Non‐canonical inflammasome activation is crucial to mount an immune response against bacteria. Two studies in this issue show that the IFN‐inducible protein Irgm2 and the ATG8 family member Gate‐16 cooperatively limit the non‐canonical inflammasome.

Subject Categories: Autophagy & Cell Death; Immunology; Microbiology, Virology & Host Pathogen Interaction
In infections caused by gram‐negative bacteria, the bacterial cell wall component lipopolysaccharide (LPS) acts as a potent pathogen‐associated molecular pattern (PAMP) that triggers the innate immune system. This is accomplished by two pattern recognition receptor systems. Toll‐like receptor 4 (TLR4) senses extracellular LPS and induces a broad pro‐inflammatory transcriptional program and also antiviral interferons. A complementary system detects intracellular LPS. As such, upon its release into the cytoplasm, LPS can directly engage the protease caspase‐4 (caspase‐11 in the murine system) and thereby trigger a pro‐inflammatory cell death program known as pyroptosis (Rathinam et al, 2019). This is mediated by active caspase‐4 cleaving its substrate gasdermin D (GSDMD). The thereby released N‐terminal fragment of GSDMD inserts into the cell membrane and forms a cytotoxic pore. As a consequence, the cell ruptures and releases its pro‐inflammatory content. In addition, the GSDMD pore results in potassium efflux that can activate the NLRP3 inflammasome. NLRP3 in turn activates caspase‐1, which matures pro‐IL‐1β and pro‐IL‐18, further perpetuating the inflammatory nature of this cell death. Given its unconventional mode of NLRP3 activation, this pathway has been coined the non‐canonical inflammasome.
Among the most prominent positive regulators of this pathway are the guanylate‐binding proteins (GBPs), which are highly induced upon type‐I and II IFN signaling, e.g., downstream of TLR4 engagement (Rathinam et al, 2019). GBPs facilitate the activation of caspase‐4 for which diverse mechanisms have been put forward, ranging from releasing LPS‐bearing bacteria from vacuoles, extraction of LPS from bacterial membranes, and—as established most recently—forming a signaling platform for the activation of human caspase‐4 (Kutsch et al, 2020; Santos et al, 2020; Wandel et al, 2020). GBPs belong to a bigger family of proteins classified as interferon‐inducible GTPases. Another subgroup of this family is the immunity‐related GTPases (IRGs) (Coers et al, 2018). The IRGs are further split into the GKS (glycine–lysine–serine) and GMS (glycine–methionine–serine) subgroup (Coers et al, 2018). This nomenclature refers to a sequence motif in the p‐loop of the nucleotide‐binding domain of the GTPases. The GKS‐IRGs ensure cell‐autonomous host defense by diverse measures. While best characterized for their role in disrupting parasitophorous vacuoles in the context of Toxoplasma gondii infection, they have also been implicated in the release of PAMPs. In concert with GBPs, the murine GKS‐IRG IRGM10 releases LPS and DNA to confer inflammasome activation in the cytoplasm (Rathinam et al, 2019). While the GKS‐IRG subfamily is absent in the human system, the GMS‐IRGs retained a single representative, namely human IRGM (hIRGM), while there are three members in the mouse (Irgm1‐3). hIRGM has gained considerable attention as genetic variants in this locus have been associated with increased risk for Crohn’s disease (Coers et al, 2018) as well as increased susceptibility to sepsis (Kimura et al, 2014). Along these lines, hIRGM and murine Irgm1 were found to negatively regulate the NLRP3 inflammasome (Mehto et al, 2019). While unlike Irgm1 and Irgm3, for which knockout mice substantiated their role in antimicrobial defense, Irgm2‐deficient mice have not been characterized. Therefore, the role of Irgm2 in the context of cell‐autonomous defense mechanisms and immunity is unclear.
In this issue of EMBO Reports, two studies independently describe Irgm2 as a novel negative regulator of caspase‐11 activation and sepsis (Eren et al, 2020; Finethy et al, 2020). Given the importance of caspase‐11 in the pathogenesis of sepsis, both studies set out to evaluate the role of the three, individual murine Irgms in regulating the non‐canonical inflammasome pathway. Doing so, both groups find that the genetic deletion of Irgm2 increases the activity of the non‐canonical inflammasome in a broad range of in vitro andin vivo experiments. In the absence of Irgm2, stimulation of cells in vitro with either bacteria‐derived outer membrane vesicles (OMVs) or a diverse set of gram‐negative bacteria, leads to increased pyroptosis (Fig 1). The activation threshold of the system in the absence of Irgm2 is down to a level at which extracellular LPS suffices to trigger caspase‐11, a process that otherwise requires LPS delivery to the cytoplasm. The augmented activation of the non‐canonical inflammasome also translates into an overshooting inflammatory response and increased lethality in murine sepsis models. In all experiments, this phenotype always remains dependent on caspase‐11, putting Irgm2 upstream of caspase‐11. Both studies further report that in the absence of Irgm2 caspase‐11 can even be activated in cells that lack the GBPs encoded on chromosome 3. This is remarkable insofar, as these GBPs are thought to be absolutely required for the activation of caspase‐11 both upon challenge with bacteria, OMVs and LPS only (Rathinam et al, 2019). In the study of Eren et al, Gate‐16 could further be identified by screening for interaction partners of Irgm2. Gate‐16 seems not only to interact with Irgm2 but also mimics its phenotype upon knockdown. Of note, the function of Gate‐16 in restricting caspase‐11 activity was simultaneously described by a third independent study (Sakaguchi et al, 2020).
Figure 1. Irgm2 and Gate‐16 fine‐tune the immune response to gram‐negative bacteria.
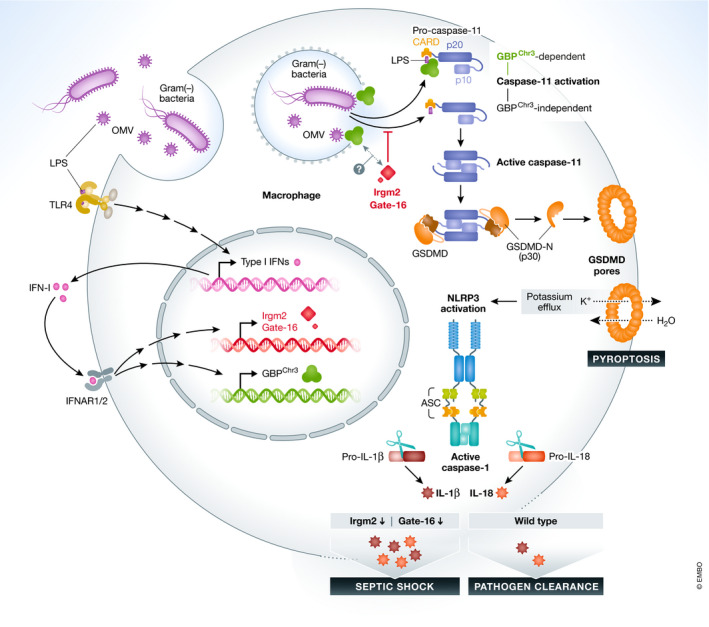
Lipopolysaccharides (LPS) from gram‐negative bacteria or bacterial‐derived outer membrane vesicles (OMVs) prime macrophages to induce type‐I‐Interferon (IFN‐I) via toll‐like receptor 4 (TLR4). Subsequently, IFN‐I induces host factors such as GBP‐proteins or Irgm2 and Gate‐16. In parallel, bacteria and OMVs that have been phagocytosed are liberated from the phago‐lysosome to the cytoplasm, where GBPs facilitate the recognition of LPS by caspase‐11. The newly identified factors Irgm2 (in mice) and Gate‐16 (in mice and humans) restrict the activation of caspase‐11. In Irgm2‐deficient cells, activation of caspase‐11 can even occur in the absence of GBPs encoded on chromosome 3. On the organismal level, the enhanced activation of caspase‐11 in the absence of Irgm2 translates into an overshooting immune response resulting in septic shock and death of the host. Therefore, Irgm2 and Gate‐16 fine‐tune the immune response to gram‐negative bacteria.
The exact mechanism of how Irgm2 and Gate16 cooperatively limit the non‐canonical inflammasome remained elusive in all of these studies. The reported findings suggest an increased release of LPS to the cytoplasm in the absence of Irgm2, while overall LPS uptake is unaltered. As such, Irgm2 and Gate16 could be involved in the degradation of LPS and LPS‐bearing pathogens, e.g., by autophagy. Indeed, autophagy has been shown to restrict caspase‐11 activity, and Irgm‐proteins (namely hIRGM and Irgm1) as well as Gate16 have shown to be involved in antimicrobial autophagy (xenophagy). However, preliminary experiments could not substantiate a defect in canonical autophagy as the cause of the described phenotype. As xenophagy also relies on many non‐canonical autophagy components, genetically dissecting different components of this apparatus could guide future studies.
Since the IRG system shows little conservation between mouse and man, it is not too surprising that targeting of hIRGM in human cells does not recapitulate the phenotype of Irgm2 deficiency. As such, it is conceivable that the silencing of human IRGM rather resembles the situation of a pan‐IRGM‐knockout in mice. Indeed, the additional deletion of Irgm1 and Irgm3, as seen in Pan‐Irgm‐deficient mice, restores the activation threshold of caspase‐11 to wild‐type levels. As different isoforms have been described for human IRGM, it will be interesting to see how these individual isoforms differentially regulate caspase‐4. The function of Gate‐16, on the other hand, seems to be conserved in humans as its knockdown in human monocytes also increases caspase‐4 activity. This suggests that the overall pathway is also operational in humans, although its exact components remain to be identified.
While further mechanistical insights are required, especially as to how the described pathway operates in the human system, the two new studies uncover a potential new avenue to bridle the detrimental activation of the non‐canonical inflammasome in sepsis.
EMBO Reports (2020) 21: e51787.
See also: E Eren et al and R Finethy et al (November 2020)
References
- Coers J, Brown HM, Hwang S, Taylor GA (2018) Partners in anti‐crime: how interferon‐inducible GTPases and autophagy proteins team up in cell‐intrinsic host defense. Curr Opin Immunol 54: 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Planès R, Bagayoko S, Bordignon PJ, Chaoui K, Hessel A, Santoni K, Pinilla M, Lagrange B, Burlet‐Schiltz O et al (2020) Irgm2 and Gate‐16 cooperatively dampen Gram‐negative bacteria‐induced caspase‐11 response. EMBO Rep 21: e50829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finethy R, Dockterman J, Kutsch M, Orench‐Rivera N, Wallace G, Piro AS, Luoma S, Haldar AK, Hwang S, Martinez J et al (2020) Dynamin‐related Irgm proteins modulate LPS‐induced caspase‐11 activation and septic shock. EMBO Rep 21: e50830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Watanabe E, Sakamoto T, Takasu O, Ikeda T, Ikeda K, Kotani J, Kitamura N, Sadahiro T, Tateishi Y et al (2014) Autophagy‐related IRGM polymorphism is associated with mortality of patients with severe sepsis. PLoS One 9: e91522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsch M, Sistemich L, Lesser CF, Goldberg MB, Herrmann C, Coers J (2020) Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J 39: e104926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehto S, Jena KK, Nath P, Chauhan S, Kolapalli SP, Das SK, Sahoo PK, Jain A, Taylor GA, Chauhan S (2019) The Crohn's disease risk factor IRGM limits NLRP3 inflammasome activation by impeding its assembly and by mediating its selective autophagy. Mol Cell 73: 429–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Zhao Y, Shao F (2019) Innate immunity to intracellular LPS. Nat Immunol 20: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Sasai M, Bando H, Lee Y, Pradipta A, Ma JS, Yamamoto M (2020) Role of gate‐16 and gabarap in prevention of Caspase‐11‐dependent excess inflammation and lethal endotoxic shock. Front Immunol 11: 561948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JC, Boucher D, Schneider LK, Demarco B, Dilucca M, Shkarina K, Heilig R, Chen KW, Lim RYH, Broz P (2020) Human GBP1 binds LPS to initiate assembly of a caspase‐4 activating platform on cytosolic bacteria. Nat Commun 11: 3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandel MP, Kim BH, Park ES, Boyle KB, Nayak K, Lagrange B, Herod A, Henry T, Zilbauer M, Rohde J et al (2020) Guanylate‐binding proteins convert cytosolic bacteria into caspase‐4 signaling platforms. Nat Immunol 21: 880–891 [DOI] [PMC free article] [PubMed] [Google Scholar]


