy, the authors attempted to uncouple the underlying mechanism(s). For the purpose of selective autophagy, autophagy cargo receptors specifically couple cargo material(s) and the autophagosomal membrane. Given the ability of nuclear receptor coactivator 4 (NCOA4) to act as selective marker for turnover of cytosolic ferritin via the process of ferritinophagy, the authors evaluated its ability to interact with FTMT. Indeed, FTMT was shown to be associated with NCOA4 in the presence of DFP with immunofluorescence staining indicating the colocalization of endogenous NCOA4 and FTMT at the mitochondria. The FTMT‐NCOA4 interaction facilitates the coupling to the LC3 as the mitochondrial damage progressed due to increased depolarisation. The authors subsequently evaluated the localisation of FTMT in order to elucidate the possible mean of mitophagy induction. It was shown that FTMT is localised on the mitochondrial outer membrane upon DFP treatment that may enable interaction with the cytosolic NCOA4, although, not a kinase, FTMT has been suggested by the authors to possible act as PINK1 in the PINK1/Parkin pathway in trafficking of damaged mitochondria, as a mechanism of action. However, it remains to be determined whether mitochondrial outer membrane localisation of FTMT is due to DFP‐mediated mitochondrial depolarisation.
Finally, the authors tested the ability of DFP to suppress tumorigenesis in two hepatocarcinogenic mouse models. Intriguingly, DFP treatment resulted in significant decrease in the abundance and maximum size of liver tumours in both mouse models. Furthermore, DFP also suppressed hepatic steatosis, fibrosis, and mitochondrial ROS, whilst it improved mitochondrial function. The authors highlight that improvement of mitochondrial function is possibly owed to enhanced iron loss‐mediated mitophagy enabling removal of damaged mitochondria. Moreover, suppressive effect of DFP on HCC was diminished upon FTMT depletion, indicating its fundamental need in order to exhibit the anti‐cancer effects of iron chelators (Fig 1). Altogether, the data suggest that iron loss‐mediated mitophagy via FTMT induction may suppress tumorigenesis and may potentially be utilised as a therapeutic strategy against liver cancer.
Figure 1. Iron loss‐induced FTMT‐dependent mitophagy suppresses the development of hepatocellular carcinoma.
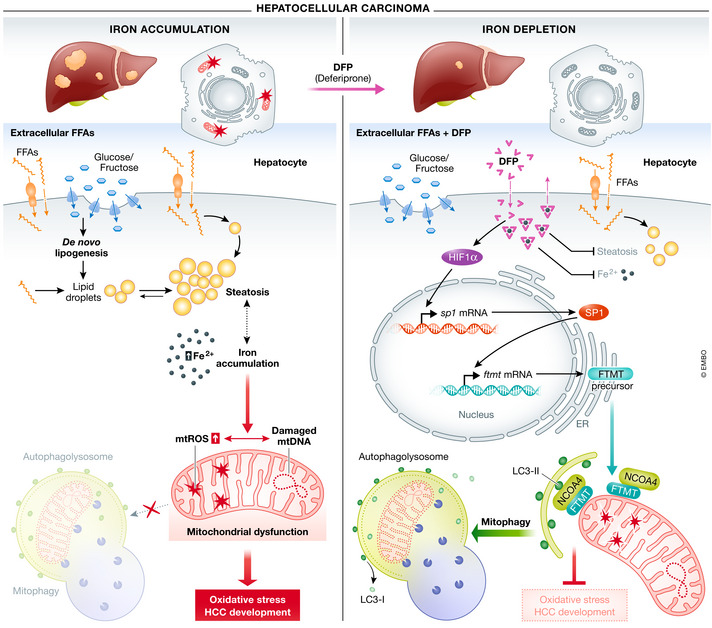
In hepatocytes, impaired de novo lipogenesis and adipose tissue lipolysis accompanied by mitochondrial dysfunction promote development of HCC (left panels). Iron depletion induced by deferiprone (DFP) triggers the HIF1α‐SP1 axis, which in turn regulates mitochondrial ferritin (FTMT). FTMT accumulation on the outer membrane of defective mitochondria induces mitophagy via specific interaction with the autophagic cargo receptor NCOA4 coupling to the LC3‐II double‐membrane phagophore. Removal of damaged mitochondria through this process protects against oxidative stress and suppresses the development of HCC. Abbreviations: DFP, deferiprone; Fe2+, Ferrous ion; FFAs, free fatty acids; FTMT, mitochondrial ferritin; HCC, hepatocellular carcinoma; HIF1α, hypoxia‐inducible factor 1‐alpha; LC3‐II, microtubule‐associated protein 1A/1B‐light chain 3 II; mtDNA, mitochondrial DNA; mtROS, mitochondrial reactive oxygen species; NCOA4, nuclear receptor coactivator 4; SP1, specific protein 1.
EMBO Reports (2020) 21: e51652.
See also: Y Hara et al (November 2020)
References
- Allen GF, Toth R, James J, Ganley IG (2013) EMBO Rep 14: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman Y, Frank J, Lautrup SH, Matysek A, Niu Z, Yang G, Shi L, Bergersen LH, Storm‐Mathisen J, Rasmussen LJ et al (2020) Mech Ageing Dev 185: 111194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF (2019) Autophagy 15: 1112–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan‐Olive MM, Caponio D, Dan X et al (2019) Nat Neurosci 22: 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yanatori I, Tanaka A, Kishi F, Lemasters J, Nishina S, Sasaki K, Hino K (2020) EMBO Rep 21: e50202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko NV, Ausubel FM, Ruvkun G (2015) Proc Natl Acad Sci USA 112: 1821–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, Tavernarakis N (2018) Nat Cell Biol 20: 1013–1022 [DOI] [PubMed] [Google Scholar]
- Scheibye‐Knudsen M, Fang EF, Croteau DL, Wilson DM III, Bohr VA (2015) Trends Cell Biol 25: 158–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi A, Maglioni S, Palikaras K, Shaik A, Strappazzon F, Brinkmann V, Torgovnick A, Castelein N, De Henau S, Braeckman BP et al (2015) Curr Biol 25: 1810–1822 [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Terai S, Sakaida I (2011) N Engl J Med 365: 576–578 [DOI] [PubMed] [Google Scholar]


