Abstract
Whether virulent human pathogenic coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) are effectively transmitted by aerosols remains contentious. Transmission modes of the novel coronavirus have become a hot topic of research with the importance of airborne transmission controversial due to the many factors that can influence virus transmission. Airborne transmission is an accepted potential route for the spread of some viral infections (measles, chickenpox); however, aerosol features and infectious inoculum vary from one respiratory virus to another. Infectious virus-laden aerosols can be produced by natural human respiratory activities, and their features are vital determinants for virus carriage and transmission. Physicochemical characteristics of infectious respiratory aerosols can influence the efficiency of virus transmission by droplets. This critical review identifies studies reporting instances of infected patients producing airborne human pathogenic coronaviruses, and evidence for the role of physical/chemical characteristics of human-generated droplets in altering embedded viruses’ viability. We also review studies evaluating these viruses in the air, field studies and available evidence about seasonality patterns. Ultimately the literature suggests that a proportion of virulent human coronaviruses can plausibly be transmitted via the air, even though this might vary in different conditions. Evidence exists for respirable-sized airborne droplet nuclei containing viral RNA, although this does not necessarily imply that the virus is transmittable, capable of replicating in a recipient host, or that inoculum is sufficient to initiate infection. However, evidence suggests that coronaviruses can survive in simulated droplet nuclei for a significant time (>24 h). Nevertheless, laboratory nebulized virus-laden aerosols might not accurately model the complexity of human carrier aerosols in studying airborne viral transport. In summary, there is disagreement on whether wild coronaviruses can be transmitted via an airborne path and display seasonal patterns. Further studies are therefore required to provide supporting evidence for the role of airborne transmission and assumed mechanisms underlying seasonality.
Keywords: Airborne transmission, Coronaviruses, SARS, Seasonality
Graphical abstract
Respiratory activities generate respirable sized aerosols; however, this does not reflect that the virus is transmittable or capable of replicating in a recipient host.
1. Introduction
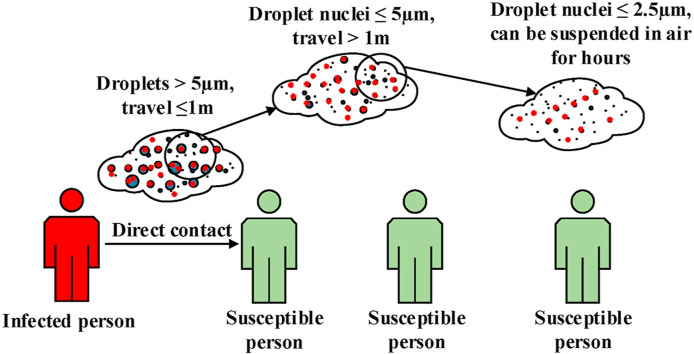
Several human coronaviruses with pandemic potential, including 2002 severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have spread in recent years (Hayden, 2006; Holshue et al., 2020; Zaki et al., 2012). The emergence of SARS-CoV-2 in Wuhan China in late 2019, which is now causing an extraordinary challenge for societies, healthcare organizations and economies globally (Guan et al., 2020) illustrates the threat of these viruses. Direct contact with large droplets expelled by infected people, and contaminated fomites are considered the primary transmission routes for respiratory viruses (Boone and Gerba, 2007; Lee, 2007).
The World Health Organisation (WHO) in several infection prevention guidelines have introduced two common and accepted transmission modes of respiratory pathogens. Firstly, a short-range transmission route, where droplets (>5 μm diameter) can carry viable virus over distances of up to 1 m. Respiratory viruses such as adenovirus, respiratory syncytial virus, influenza and SARS-CoV can be transmitted via this mode. Secondly, the prolonged airborne suspension of respiratory droplet nuclei smaller than 5 μm containing viable pathogens can result in airborne transmission over distances larger than 1 m (WHO, 2009, 2014). However, the role of this latter mode of transmission in spreading respiratory viruses has been hotly debated, and the optimal person-to-person physical distance to prevent transmission is uncertain (Otter et al., 2016).
A more recent WHO report dated March 27, 2020 (WHO, 2020) emphasized that large expelled droplets which can contaminate nearby surfaces and transfer the viruses through direct and indirect contact (within 1 m) are the main route of transmission of SARS-CoV-2. Therefore, regular handwashing and maintaining a minimum distance of 1 m (or an “arm’s length”) are considered as the most important protective measures for the public against contracting the infection (Cucinotta and Vanelli, 2020). Other organizations such as the US Centres for Disease Control and Prevention stated that 6 feet (approximately 2 arms’ length or 1.82 m) is the minimum safe social distancing space from other people who are not from your household in both indoor and outdoor spaces (CDC, 2020).
Three major questions which remain unanswered are, firstly, what is the importance of the airborne transmission mode in promoting the pandemic potential of coronaviruses, secondly, how long can the expelled viruses survive in droplet nuclei suspended in air, and finally what are the physicochemical factors affecting their survival? It is frequently implied that airborne viruses are naked particles suspended in the air, however it is important to understand that these airborne viruses are usually embedded in respiratory fluid when expelled through coughing, sneezing, talking and breathing (Vejerano and Marr, 2018) and that the composition of that fluid can influence how the ambient environment interacts with the droplet, and ultimately influence the viability of the embedded virus.
The mechanisms by which viral infectivity becomes degraded in aerosols remain poorly understood, however there is scientific evidence that suggests desiccation could be a cause of virus degradation (Li et al., 2007; Prussin et al., 2018; Zhao et al., 2012). It was highlighted that SARS-CoV-2 might display a seasonal pattern as observed for other coronaviruses, especially SARS-CoV, which could lower the rate of infection. Several studies have examined seasonal fluctuations of SARS-CoV-2 and the impact of regional climatic parameters, including temperature, humidity, ultraviolet (UV) radiation intensity and other factors (Bashir et al., 2020; Xie and Zhu, 2020; Yao et al., 2020).
This review focuses on: a) the ability of an infected person to produce viable aerosolized viruses, b) the physical and chemical characteristics of generated droplets from breathing, coughing, sneezing and talking and their effects on embedded virus viability, c) human coronavirus survival in ambient air and environmental settings, d) the existence and possible causes of seasonality of human coronaviruses.
2. Definitions
-
-
Airborne transmission: the spread of a pathogen embedded in an aerosol from a source to a susceptible host, causing infection of the host with or without the consequent disease (Hinds, 1999).
-
-
Aerosol: a collection of particles (liquid or solid) ranging in size from 0.001 μm to over 100 mm suspended in a gas (Wells, 1934).
-
-
Droplet nucleus: The airborne residue (with or without embedded pathogens) of a respiratory droplet containing non-volatile solutes, from which water has evaporated to the point of equilibrium with the ambient relative humidity (Wells, 1934).
-
-
Short-range airborne infection route: the transmission of infectious aerosols (>5 μm diameter) between individuals, generally less than 1m (WHO, 2009).
-
-
Long-range airborne infection route: the transmission of infectious aerosols (≤5 μm diameter) within a room, between rooms or between distant locations, typically greater than 1m distances (WHO, 2009).
Airborne transmission has been further classified into obligate and preferential;
-
-
Obligate airborne transmission: pathogens that are transmitted only through the airborne route (e.g., pulmonary tuberculosis) (WHO, 2014).
-
-
Preferential airborne transmission: pathogens that can initiate infection through non-aerosol routes, but are mainly transmitted through droplet nuclei, such as measles and chickenpox (WHO, 2014).
3. Methodology
This critical review evaluates, in detail, a cross-sample of the studies, and only includes the most relevant papers on the mechanisms of generation of human pathogenic coronaviruses, evidence for the role of physical/chemical characteristics of human-generated droplets in altering embedded viruses’ viability, evaluating these viruses in the air/field studies and available evidence about their seasonality patterns. We searched original research studies published in four databases, including EMBASE, Scopus, Web of Science and PubMed databases with no restriction on year or country up to July 31, 2020. Combinations of search keywords from four categories (virulent human pathogenic coronaviruses, environmental transmission, bioaerosols physicochemical characteristics AND climatic variables) were applied in our search for the relevant literature. Search terms for virulent human pathogenic coronaviruses were ‘SARS’ OR ‘SARS-CoV’ OR ‘SARS-CoV-1’ OR ‘MERS-CoV’ OR ‘SARS-CoV-2’ OR ‘COVID-19’ and environmental transmission keywords were ‘airborne transmission’ OR ‘aerosols transmission’ OR ‘airborne suspension’ OR ‘aerosol suspension’ OR ‘nebulized aerosols OR ‘droplet transmission’ OR ‘droplet nuclei transmission’ OR ‘environmental survival’ OR ‘environmental settings’. Search terms for physicochemical characteristics included ‘physical properties’ OR ′physical characteristics’ OR ‘chemical properties’ OR chemical characteristics’ OR physiochemistry features. Climatic variables keywords consisted of ‘relative humidity’ OR RH OR ‘absolute humidity’ OR AH OR ‘temperature’ OR ‘ultraviolet (UV) radiation’ OR UV.”
4. The source regions and generation mechanisms of airborne human respiratory aerosol
Respiratory droplets are generated through typical human exhalation activities, such as breathing, speaking, coughing and sneezing (Chao et al., 2009; Tellier, 2006). Many of these droplets evaporate rapidly to half of their original size before reaching equilibrium with the ambient relative humidity. The resulting particles are then referred to as droplet nuclei (Duguid, 1946; Nicas et al., 2005). This evaporation process can be considered most relevant for droplets with an initial diameter smaller than 20 μm because these particles achieve equilibrium before settling or impacting on surfaces.
The capacity of exhalation manoeuvres to generate airborne pathogen laden droplet nuclei has remained uncertain and is likely to vary with the type and severity of the infection. Johnson et al. (2011) measured the size distribution modes of speech and cough of healthy individuals using a combination of aerodynamic particle sizing and droplet deposition analysis techniques. This work suggested that there are three modes of aerosol production (Fig. 1 ) active in the respiratory system: a bronchiolar mode comprised of small droplet nuclei arising from droplets produced through fluid-film bursts (FFB) that can occur during normal breathing; a laryngeal mode in which aerosols are generated through of voicing and coughing, and an oral mode in which larger droplets are produced during speech and coughing. Three different droplet size distribution modes were reported for speech with count median diameters measured at 1.6 (bronchiolar mode), 2.5 (laryngeal mode) and 145 (oral mode) μm. In the case of voluntary coughing, the modes were at 1.6, 1.7 and 123 μm. The smaller laryngeal and oral mode droplets are small enough to achieve equilibrium with the ambient relative humidity. These droplets therefore form airborne droplet nuclei while the much larger oral mode droplets settle or impact with surfaces before equilibrium can be achieved.
Fig. 1.
The origin and generation mechanism of respiratory droplets.
The average mass concentration of these modes varies with the respiratory manoeuvre. For speech it was 0.21, 2.2 and 7500 μg m−3, while these figures for coughing were 0.22, 1.09 and 6900 μ gm−3. Further studies have supported Johnson’s results, such as the findings of Holmgren et al. (2013) which confirmed that the particle number concentration in the exhaled aerosol decreases when the volunteer held their breath after inhaling. The consistent findings of Johnson and Holmgren that fewer particles are observed after breath-holding, this would suggest that the generated particles have adequate time to deposit on the airways before being exhaled, consistent with the FFB model proposed by Johnson. Based on previous literature, healthy subjects can produce particles between 0.01 and 500 μm (some up to 1000 μm) in diameter (Johnson et al., 2011; Johnson and Morawska, 2009), and those individuals with viral infections generate particles between 0.05 and 500 μm (Gralton et al., 2011). Many of these particles are large enough in diameter to contain a variety of respiratory pathogens, including coronaviruses (80–200 nm) (Lin et al., 2004) influenza virus (100 nm) (Rossman and Lamb, 2011) measles virus (50–500 nm) (Liljeroos et al., 2011).
5. The physicochemical properties of human respiratory droplet nuclei
5.1. Physical properties of human respiratory droplet nuclei
The aerosols generated through speech, coughing, sneezing, and breathing have been surveyed in several studies (Table 1 ). However, in most of these studies, healthy individuals were used, and few have examined the aerosol production of participants with a respiratory virus infection, including influenza (Fabian et al., 2008; Lindsley et al., 2010, 2012; Stelzer-Braid et al., 2009) and rhinovirus (Fabian et al., 2011). Exhaled particle concentrations had large interpersonal variability and with the depth of breathing and loudness of vocalization (Almstrand et al., 2010; Johnson and Morawska, 2009). To better understand the role of airborne transmission in spreading influenza virus, Lindsley et al. (2010) measured influenza viral gene copies and the size distribution of aerosols containing influenza viruses produced by patients’ coughs. The aerosol collection system consisted of an ultrasonic spirometer which was connected to a 10 L piston-style spirometer. The aerosols were extracted into a two-stage NIOSH sampler (cyclone aerosol sampler) and an SKC BioSampler (impinger type sampler). It was reported that 42% of detected influenza gene copies were found in droplet nuclei <1 μm, 23% in droplet nuclei of 1–4 μm, and 35% in droplet nuclei >4 μm. However, only the viral gene copies within the aerosols that remained airborne in the spirometer piston were extracted. It should be noted that most of the fluid emitted through the patient’s cough would have been deposited on the walls of the setup and therefore gone unrepresented in the sample. In 2012 in another study with the same collection system, they showed the number and size distribution of cough aerosols produced by influenza patients before and after recovery and reported that people who have influenza infection generated a larger volume of aerosols than after they have recuperated (Lindsley et al., 2013). The number of cough-generated particles produced by patients differed significantly from person to person, while individuals with an influenza infection produced 400–516,800 particles/cough and 300–362,700 particles/cough after recovery. The size of generated aerosols was measured via a laser aerosol particle spectrometer. This instrument was able to cover a range of 0.35–10 μm and it was found that 63% of detected cough particles from influenza patients were in the respirable particle size range. However, as discussed above, cough aerosols have a much wider size distribution than was covered by their instrument, and thus, this does not mean that 63% of the whole cough aerosol was in the respirable fraction. It should be noted that for measuring the infection risk, besides the number, it is essential to report the mass or volume fraction of particles generated by different natural respiratory activities. For example, a 5 μm virus-laden droplet has a volume of approximately 6.5 × 10−5 nL, which is 1000 times greater than the volume of a 0.5 μm droplet. Therefore, the large airborne carrier aerosols can potentially carry more virions compared to small aerosols. It takes approximately 13 min for an 8 μm droplet to fall 1.5 m in still air and as droplet size decreases the settling time quickly increases (Hinds, 1999). Larger particles (8 μm>), therefore because of their greater size and volume as well as subsequent viral content can be theoretically important when susceptible persons have close contact with the infected person. Additionally, smaller respirable virus-laden aerosols have the potential to reach the alveolar region of the lung during inhalation, and recent studies have reported that the required infectious dose for influenza to develop an infection in this region is much lower than in the nasal region (Tellier, 2006). That it also depends on the locations of cells with suitable receptor sites for the specific virus. Lindsley et al. in another study (Lindsley et al., 2015) with the same setup measured the total infectious influenza virus in collection media of the SKC BioSampler, the deposited aerosols in the piston (not in the spirometer) and SKC BioSampler elbow as its inlet. Some previous studies reported that aerosols in the range of 10–15 μm would be accumulated in the elbow of the SKC BioSampler (Tsai and Pui, 1990), and aerosols between 0.3 and 8 μm size would be collected in the collection cup (Kesavan et al., 2010). Their results have shown that the highest number of viable influenza A was identified in the collection media of the BioSampler (0.3–8 μm), as opposed to in the sampler elbow or in droplets deposited in the tray. This suggests that respirable aerosols may play an important role in the transmission of influenza virus. However, it is not obvious whether the dry surfaces of the sampler elbow or tray impact the viability of collected viruses or that larger particles were not collected efficiently. These studies have contributed significantly to the understanding of the production modes of respiratory aerosols. The number, size, and mass of potentially infectious aerosols produced by different activities such as speaking, breathing, coughing and sneezing have not been well characterized. This has therefore caused conflict over the potential for the airborne transmission of respiratory viruses. Further studies should be conducted to measure the number of viable and total viruses in a wider size range of generated aerosols that better reflects the observed droplet sizes produced under different natural respiratory activities for each specific type of respiratory infection.
Table 1.
Investigations of the diameter of particles generated from human respiratory activities.
| Particle size range (μm) of natural respiratory activities and the methods of measuring |
|||||||
|---|---|---|---|---|---|---|---|
| Type of infection | Breathing | talking | coughing | sneezing | Methods of measuring size | Study | Year |
| Healthy subjects | <0.6 | <0.6 | <0.6 | – | Optical Particle Counter (OPC) (Optical diffraction) |
Papineni et al. (Papineni and Rosenthal, 1997) | 1997 |
| Mycobacterium tuberculosis infection | – | ≤3.3 | – | Andersen Cascade Impactor (Pacitto et al.) (Impaction) |
Fennelly et al. (Fennelly et al., 2004) | 2004 | |
| Healthy subjects | 015–0.19 | – | – | – | OPC (Optical diffraction) | Edwards et al. (Edwards et al., 2004) | 2004 |
| Healthy subjects | – | 0.62–15.9 DN: (0.58–5.42) |
– | Aerodynamic Particle Sizer (George et al.) (Time-of-flight, TOF) Scanning Mobility Particle Sizer (SMPS) (Electrical mobility) |
Yang et al. (Yang et al., 2007) | 2007 | |
| Unknown viral spp. | 0.3–0.5 | – | – | – | OPC (Optical diffraction) | Fabian et al. (Fabian et al., 2008) | 2008 |
| Healthy subject | – | <1 | – | APS (TOF) | Fang et al. (Fang et al., 2008) | 2008 | |
| Healthy subjects and infected subjects by unknow viral spp. | H:0.09-<0.16 I:0.09->9.97 |
– | – | – | Electrical Low-Pressure Impactor (ELPI) (Impaction) |
Hersen et al. (Hersen et al., 2008) | 2008 |
| Healthy subjects | 0.1–1 | 0.1–1 | 0.1–1 | – | APS (TOF) | Morawska et al. (Morawska et al., 2009) | 2008 |
| Heathy subjects | ≈0.3–3 | – | – | – | APS (TOF) | Johnson et al. (Johnson and Morawska, 2009) | 2009 |
| Healthy subjects | – | 4–8 | 4–8 | – | Interferometric Mie Imaging (IMI) (Mie scattering) |
Chao et al. (Chao et al., 2009) | 2009 |
| Healthy subjects | – | 50–75 | 50–75 | – | Droplet deposition (Analytical microscopy) Dust Monitor (Optical diffraction) |
Xie et al. (Xie et al., 2009) | 2009 |
| Healthy subjects | 0.4–1.1 | – | 0.4–10 | 0.4–4 | APS (TOF) | Morawska et al. (Morawska et al., 2008) | 2009 |
| Bacterial, unknown infection | – | – | ≤3.3 | – | ACI (Impaction) | Wainwright et al. (Wainwright et al., 2009) | 2009 |
| Healthy subjects | 0.3–0.4 | – | – | – | OPC (Optical diffraction) | Almstrand et al. (Almstrand et al., 2010) | 2010 |
| Healthy subjects | 0.1–7 | – | – | – | Laser Spectrometer (TOF) | Haslbeck et al. (Haslbeck et al., 2010) | 2010 |
| Healthy subjects | OPC: 0.4–4 SMPS:0.01–0.3 |
– | – | – | OPC (Optical diffraction) SMPS (Electrical mobility) | Holmgren et al. (Holmgren et al., 2010) | 2010 |
| Heathy subjects | – | 0.1–1000 | 0.01–1000 | – | APS (TOF) Droplet deposition analysis |
Johnson et al. (Johnson et al., 2011) | 2011 |
| 3 healthy and 16 human rhinovirus (HRV)-infected subjects | 0.3–0.5 | – | – | – | OPC with nominal diameter-size bins ranging between 0.3 μm and 10 mm | Patricia Fabian et al. (Fabian et al., 2011) | 2011 |
| Influenza, before and after recovery | – | – | H: 0.57–0.89 I: 0.57–0.71 |
– | Laser Spectrometer (TOF) | Lindsley et al. (Lindsley et al., 2012) | 2012 |
| Influenza A and B, parainfluenza 1, 2 and 3, respiratory syncytial virus (RSV), human metapneumovirus and human rhinoviruses (hRV) | 58% of participants produced particles >5 μm and 80% produced particles (≤5 μm) | 57% of participants produced particles >5 μm and 82% produced particles (≤5 μm) | – | ACI (Impaction) | Jan Gralton et al. (Gralton et al., 2013) | 2013 | |
| Healthy subjects | – | – | – | First mode: 40–200, Second mode: 200-1000 | Laser Spectrometer (TOF) | Z. Y. Han et al. (Han et al., 2013) | 2013 |
| Healthy subjects | – | ≈0.5–10 | – | – | APS (TOF) | Sima Asadi et al. (Asadi et al., 2019) | 2019 |
Key: H: Healthy; I: Infected; DN: Droplet nuclei.
5.2. Chemical composition of human respiratory droplets
Our understanding of the chemical properties of respiratory aerosols and the influences that this physicochemistry has on the viability of embedded viruses remains incomplete (Eames et al., 2009). The infectivity of a virus is likely to be altered by the chemical microenvironment provided by the aerosol droplet and droplet nuclei (Benbough, 1971). As distinct mechanisms by which humans independently produce aerosol from each of the lower (alveolar), middle (laryngeal), and upper (oral cavity) regions of the respiratory tract, the composition of carrier droplets would depend on their sources in the respiratory tract (Johnson et al., 2011; Johnson and Morawska, 2009). A simplified respiratory fluid composition model has shown salt, protein, and surfactant are the major components in respiratory fluids (Effros et al., 2002; Yang and Marr, 2011). Furthermore, Potter et al. (1963) examined the human pulmonary secretions of laryngectomized patients (called “normal secretions”), patients with bronchiectasis, and patients with chronic Cystic Fibrosis (CF) infections, and their findings are summarised in Table 2 . The highest and lowest protein concentrations were measured in CF and normal secretions, respectively. Solids percentage in the secretions of the “normal” and bronchiectasis groups were reported to be approximately half that was observed in the CF secretions. The overall chemical composition of secretions was influenced by diffuseness of the infectious process. A surge in exudation and necrosis would occur due to infection in the tracheobronchial tree that was most obvious in CF patients. The low level of inorganic components, such as sodium and chloride ions, in the secretions of CF and bronchiectasis patients is assumed to be a result of maintaining the osmolar relationship. The elevation of potassium ions may be because of the existence of largely intracellular site in the secretions, while the augmented phosphorus content is likely due to the phospholipid and DNA constituents of the secretions.
Table 2.
Organic and inorganic analysis of human pulmonary secretions (Potter et al., 1963).
| Infection type | Number of examined patients | mg protein/g secretion | mg lipid/g secretion | % Solids (g solids/l00g secreted fluid) | μEquivalent/gm of whole pulmonary secretions |
||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Cl | Ca | P | K | |||||
| Cystic fibrosis | 24 | 55.7 ± 20.3 | 31.4 ± 9.7 | 10.68 ± 2.23 | 101 ± 27 | 75 ± 12 | 7.3 ± 1.9 | 127 ± 44 | 28 ± 8.2 |
| Bronchiectasis | 8 | 20.4 ± 4.5 | 11.7 ± 4.3 | 5.2 ± 1.21 | 116 ± 15 | 97 ± 14 | 9.3 ± 2.6 | 37 ± 9 | 18.7 ± 3.2 |
| Laryngectomized | 29 | 10.0 ± 3.0 | 8.4 ± 2.7 | 5.21 ± 1.69 | 165 ± 42 | 162 ± 60 | 6.2 ± 2.0 | 27 ± 16 | 13.2 ± 5.4 |
Note: The number of patients for measuring protein and lipid contents were 8 in each group.
Leon CP.M. Schenkels et al. (1995) and Mehboob Ali et al. (2011) described the biochemical constituents of pulmonary secretions (Table 3 ). The main non-volatile cations and anions are sodium and chloride, respectively. However, lactate and glycoprotein are the primary organic substances existing in exhaled condensates (Effros et al., 2002). Aerosolized respiratory mucus was measured to contain primarily sodium chloride (NaCl) (150 ± 20 mM, which is approximately equivalent to 8.8 g/L) and protein (76 ± 18 g/L) (Nicas et al., 2005). However, it has been demonstrated that sputum and secretions from the nasal, oropharyngeal, tracheobronchial and bronchoalveolar regions have differing compositional profiles (Reynolds and Chrétien, 1984). Composition has also been demonstrated to change in response to disease and inflammation (Reynolds and Chrétien, 1984).
Table 3.
Distribution of proteins of human mucosal fluids (Ali et al., 2011; Schenkels et al., 1995).
| Fluid type Protein type |
Saliva | Bronchial Mucus | Nasal Mucus | Airway secretions |
|---|---|---|---|---|
| Mucins (% of total protein) | >15% | >15% | >15% | >15% |
| Acidic proline-rich proteins (% of total protein) | >15% | ND | ND | ND |
| a-Amylase (% of total protein) | >15% | <1% | <1% | <1% |
| Basic proline-rich proteins (% of total protein) | 5–15% | <1% | <1% | 1–5% |
| Basic proline rich glycoprotein (% of total protein) | 5–15% | ND | ND | ND |
| Secretory Immunoglobulin A (% of total protein) | 5–15% | ND | >15% | >15% |
| Cystatins (% of total protein) | 1–5% | ND | <1% | ND |
| Statherin (% of total protein) | 1–5% | <1% | <1% | <1% |
| Immunoglobulin G (% of total protein) | <1% | <1% | <1% | <1% |
| Extra-parotid glycoprotein (% of total protein) | <1% | ND | <1% | ND |
| Histatins (% of total protein) | <1% | ND | ND | ND |
| Lysozyme (% of total protein) | <1% | >15% | >15% | >15% |
| Kallikrein (% of total protein) | <1% | ND | <1% | ND |
| Lactoferrin (% of total protein) | <1% | >15% | >15% | >15% |
| Lactoperoxidase (% of total protein) | <1% | ND | <1% | ND |
| Haptocorrin (% of total protein) | <1% | ND | <1% | ND |
| B-Microseminoprotein (% of total protein) | <1% | 5–15% | <1% | <1% |
| Immunoglobulin M (% of total protein) | <1% | <1% | 5–15% | <1% |
| Albumin (% of total protein) | <1% | <1% | <1% | ND |
| Zn-a2 glycoprotein (% of total protein) | <1% | ND | ND | ND |
Using time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis on respiratory exhaled particles, Almstrand identified that phospholipids feature prominently in the pulmonary surfactant (Almstrand et al., 2009). Among these phospholipids, Dipalmitoylphosphatidylcholine (DPPC) was the main constituent of pulmonary surfactants. Further studies (Bredberg et al., 2012) have further confirmed the presence of these pulmonary surfactants through a combination of sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), liquid chromatography-mass spectrometry (LC-MS), and triple quadrupole mass spectrometry (TQMS), and have even observed that DPPC concentrations vary with particle size, consistent with the multimodal production mechanisms proposed by Johnson.
6. The influence of droplet physicochemistry on virus viability
When measuring the viability of viruses embedded in droplets and droplet nuclei in the air and environmental settings, it is important to consider the characteristics of carrier aerosols that were initially released from the human respiratory tract. There is an apparent relationship between transmission rates and environmental conditions for a subset of respiratory diseases (Lin and Marr, 2020). RH, absolute humidity (AH), temperature, and sunlight intensity are the main environmental factors that have been reported to affect the viability of airborne respiratory viruses (Pica and Bouvier, 2012). The effects of these environmental factors on the viability of viruses can be attributed to either direct impacts on the embedded viruses in aerosols or through their indirect impacts by impairing host response and immune system. For example, Loosli et al. (1943) reported that exposure to humidity levels of 80–90% for 30 min could render the influenza virus non-infectious to mice, while the virus remained highly infectious after exposure to lower humidity levels (17–24%). Nevertheless, results from a more recent study using a mice model have suggested that prolonged exposure to low RH negatively affects the immune response to influenza infections in mice, and also impairs antiviral defense and tissue repair (Kudo et al., 2019).
Recent studies have demonstrated that modulating RH can influence the viability of airborne viruses, transmission potential, and seasonality (Pica and Bouvier, 2012; Sobsey and Meschke, 2003; Yang and Marr, 2012). Nevertheless, early studies suggest these features could instead be mechanistically be explained by AH for the influenza virus (Deyle et al., 2016; Marr et al., 2019; Shaman et al., 2010). Shaman and Kohn (2009) reported that water vapor content significantly influences airborne influenza virus transmission rates when compared to either RH or temperature through analysis of guinea pig influenza transmission data (Lowen et al., 2007). Their findings illustrated that approximately 50% of influenza virus transmission and approximately 90% of influenza virus survival could be explained by AH, while RH could describe only 12% and 36%, respectively. In another experimental study with aerosolized Gumboro virus, Zhao et al. (2012) suggested that temperature and RH best predicts virus viability, and that viability was not meaningfully attributed to AH. In their findings, very broadly, it is suggested that the predictive power of AH on virus viability can instead be attributed to the effects of temperature, and not strictly the water vapor content, as high AH is typically achieved through increased temperature. It has been previously demonstrated that virus viability is negatively correlated with temperature (Yamaya et al., 2019). Thereby, while evaluating the influence of humidity and only exploring the variables under deliberation to AH and RH, and not temperature, the hindrance is that high values of AH are only achievable at high temperature.
Ultraviolet (UV) wavelengths present in sunlight could potentially penetrate the carrier aerosols and eradicate the viruses. H1N1 influenza virus inactivation with low doses of 222 nm UV light has recently reported (Welch et al., 2018). In a recent study, 0.12 min−1 (1 log reduction: 19 min) and 0.37 min−1 (1 log reduction: 6 min) decay rates were reported for SARS-CoV-2 in simulated saliva, under simulated sunlight levels representative of late winter/early fall and summer, respectively, indicating that the potential for aerosol transmission of SARS-CoV-2 may be dependent on sunlight (Schuit et al., 2020).
In a physical system involving a human coughing, virus-laden aerosols are released from the respiratory tract (RH = 100%) into ambient air, where the RH is typically much lower. Water is then evaporated from the aqueous solution in the aerosol droplets to reach a thermodynamic equilibrium with the atmosphere. The rate of this evaporation increases with temperature and decreases with ambient humidity, but for a pure water droplet surrounded by air, at a relative humidity of less than 100%, the evaporation will typically progress to the point where all of the water has been removed. The rate of evaporation, however, can also be affected by the presence of solutes. A variety of physicochemical alterations can be induced due to the evaporation of the droplets, as has been presented for atmospheric aerosol (Vejerano and Marr, 2018), which can then affect the viability of any embedded pathogens. As more water content has been evaporated, the resulting concentration of lower volatility solutes, such as salts and biological matter, will increase. Multiple hypotheses have been developed to explain the relationship between these physicochemical changes and virus viability. These include water activity, surface inactivation, salt toxicity (Yang and Marr, 2012), and phase separation of carrier aerosols components (Song et al., 2018). The hypothesis of viral inactivation due to the removal of structural water molecules from the virus capsid has been introduced and tested with bacteria (Webb, 1960). However, the results for bacteria viability cannot be directly extended to viruses due to structural differences between the pathogens. The structure of viruses embedded in carrier aerosols might be altered due to the effects of surface tension, shear stress, and conformational rearrangement driven by hydrophobicity. Laboratory investigations have shown that the viability of an influenza virus decreases with an increasing salt concentration in proteinaceous droplets (Yang et al., 2012). A probable explanation lies in the fact that the concentrations of different respiratory aerosols components and their evaporation can vary strongly with the ambient RH. Hygroscopic salts influence the transport of water vapor, and allow for humidity dependent droplet sizes as described by Köhler theory (Köhler, 1936). Because of their hygroscopic behavior, inorganic salts such as NaCl can play an essential role in controlling the water uptake and loss when present in an aerosol, and consequently limit or promote virus viability.
NaCl is a prominent constituent found in human respiratory aerosol. Some hygroscopic salts, including NaCl, are observed to exhibit humidity-dependent hysteresis behavior, in which phase transitions occur at different RH values depending on the history of the particle (Seinfeld and Pandis, 2016). The previously discussed aerosol interactions with the water vapor present in the surrounding air may play a significant role in the observed seasonality of viral respiratory tract infections. The viability of some enveloped viruses such as influenza A degrades with decreasing RH, reaching a minimum at approximately 47% humidity. Below 47% RH, the viability of the virus increases with decreasing RH. However, once above approximately 75% humidity, the trend is reversed, with survival increasing with RH (Yang et al., 2012). Between these two RH extremes lies a region in which various research groups have reported contradictory findings. This region of contradiction corresponds to the RH range in which human comfort is highest and is therefore commonly found in indoor environments, where cross-infection also is greatest. One hypothesis that attempts to explain the above RH dependency of virus survival and the contradictory findings in the literature, relies on the hysteresis behavior of the NaCl present within the aerosol carrying the virus. Such salt laden aerosols exhibit critical values in their water uptake profile that corresponds to the boundaries that delineate those humidity ranges. Outside of these critical values the literature is typically in agreement with virus viability, and between the critical values there are conflicting results.
The hypothesis of phase separation of organic and inorganic components of aerosols has been introduced recently by several studies (Freedman, 2017; Vejerano and Marr, 2018; Znamenskaya et al., 2012). It was demonstrated that the presence of proteins and other organics with slower rehydration process than inorganic constituents could enable phase separation in internally mixed aerosols. It is then hypothesized droplets which undergo phase separation at high RH can then undergo structural rearrangement during subsequent dehydration. An observed augmented structure, named ‘core-shell morphology’, has been shown to allow for internally mixed organic-inorganic aerosols to undergo transition to a glassy or gel-like state at low RH as the droplet viscosity increases. This will inhibit molecular diffusion and evaporation (Runnsjo et al., 2016; Znamenskaya et al., 2012), potentially preventing water inside the droplet’s saline core from evaporating and thus protecting the virus from desiccation.
7. Evidence for the airborne transmission of coronavirus infection
It has been debated within the scientific community whether coronaviruses, especially SARS-CoV-2, exhibit effective transmission through the airborne route. As discussed earlier, a person with a viral respiratory infection can produce respirable size virus-laden aerosols (Ho et al., 2020) that can easily be inhaled into the most susceptible pulmonary region. Furthermore, substantial numbers of influenza viral genome copies were found in air sampled from aircraft, a health center, and a day-care facility, primarily found in particles ≤2.5 μm in diameter (Yang et al., 2011). Modelled airflow and air sampled studies demonstrated that SARS-CoV can be transmitted via an airborne path (Wong et al., 2004; Yu et al., 2004). Some studies reported positive air samples of coronaviruses’ viral RNA collected from patients’ rooms in hospitals (Table 4 ). During the Toronto SARS outbreak, two air samples collected from a room occupied by a patient with SARS-CoV were positive in terms of viral RNA (Booth et al., 2005). Air sampled from a hospital ward containing patients with SARS-CoV-2 infections showed that the rates of positivity varied by air sampling site, ranging from 35.7% (5/14) near air outlets, 44.4% (8/18) in patients’ rooms, to 12.5% (1/8) within the doctors’ office area (Guo et al., 2020). In another study in Singapore, 2 of 3 air samples from patients’ rooms were positive for SARS-CoV-2, in particle sizes >4 μm and 1–4 μm in diameter (Santarpia et al., 2020). However, all collected air samples at the SARS-CoV-2 outbreak center in Singapore, occupied by three symptomatic patients, were negative, while 2 of 3 swabs taken from the air exhaust outlets were positive (Ong et al., 2020). In a study in the largest hospital in Iran, ten air samples were collected using impinger setups located between 2 and 5 m away from patients’ beds, who had confirmed CoVID-19 cases, and all samples were negative in terms of SARS-CoV-2 RNA (Faridi et al., 2020). A hospital study in Hong Kong reported no SARS-CoV-2 positive air samples collected at a distance of 10 cm from the patient’s chin, with or without wearing a surgical mask for all 8 samples (Cheng et al., 2020). The culture experiments for detecting viable viruses from air samples in these studies were unsuccessful. Therefore, the presence of viral RNA does not necessarily indicate that the virus is transmittable, capable of replicating, or sufficient inoculum to initiate an invasive infection.
Table 4.
Studies of air sampling for coronaviruses (methods and conditions).
| Sampling site and location | Infection type | Sampling method | Sampling flow rate (sampling volume) | # of collected samples | # of positive samples | Study | Year |
|---|---|---|---|---|---|---|---|
| Patients’ room and rooms as control areas without housing SARS patients | SARS-CoV | PTFE membrane filter with a pore size of 0.3 μm | 2 L/min (1260–1560 L) | 40 | 2 (5%) | Booth et al. (Booth et al., 2005) | 2005 |
| Camels’ barn | MERS-CoV | MD-8 airscan sampling device (Sartorius) and sterile gelatine filters | 50 L/min (1000 L) | 3 | 1 | Azhar et al. (Azhar et al., 2014) | 2014 |
| Patients’ rooms | MERS-CoV | MD-8 airscan sampler | 50 L/min (1000 L) | 7 | 4 | Kim et al. (Kim et al., 2016) | 2016 |
| Patients’ rooms | SARS-CoV-2 | 37-mm filter cassettes and 0.3-μm polytetrafluoroethylene Filters/MD-8 airscan sampler | 6 m3/h (400 L)/6 m3/h (1500 L) | unknown | 0 | Ong et al. (Ong et al., 2020) | 2020 |
| Patient areas, medical staff areas and public areas | SARS-CoV-2 | Filters (25 mm in diameter) loaded into styrene filter cassettes (SKC), miniature cascade impactor (Sioutas Impactor, SKC) and filters (80 mm in diameter) packed into a holder | 5 L/min (300 L) | 11,13 and 11 samples from patient areas, medical staff areas and public areas, respectively | 7,8 and 8 positive samples for patient areas, medical staff areas and public areas, respectively | Liu, Y. et al. (Liu et al., 2020) | 2020 |
| Negative pressure isolation rooms | SARS-CoV-2 | MD8 airscan and personal air samplers | 50 L/min (750 L). | unknown | 63.2% | Santarpia JL et al. (Santarpia et al., 2020) | 2020 |
| Hospital wards | SARS-CoV-2 | Impinger attached to a personal sample pump | 1 L/min (60 L) | 10 | 0 | Faridi et al. (Faridi et al., 2020) | 2020 |
| Hospital rooms | SARS-CoV-2 | NIOSH BC 251 bioaerosol samplers | 3.5 L/min (840 L) | 3 | 2 | Po Ying Chia, MBBS et al. (Chia et al., 2020) | 2020 |
| Hospital rooms | SARS-CoV-2 | SAS Super ISO 180 model 86,834 (VWR International PBI Srl, Milan, Italy) | 180 L/min (1000 L) | 8 | 0 | VCC Cheng et al. (Cheng et al., 2020) | 2020 |
When it comes to monitoring airborne viral spread, several important factors can affect the final results and their interpretation, including patient distance from the sampler, the height of used samplers from the floor, the type of sampler, used flow rate, patient activities, patients’ respiratory activities (cough or sneeze) during sampling time, air movement, air conditioning, sample storage and transferring conditions (Rahmani et al., 2020). The different outcomes in the two above discussed groups can be potentially attributed to any changes in these factors. There has been no standardized method for the sampling and determination of viral content in indoor air. The discussed acknowledged research gaps associated with the airborne transmission of the coronaviruses should be addressed by high-quality research, including randomized trials in multiple settings.
8. Environmental contamination by human coronaviruses in field settings
Several studies have assessed environmental samples of wild coronaviruses collected in field settings (Table 5 ). A significant limitation of these studies is the inability to quantify the number of viable viruses, relying only on the total number of viruses. In a study conducted in Bangkok and Taipei in 2004, the potential role of contaminated hospital surfaces in SARS-CoV transmission was explored by swabbing surfaces and quantifying the virus present by reverse-transcriptase polymerase chain reaction (RT-qPCR) (Dowell et al., 2004). It was measured that 38.1% of 63 sites and 6.4% of 31 public areas were contaminated with SARS-CoV RNA. Timothy F. Booth et al. (2005) investigated environmental contamination of rooms occupied by SARS-CoV patients during the Toronto 2003 SARS outbreak via conventional surface swabbing. They reported positive surface swabs that were taken from regularly touched surfaces in rooms. In Jeddah Airport, Saudi Arabia, three (7.5%) of 40 surface samples were identified as contaminated with human coronavirus RNA (Memish et al., 2014).
Table 5.
Field sampling for human coronaviruses.
| Sampling site and location | Infection type | Sampling method | # of collected samples | # of positive samples | Notes | Study | Year |
|---|---|---|---|---|---|---|---|
| SARS patients’ rooms and some rooms as control areas without housing SARS patients | Human coronavirus | Moisturised swabs; PCR for viral RNA and viral culture | 85 | 3 (3.5%) | A bedside table, remote control and a refrigerator handle at a nurses’ medication station were positive. All swabs were culture negative. | Booth et al. (Booth et al., 2005) | 2005 |
| patient parts (patient rooms, nursing stations, emergency department) | SARS-CoV | Moisturised swabs; PCR for viral RNA and viral culture | 63 | 24 (38.1%) | 38.1% of 63 sites and 6.4% of 31 public areas were positive for SARS-CoV RNA. All swabs were culture negative. | Dowell et al. (Dowell et al., 2004) | 2004 |
| Jeddah airport, Saudi, Arabia | Human coronavirus (OC43/HKU1) | Moisturised swabs; PCR for viral RNA and viral culture | 40 | 3 (7.5%) | RNA was identified from surfaces. | Memish et al. (Memish et al., 2014) | 2014 |
| Negative pressure isolation rooms | SARS-CoV-2 | Moisturised swabs; PCR for viral RNA and viral culture | 163 | 126 (77.3%) | 80.4% of all room surfaces, 76.5% of all personal items sampled and 81.0% Samples of the toilets in the room positive for SARS-CoV-2 RNA. All samples were culture negative | Santarpia JL et al. (Santarpia et al., 2020) | 2020 |
| Patients’ room in hospital | SARS-CoV-2 | Pre-moistened macrofoam sterile swabs Air samples were collected by NIOSH samplers |
245 | 56.7% of the rooms had at least one environmental surfaces contamination. 18.5% of the toilet seats and button showed positive RT-qPCR results | Po Ying Chia, MBBS et al. (Chia et al., 2020) | 2020 | |
| COVID-19 isolation ward | SARS-CoV-2 | Moisturised swabs; PCR for viral RNA | 37 | 9 (24.3%) | The most contaminated surfaces were hand sanitizer dispensers (100.0%), medical equipment (50.0%), medical equipment touch screens (50.0%), shelves for medical equipment (40.0%), bedrails (33.3%), and door handles (25.0%). | Katia Razzini et al. (Razzini et al., 2020) | 2020 |
| Quarantine room | SARS-CoV-2 | Moisturised swabs; PCR for viral RNA | 23 | 11 (47.8%) | 70% of samples from the bedroom followed by 50% of samples from the bathroom and that of 33% from the corridor were positive for SARS-CoV-2 | Xiaowen Hu et al. (Hu et al., 2020) | 2020 |
Environmental surfaces and personal protective equipment (PPE) were tested for contamination by SARS-CoV-2 RNA from three symptomatic patients in the SARS-CoV-2 outbreak center in Singapore (Ong et al., 2020). Only 1 PPE swab (the surface of a shoe front) was positive. All other PPE swabs were negative. All collected samples from two patients’ rooms after daily cleaning were negative, while in a patient’s room before daily cleaning 13 (87%) of 15 room sites (including air outlet fans) and 3 (60%) of 5 toilet sites (toilet bowl, sink, and door handle) showed positive results.
A study at the University of Nebraska Medical Center collected surfaces samples in eleven negative pressure isolation rooms during the initial isolation of 13 persons confirmed positive with COVID-19 infections (Santarpia et al., 2020). The samples of surfaces and personal items were collected by using sterile gauze pads prewetted with 3 mL of phosphate-buffered saline (PBS) solution. RT-qPCR was used to quantify the copy number of the virus per μl of the recovered buffer. In total, 126 (77.3%) out of the 163 samples, which were collected from air and surfaces, showed positive PCR results for SARS-CoV-2. Positive SARS-CoV-2 RNA detection in samples for of all surfaces, personal items and toilets in the room were 80.4, 76.5 and 81.0%, respectively. Whilst, 63.2% of air samples collected within the rooms showed positive results.
In another study at the national center for infectious diseases in Singapore, 245 surface samples were collected from 30 rooms of patients who were positive for COVID-19. In general, 56.7% of the rooms had at least one environmental surface contamination and 18.5% of the toilet seats and buttons showed positive RT-qPCR results. Environmental surface positivity decreased significantly after seven days since the initial diagnosis (Chia et al., 2020). The culture experiments for detecting viable viruses from any of the surfaces in these studies were unsuccessful.
9. Survival of human coronaviruses in air using simulated respiratory droplets
Several studies have evaluated the viability of nebulized coronaviruses in different temperatures and RH conditions (Table 6 ). Ijaz et al. (1985) used a 6-jet Collison nebulizer to aerosolize human coronavirus 229E (HCV/229E) into a 300-Liter rotating drum to investigate airborne survival under different conditions of temperature and RH. At 20 ± 1 °C, the highest viability was found at an intermediate RH level (50%) with a half-life of 67.33 ± 8.24 h, whereas at 30% RH the viability half-life was 26.76 ± 6.21 h. Minimum viability was found at a high RH level (80 ± 5%) with a half-life of 3 h. HCoV-229E aerosols remained infectious for 6 day at 20 °C and 50% RH. Another study used the Aerosol AeroMP (Biaera Technologies, USA) aerosol management platform to evaluate the viability of airborne MERS-CoV at 20 °C and 40% or 70% RH (van Doremalen et al., 2013). The virus was aerosolized in triplicate for 10 min, and aerosols were collected simultaneously during nebulization. It was shown that the viability decreased by 7% at 40% RH, while at 70% RH, this figure decreased significantly by 89%. Airborne MERS-CoV viability has previously been reported under different climatic conditions by Pyankov et al. (2018). They tested the viability of MERS-CoV in an environment with 38 °C and 24% RH, similar conditions to the geographical origin of the virus, and in a typical indoor office environment at 25 °C and 79% RH. In the typical office environment, nebulized viruses demonstrated long-term viability, with ∼63.5% of the viruses remaining viable for 60 min post-aerosolization, whereas in the higher temperature environment with drier air, just 4.7% virus viability was observed after 60 min. Doremalen et al. (van Doremalen et al., 2020) in a recent publication using a 3-jet Collison nebulizer and Goldberg drum to study the viability of SARS-CoV-2 compared to SARS-CoV. After 180 min, the viability of airborne SARS-CoV-2 decreased by an infectious titer from 103.5 to 102.7 TCID50/mL, at 3 h post-nebulization. This reduction is comparable to the decrease observed in SARS-CoV infectivity, from 104.3 to 103.5 TCID50/mL.
Table 6.
Studies of viability of coronaviruses in simulated aerosols.
|
Coronavirus strain |
Viability at different temperature and RH conditions | Type of spraying media | Aerosolization system | Collection system | study | Year |
|---|---|---|---|---|---|---|
| 229E (HCV/229E) | At 20 ± 1 °C and 50% RH (half-life of 67.33 ± 8.24 h), at 20 ± 1 °C and 30% RH (half-life of 26.76 ± 6.21 h), at 20 ± 1 °C and 80 ± 5% RH (half-life of 3 h) | Tryptose phosphate broth with 2.5 mg of rhodamine B per mL | Collison nebulizer | Glass impinger | M. K. IJAZ et al. (Ijaz et al., 1985) | 1985 |
| MERS-CoV | At 20 °C and 40% RH 7% reduction, at 20 °C and 70% RH 89% reduction | Unknown media | Collison nebulizer | Glass impinger | N van Doremalen1 et al. (van Doremalen et al., 2013) | 2013 |
| MERS-CoV | At 25 °C and 79% RH 63.5% survival after 60 min, at 38 °C and 24% RH 4.7% survival after 60 min | Unknown media | Collison nebulizer | Personal bioaerosol samplers with 40 mL of collecting liquid and 4 L/min flow rate | Oleg V. Pyankov et al. (Pyankov et al., 2018) | 2017 |
| SARS-CoV | At 21–23 °C and 65% RH half time of 3 h | Unknown media | Collison nebulizer | Viruses were collected on a 47 mm gelatine filter and then dissolved in 10 mL of DMEM containing 10% FBS | Neeltje van Doremalen et al. (van Doremalen et al., 2020) | 2020 |
| SARS-CoV-2 | At 21–23 °C and 65% RH half time of 3 h | Unknown media | Collison nebulizer | Viruses were collected on a 47 mm gelatine filter and then dissolved in 10 mL of DMEM containing 10% FBS | Neeltje van Doremalen et al. (van Doremalen et al., 2020) | 2020 |
The Collison nebulizer has typically been used as the standard for aerosol production in infectious respiratory disease investigations (Chan et al., 2011; Hermann et al., 2007; Kormuth et al., 2019). The size distribution of generated aerosols by a Collison nebulizer is dependent on the type and viscosity of used pathogen suspension. It is possible to produce small-sized aerosols by through the dilution of the virus suspension. Furthermore, multiple size distributions were reported for Collison nebulizers between different studies. For example, it was reported that a Collison nebulizer produces high aerosol number concentrations, relatively monodisperse with a mass median aerodynamic diameter between 1 and 2 μm (Swearengen, 2012). It has also shown that the impaction, shear forces, and recirculation of the infectious sample can damage microorganisms, potentially decreasing pathogen viability or infectivity (Brown et al., 2015; Fennelly et al., 2015).
As discussed above, the physicochemical characteristics of human-generated carrier aerosols depend on the aerosol formation mechanisms and frequency of the respiratory activity, type, and location of infection and virus load. Additional, relative humidity, particle aggregation, and respiratory fluid properties can influence expelled particle size and subsequent transmission probability. Furthermore, the different aerosol production mechanisms could produce carrier droplets in different size modes compared to a Collision nebulizer, indicating that aerosols produced with a Collison nebulizer may not be sufficiently physicochemically representative of human respiratory aerosols. The aerosol generation procedures of these experimental works may not appropriately reflect the conditions of human respiratory activities and could be a potential cause of inconsistent conclusions.
10. Implications for the seasonality of airborne transmission
To find the seasonality of any virus several elementary facts are required to be in place: a vulnerable population in which the virus can propagate and replicate, a precise, delicate and particular diagnostic assessment to distinguish its existence, and a consistent, vigorous reporting method to provide the opportunity for determination and examination of seasonality cycles and trends. Seasonality is defined as “a time-based cycle of systematic, regular fluctuation within a fixed pattern that could be described by peak timing, amplitude, and interval” (Moorthy et al., 2012). Seasonal influenza peaks in the northern and southern temperate regions are the clearest example of this phenomenon that is not observed in tropical regions. Therefore, the factors which drive these seasonal cycles of influenza infections in temperate climates must not be present, or at least significantly varied, in tropical regions. The most noticeable change is the meteorological variables, such as relative and absolute humidity, temperature, rainfall, sunlight intensity, and wind speed. However, causes of the seasonality of respiratory infections may not be related to climatic variables. For example, the reason for these patterns could be related to fluctuations in indoor and outdoor climatic conditions, and their influence on virus viability, host behavioral patterns, host insusceptibility, intrinsic oscillations of the virus circulation and infection and factors affecting susceptible host proximity.
Some studies were performed to explain whether human coronaviruses exhibit seasonal variability (Table 7 ). In a study in Beijing (2003), the SARS-CoV epidemic peaked during the early springtime, and a correlation between the disease spread rate and climate variables were reported (Yuan et al., 2006). Likewise, in Hong Kong (2003), SARS-CoV incidence negatively correlated with higher temperatures (Lin et al., 2006). Contrasting with SARS-CoV, the highest global seasonal incidence of the MERS-CoV happened between the spring summer seasons in countries with typically warm climates, such as Saudi-Arabia, with high temperature and low RH (Al-Ahmadi et al., 2019; Altamimi and Ahmed, 2020; Nassar et al., 2018).
Table 7.
Relationship between incidence of coronaviruses and climatic variabilities.
| Study location | Study period | Study population | Infection type and Diagnostic test | Association with T | Association with RH | Other association | Statistical method | Author | Year |
|---|---|---|---|---|---|---|---|---|---|
| Guangzhou, China | January 2 to April 15, 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax_7 (R = −0.438, P-value≤0.001) Tmin_7 (R = −0.193 P-value≤0.05) |
RH_7 (R = −0.271, P-value≤0.001) | P_7 (R = −0.361, P-value≤0.001) | The lag = 7, simple correlation | Jianguo Tan et al. (Tan et al., 2005) | 2004 |
| Beijing, China | March 5 to May 31, 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax_7 (R = 0.528, P-value≤0.001) Tmin_7 (R = −0.475, P-value≤0.001) |
RH_7 (R = −0.448, P-value≤0.001) | P_7 (R= −0.513, P-value≤0.001) | The lag = 7, simple correlation | Jianguo Tan et al. (Tan et al., 2005) | 2004 |
| Taiyuan, China | March 7 to May 12, 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax_7 (R = −0.310, p˂0.001) Tmin_7 (R = −0.214, NS) |
RH_7 (R = −0.321, P-value≤0.001) | P_7 (R = −0.488, P-value≤0.001) | The lag = 7, simple correlation | Jianguo Tan et al. (Tan et al., 2005) | 2004 |
| Hong Kong | February 15 to 31 May 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax_7 = −0.453 Tmin_7 = −0.425 |
RH_7 (R = 0.067, NS) | P_7 (R = 0.364, P-value≤0.001) | The lag = 7, simple correlation | Jianguo Tan et al. (Tan et al., 2005) | 2004 |
| Hong Kong | 11 March to 22 May 2003 | hospital staff | SARS-CoV, clinical signs, chest X-ray, diagnostic tests in some patients and/or autopsy | An increase of 1 °C in air temperature was related to an average reduction of 0.7 staff patients. | – | – | regression analysis (odd ratio). | Kun Lin et al. (Lin et al., 2006) | 2005 |
| Beijing, China | April 3 to June 11, 2003 | laboratory-confirmed cases | clinical diagnosis | Temperature range (R = 0.337) Temperature (R = −0.718) |
Relative humidity (R = −0.784) | Wind velocity (R = 0.617), Barometric pressure (R = 0.210), Cloudiness (R = −0.569), and Precipitation (R = −0.379) |
Correlation | Jingsong Yuan et al. (Yuan et al., 2006) | 2006 |
| Hong Kong | April 21 to May 20, 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax (R = −0.79), Tmin (R = −0.76) |
RH (R = 0.24) | (R = 0.57) | Pearson’s correlation | P. Bi et al. (Bi et al., 2007) | 2007 |
| Beijing, China | April 21 to May 20, 2003 | laboratory-confirmed cases | SARS-CoV, clinical diagnosis | Tmax (R = NS) Tmin (R = −0.41) |
RH (R = −0.5) | (R = NS) | Pearson’s correlation | P. Bi et al. (Bi et al., 2007) | 2007 |
| Worldwide | June 2012 to the Dec 2017. | Worldwide 2048 laboratory confirmed Cases |
MERS-CoV, clinical diagnosis | The highest global seasonal occurrence was found in the month of June, while the lowest was found in the month of January | – | – | M.S. Nassar et al. (Nassar et al., 2018) | 2018 | |
| Hubei Province, China | from January 23, 2020 to February 10, 2020. | SARS-CoV-2, clinical diagnosis | Tmean (R = −1.05 and P-value = 0.008) | – | Absolute Humidity (R = 0.761 and P-value = 0.048) | Loess regression and an exponential fit | Wei Luo et al. (Luo et al., 2020) | 2020 | |
| 31 provincial-level regions in mainland China | between Jan 20 and Feb 29, 2020 | the number of new confirmed and probable cases were obtained from 101 the China National Health Commission (CNHC) | SARS-CoV-2, clinical diagnosis | – | – | No significant association between COVID-19 incidence and absolute humidity | regression and smoothing scatterplot | Peng Shi et al. (Shi et al., 2020) | 2020 |
| Worldwide | COVID-19 Global Cases up to March 19, 2020 (13 and 7 countries with cold and warm climates. 4 countries considered as none | Worldwide laboratory confirmed Cases |
SARS-CoV-2, clinical diagnosis | Correlation between rate of spread and T (R = −0.72, P-value≤0.001) | Correlation between rate of spread and morning humidity (R = 0.2, P-value = 0.39) Correlation between rate of spread and evening humidity (R = 0.11, P-value = 0.65) |
Correlation between rate of spread and precipitation (R = −0.04, P-value = 0.87) Correlation between rate of spread and dew point (R = −0.62, P-value = 0.008) |
Pearson and Spearman correlation | Gil Caspi et al. (Caspi et al., 2020) | 2020 |
Luo et al. in a preprint article on weather reports and statistics on COVID-19 incidence between January 23 and February 10, 2020, investigated the effects of temperature and RH on the SARS-CoV-2 transmission in China, Thailand, Singapore, Japan, South Korea, and Taiwan (Luo et al., 2020). No significant difference was found in the transmission rate between cold and dry provinces, concluding that decreases in case counts did not necessarily happen in higher temperatures and humidity. However, Bu et al. (2020) from a data analysis of Wuhan incidence rates (the Chinese city in which SARS-CoV-2 emerged) reported that the virus appears to spread thoroughly in summery weather, with an ideal temperature of 19 °C and RH of 75% and lower than 30 mm of monthly precipitation. Another new analysis of 80,981 cases of COVID-19 through mainland China between January 20 and February 29, 2020, reported that the best temperature for virus transmission is 10 °C and that lower or higher temperatures repress it (Shi et al., 2020). Another study with consideration of each worldwide confirmed case up to February 29, 2020, reported that higher temperatures are related to less disease incidence. However, the researchers believed any assumptions are conditional due to limited data (Bannister-Tyrrell et al., 2020). To date, there are many conflicting results regarding SARS-CoV-2 incidence, and it is poorly understood if there is any seasonal behavior exhibited by the virus. These conflicts can potentially be attributed to the difference between indoor and outdoor meteorological conditions, the effects of confounder variables, and differing statistical analyses between studies. As the susceptible population is a core driver for the pandemic, it has remained unclear whether geographic and seasonal alterations in climate can significantly change the pandemic path (Baker et al., 2020). Since the most reviewed studies period was limited to a short time, it is difficult to predict the novel coronavirus seasonality. A more accurate view can be obtained through a dynamic reporting system of COVID-19 cases over time. The WHO believes that the virus can be transmitted in all areas, regardless of climatic conditions. It is predicted that COVID-19 incidence will align similarly to SARS-CoV, potentially showing seasonality.
11. Knowledge gaps and future recommendation
-
1
Further randomized clinical studies should be conducted to measure the viable and total viruses in the broader size range of actual human-generated aerosols that better reflect droplets size, mass and volume produced under different natural respiratory activities, for each relevant virus.
-
2
The importance of airborne transmission could be examined by measuring the viable and total viruses of actual human aerosols (≤5 μm) at different aging times of aerosol.
-
3
Further studies need to be conducted to find the detailed chemical compositions of respiratory droplets and droplet nuclei of each mode of production for different viral infections, in comparison to healthy individuals.
-
4
Further studies need to be conducted to find the effects of water activity, surface inactivation, salt toxicity and phase separation of carrier aerosols on embedded viruses.
-
5
The relationship between RH or AH and the survival of actual expelled human-generated viruses should be assessed at a constant temperature to avoid confounding effects.
12. Conclusion
In this critical review, we reviewed the ability of infected individuals to produce droplet laden-virus with pandemic potential, including SARS-CoV, MERS-CoV, and SARS-CoV-2. There is evidence explaining the production mechanisms of airborne pathogenic bioaerosols through human respiratory activities, which can travel distance over several meters in the air and remain infectious. Studies investigating respiratory droplets and droplet nuclei generation during respiratory activities have shown that the produced droplets are of adequate size to support an infectious virus. Additionally, viral RNA was found in the air of patients’ rooms under different conditions. The airborne transmission was thought to play an essential role in the epidemiology of several highly transmissible coronaviruses that emerged this century. However, artificial generation of respirable aerosols, or measurements of viral RNA air samples may not fully demonstrate the importance of the airborne transmission route for these viruses. Besides, infection depends on the route of exposure, the size of the inoculum, the duration of exposure, and host defences. If human coronaviruses are primarily spread by large respiratory droplets, the use of proper PPE (including gloves, face masks or face shields) and maintaining an appropriate distance from other persons while maintain high personal hygiene should be sufficient to reduce risk of transmission. If, however, these viruses are carried by droplet nuclei that can remain airborne for prolonged periods, medical masks may be insufficient, as droplet nuclei can potential penetrate or circumnavigate the mask. It was reported that all three recent coronaviruses with pandemic potential could survive under controlled conditions in laboratory-generated aerosols for a significant length of time (>24 h). However, there is no evidence of this in human-generated respiratory aerosol. The physicochemical characteristics of carrier aerosols, which are originally expelled from the human respiratory tract, may play a significant role in virus survival, however, that role is not fully understood yet.
The mechanisms by which the composition and morphology of human respiratory aerosols influence the viability of embedded viruses is not well understood. Laboratory generated virus-laden aerosols studies might not be an appropriate simulation in studying the transportation and viability of airborne viruses in the environment. The components of human respiratory fluid create the viruses’ microenvironment and therefore, can affect viral survival in the air and environment. The composition of viral bioaerosols display wide inter and intrapersonal variance with the infected human source’s respiratory health status and the location in the respiratory tract from which the aerosol arises. Thus, these aerosols’ chemical compositions will likely be mixtures of the various chemicals present in mucus or lining secretions. This mixture status will finally affect the virus’s survival embedded in human respiratory droplets. Environmental field sampling has been collected for human coronaviruses in multiple locations. RT-qPCR has been used to detect the presence of viral RNA, which does not necessarily represent the existence of viable viruses but is an indicator of virus shedding and airborne transport of the viral RNA in respiratory droplet nuclei. The frequency of sites contaminated with human coronaviruses RNA ranged from <10% to >60%, which suggests that airborne transport of viral RNA is probable. The infection prevention and control implications of these discoveries emphasize the importance of the use of appropriate PPE to avoid close contact, droplet, and airborne transmissions.
Declaration of competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the Australian Research Council (ARC), Australia with DP170102733 grant number.
Footnotes
This paper has been recommended for acceptance by Pavlos Kassomenos
References
- Al-Ahmadi K., Alahmadi S., Al-Zahrani A. Spatiotemporal clustering of Middle East respiratory syndrome coronavirus (MERS-CoV) incidence in Saudi Arabia, 2012-2019. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Lillehoj E.P., Park Y., Kyo Y., Kim K.C. Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 2011;9:4. doi: 10.1186/1477-5956-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almstrand A.-C., Bake B., Ljungström E., Larsson P., Bredberg A., Mirgorodskaya E., Olin A.-C. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010;108:584–588. doi: 10.1152/japplphysiol.00873.2009. [DOI] [PubMed] [Google Scholar]
- Almstrand A.-C., Ljungström E., Lausmaa J., Bake B., Sjövall P., Olin A.-C. Airway monitoring by collection and mass spectrometric analysis of exhaled particles. Anal. Chem. 2009;81:662–668. doi: 10.1021/ac802055k. [DOI] [PubMed] [Google Scholar]
- Altamimi A., Ahmed A.E. Climate factors and incidence of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2020;13:704–708. doi: 10.1016/j.jiph.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S., Wexler A.S., Cappa C.D., Barreda S., Bouvier N.M., Ristenpart W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci. Rep. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar E.I., Hashem A.M., El-Kafrawy S.A., Sohrab S.S., Aburizaiza A.S., Farraj S.A., Hassan A.M., Al-Saeed M.S., Jamjoom G.A., Madani T.A. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. mBio. 2014;5 doi: 10.1128/mBio.01450-14. e01450-01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.E., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369:315–319. doi: 10.1126/science.abc2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister-Tyrrell M., Meyer A., Faverjon C., Cameron A. medRxiv; 2020. Preliminary Evidence that Higher Temperatures Are Associated with Lower Incidence of COVID-19, for Cases Reported Globally up to 29th February 2020. 2020.2003.2018.20036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir M.F., Ma B., Bilal Komal.B., Bashir M.A., Tan D., Bashir M. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci. Total Environ. 2020;728:138835. doi: 10.1016/j.scitotenv.2020.138835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbough J. Some factors affecting the survival of airborne viruses. J. Gen. Virol. 1971;10:209–220. doi: 10.1099/0022-1317-10-3-209. [DOI] [PubMed] [Google Scholar]
- Bi P., Wang J., Hiller J.E. Weather: driving force behind the transmission of severe acute respiratory syndrome in China? Intern. Med. J. 2007;37:550–554. doi: 10.1111/j.1445-5994.2007.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007;73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plummer F. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Gobom J., Almstrand A.C., Larsson P., Blennow K., Olin A.C., Mirgorodskaya E. Exhaled endogenous particles contain lung proteins. Clin. Chem. 2012;58:431–440. doi: 10.1373/clinchem.2011.169235. [DOI] [PubMed] [Google Scholar]
- Brown J.R., Tang J.W., Pankhurst L., Klein N., Gant V., Lai K.M., McCauley J., Breuer J. Influenza virus survival in aerosols and estimates of viable virus loss resulting from aerosolization and air-sampling. J. Hosp. Infect. 2015;91:278–281. doi: 10.1016/j.jhin.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Bu J., Peng D.-D., Xiao H., Yue Q., Han Y., Lin Y., Hu G., Chen J. medRxiv; 2020. Analysis of Meteorological Conditions and Prediction of Epidemic Trend of 2019-nCoV Infection in 2020. 2020.2002.2013.20022715. [Google Scholar]
- Caspi G., Shalit U., Kristensen S.L., Aronson D., Caspi L., Rossenberg O., Shina A., Caspi O. medRxiv; 2020. Climate Effect on COVID-19 Spread Rate: an Online Surveillance Tool. 2020.2003.2026.20044727. [Google Scholar]
- CDC . 2020. Social Distancing. [Google Scholar]
- Chan K.H., Peiris J.S., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.Y.H., Wan M.P., Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Li Y., Xie X., Katoshevski D. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Wong S.C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., Sridhar S., Chan J.F.W., Ho P.L., Yuen K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.S., Ng O.T., Wong M.S.Y., Marimuthu K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyle E.R., Maher M.C., Hernandez R.D., Basu S., Sugihara G. Global environmental drivers of influenza. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:13081–13086. doi: 10.1073/pnas.1607747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.S., Chaovavanich A., Javadi M., Yang J.Y., Anderson L.J., Tong S., Ho M.S. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin. Infect. Dis. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J.P. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiol. Infect. 1946;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames I., Tang J.W., Li Y., Wilson P. Airborne transmission of disease in hospitals. J. R. Soc. Interface. 2009;6(Suppl. 6):S697–S702. doi: 10.1098/rsif.2009.0407.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.A., Man J.C., Brand P., Katstra J.P., Sommerer K., Stone H.A., Nardell E., Scheuch G. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R.M., Hoagland K.W., Bosbous M., Castillo D., Foss B., Dunning M., Gare M., Lin W., Sun F. Dilution of respiratory solutes in exhaled condensates. Am. J. Respir. Crit. Care Med. 2002;165:663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- Fabian P., Brain J., Houseman E.A., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J. Aerosol Med. Pulm. Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian P., McDevitt J.J., DeHaan W.H., Fung R.O., Cowling B.J., Chan K.H., Leung G.M., Milton D.K. Influenza virus in human exhaled breath: an observational study. PloS One. 2008;3 doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Lau A.P.S., Chan C.K., Hung C.T., Lee T.W. Aerodynamic properties of biohazardous aerosols in hospitals. Hong Kong Med. J. 2008;14:26–28. [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghniiat K., Nabizadeh R., Yunesian M., Momeniha F., Mokamel A., Hassanvand M.S., MokhtariAzad T. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P., Martyny J.W., Fulton K.E., Orme I.M., Cave D.M., Heifets L.B. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 2004;169:604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- Fennelly K.P., Tribby M.D., Wu C.Y., Heil G.L., Radonovich L.J., Loeb J.C., Lednicky J.A. Collection and measurement of aerosols of viable influenza virus in liquid media in an Andersen cascade impactor. Virus Adapt. Treat. 2015;7:1–9. [Google Scholar]
- Freedman M.A. Phase separation in organic aerosol. Chem. Soc. Rev. 2017;46:7694–7705. doi: 10.1039/c6cs00783j. [DOI] [PubMed] [Google Scholar]
- Gralton J., Tovey E., McLaws M.-L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralton J., Tovey E.R., McLaws M.L., Rawlinson W.D. Respiratory virus RNA is detectable in airborne and droplet particles. J. Med. Virol. 2013;85:2151–2159. doi: 10.1002/jmv.23698. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R.B., Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, wuhan, China, 2020. Emerg. Infect. Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interface. 2013;10:20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck K., Schwarz K., Hohlfeld J.M., Seume J.R., Koch W. Submicron droplet formation in the human lung. J. Aerosol Sci. 2010;41:429–438. [Google Scholar]
- Hayden F.G. Respiratory viral threats. Curr Opin Infect Dis. 2006;19:169–178. doi: 10.1097/01.qco.0000216628.51563.b1. [DOI] [PubMed] [Google Scholar]
- Hermann J., Hoff S., Muñoz-Zanzi C., Yoon K.J., Roof M., Burkhardt A., Zimmerman J. Effect of temperature and relative humidity on the stability of infectious porcine reproductive and respiratory syndrome virus in aerosols. Vet. Res. 2007;38:81–93. doi: 10.1051/vetres:2006044. [DOI] [PubMed] [Google Scholar]
- Hersen G., Moularat S., Robine E., Géhin E., Corbet S., Vabret A., Freymuth F. Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean. 2008;36:572–577. doi: 10.1002/clen.200700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds W.C. John Wiley & Sons; 1999. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. [Google Scholar]
- Ho K.-F., Lin L.-Y., Weng S.-P., Chuang K.-J. Medical mask versus cotton mask for preventing respiratory droplet transmission in micro environments. Sci. Total Environ. 2020;735:139510. doi: 10.1016/j.scitotenv.2020.139510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren H., Gerth E., Ljungström E., Larsson P., Almstrand A.C., Bake B., Olin A.C. Effects of breath holding at low and high lung volumes on amount of exhaled particles. Respir. Physiol. Neurobiol. 2013;185:228–234. doi: 10.1016/j.resp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Holmgren H., Ljungström E., Almstrand A.-C., Bake B., Olin A.-C. Size distribution of exhaled particles in the range from 0.01 to 2.0μm. J. Aerosol Sci. 2010;41:439–446. [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Xing Y., Ni W., Zhang F., Lu S., Wang Z., Gao R., Jiang F. Environmental contamination by SARS-CoV-2 of an imported case during incubation period. Sci. Total Environ. 2020;742:140620. doi: 10.1016/j.scitotenv.2020.140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Brunner A.H., Sattar S.A., Nair R.C., Johnson-Lussenburg C.M. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66(Pt 12):2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- Johnson G., Morawska L., Ristovski Z., Hargreaves M., Mengersen K., Chao C.Y.H., Wan M., Li Y., Xie X., Katoshevski D. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011;42:839–851. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G.R., Morawska L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- Kesavan J., Schepers D., McFarland A.R. Sampling and retention efficiencies of batch-type liquid-based bioaerosol samplers. Aerosol. Sci. Technol. 2010;44:817–829. [Google Scholar]
- Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H., Choi J.P., Choi W.S., Min J.Y. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler H. The nucleus in and the growth of hygroscopic droplets. Royal Society of Chemistry. 1936;32:1152–1161. [Google Scholar]
- Kormuth K.A., Lin K., Qian Z., Myerburg M.M., Marr L.C., Lakdawala S.S. 2019. Environmental Persistence of Influenza Viruses Is Dependent upon Virus Type and Host Origin. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo E., Song E., Yockey L.J., Rakib T., Wong P.W., Homer R.J., Iwasaki A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116:10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.V. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7:760–761. doi: 10.1016/S1473-3099(07)70270-0. author reply 761-763. [DOI] [PubMed] [Google Scholar]
- Li Y., Leung G.M., Tang J., Yang X., Chao C.Y.H., Lin J.Z., Lu J., Nielsen P.V., Niu J., Qian H. Role of ventilation in airborne transmission of infectious agents in the built environment–a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Liljeroos L., Huiskonen J.T., Ora A., Susi P., Butcher S.J. Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18085–18090. doi: 10.1073/pnas.1105770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Marr L.C. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environ. Sci. Technol. 2020;54:1024–1032. doi: 10.1021/acs.est.9b04959. [DOI] [PubMed] [Google Scholar]
- Lin K., Yee-Tak Fong D., Zhu B., Karlberg J. Environmental factors on the SARS epidemic: air temperature, passage of time and multiplicative effect of hospital infection. Epidemiol Infect. 2006;134:223–230. doi: 10.1017/S0950268805005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Yan X., Cao W., Wang C., Feng J., Duan J., Xie S. Probing the structure of the SARS coronavirus using scanning electron microscopy. Antivir. Ther. 2004;9:287–289. [PubMed] [Google Scholar]
- Lindsley W.G., Blachere F.M., Thewlis R.E., Vishnu A., Davis K.A., Cao G., Palmer J.E., Clark K.E., Fisher M.A., Khakoo R. Measurements of airborne influenza virus in aerosol particles from human coughs. PloS One. 2010;5 doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Noti J.D., Blachere F.M., Thewlis R.E., Martin S.B., Othumpangat S., Noorbakhsh B., Goldsmith W.T., Vishnu A., Palmer J.E., Clark K.E., Beezhold D.H. Viable influenza A virus in airborne particles from human coughs. J. Occup. Environ. Hyg. 2015;12:107–113. doi: 10.1080/15459624.2014.973113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Pearce T.A., Hudnall J.B., Davis K.A., Davis S.M., Fisher M.A., Khakoo R., Palmer J.E., Clark K.E., Celik I.J.J.o.o., hygiene e. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Reynolds J.S., Szalajda J.V., Noti J.D., Beezhold D.H. A cough aerosol simulator for the study of disease transmission by human cough-generated aerosols. Aerosol. Sci. Technol. 2013;47:937–944. doi: 10.1080/02786826.2013.803019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Loosli C., Lemon H., Robertson O., Appel E. Experimental air-borne influenza infection. I. Influence of humidity on survival of virus in air. Proceedings of the Society for Experimental Biology and Medicine. 1943;53:205–206. [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:e151. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Majumder M.S., Liu D., Poirier C., Mandl K.D., Lipsitch M., Santillana M. medRxiv; 2020. The Role of Absolute Humidity on Transmission Rates of the COVID-19 Outbreak. 2020.2002.2012.20022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr L.C., Tang J.W., Van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface. 2019;16 doi: 10.1098/rsif.2018.0298. 20180298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish Z.A., Almasri M., Assirri A., Al-Shangiti A.M., Gray G.C., Lednicky J.A., Yezli S. Environmental sampling for respiratory pathogens in Jeddah airport during the 2013 Hajj season. Am. J. Infect. Contr. 2014;42:1266–1269. doi: 10.1016/j.ajic.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy M., Castronovo D., Abraham A., Bhattacharyya S., Gradus S., Gorski J., Naumov Y.N., Fefferman N.H., Naumova E.N. Deviations in influenza seasonality: odd coincidence or obscure consequence? Clin Microbiol Infect. 2012;18:955–962. doi: 10.1111/j.1469-0691.2012.03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Johnson G., Ristovski Z., Hargreaves M., Mengersen K., Chao C., Wan M., Li Y., Xie X., Katoshevski D. In: Proceeding of the 11th International Conference on Indoor Air Quality and Climate Change. Olesen B.W., Toftum J., Wargocki P., Zukowska D., Strom-Tejsen P., editors. University of Denmark; Denmark: 2008. Droplets expelled during human expiratory activities and their origin; pp. 1–8. [Google Scholar]
- Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar M.S., Bakhrebah M.A., Meo S.A., Alsuabeyl M.S., Zaher W.A. Global seasonal occurrence of middle east respiratory syndrome coronavirus (MERS-CoV) infection. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3913–3918. doi: 10.26355/eurrev_201806_15276. [DOI] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Current opinion in virology. 2012;2:90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J., Matthews L., Lemm J., Spector S. Human pulmonary secretions in health and disease. Annals of the New York Academy of Sciences. 1963;106:692–697. doi: 10.1111/j.1749-6632.1963.tb16677.x. [DOI] [PubMed] [Google Scholar]
- Prussin A.J., Schwake D.O., Lin K., Gallagher D.L., Buttling L., Marr L.C. Survival of the enveloped virus Phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00551-18. e00551-00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A.R., Leili M., Azarian G., Poormohammadi A. Sampling and detection of corona viruses in air: a mini review. Sci. Total Environ. 2020;740:140207. doi: 10.1016/j.scitotenv.2020.140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.Y., Chrétien J. Respiratory tract fluids: analysis of content and contemporary use in understanding lung diseases. Disease-a-Month. 1984;30:3–103. doi: 10.1016/0011-5029(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Rossman J.S., Lamb R.A. Influenza virus assembly and budding. Virology. 2011;411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnsjo A., Dabkowska A.P., Sparr E., Kocherbitov V., Arnebrant T., Engblom J. Diffusion through pig gastric mucin: effect of relative humidity. PloS One. 2016;11 doi: 10.1371/journal.pone.0157596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. medRxiv; 2020. Aerosol and Surface Transmission Potential of SARS-CoV-2. 2020.2003.2023.20039446. [Google Scholar]
- Schenkels L.C., Veerman E.C., Nieuw Amerongen A.V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit. Rev. Oral Biol. Med. 1995;6:161–175. doi: 10.1177/10454411950060020501. [DOI] [PubMed] [Google Scholar]
- Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld J.H., Pandis S.N. John Wiley & Sons; 2016. Atmospheric Chemistry and Physics: from Air Pollution to Climate Change. [Google Scholar]
- Shaman J., Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Pitzer V.E., Viboud C., Grenfell B.T., Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P., Dong Y., Yan H., Li X., Zhao C., Liu W., He M., Tang S., Xi S. medRxiv; 2020. The Impact of Temperature and Absolute Humidity on the Coronavirus Disease 2019 (COVID-19) Outbreak - Evidence from China. 2020.2003.2022.20038919. [Google Scholar]
- Sobsey M.D., Meschke J.S. Report for the World Health Organization; Geneva, Switzerland: 2003. Virus Survival in the Environment with Special Attention to Survival in Sewage Droplets and Other Environmental Media of Fecal or Respiratory Origin; p. 70. [Google Scholar]
- Song M., Ham S., Andrews R.J., You Y., Bertram A.K. Liquid–liquid phase separation in organic particles containing one and two organic species: importance of the average O : C. Atmos. Chem. Phys. 2018;18:12075–12084. [Google Scholar]
- Stelzer-Braid S., Oliver B.G., Blazey A.J., Argent E., Newsome T.P., Rawlinson W.D., Tovey E.R. Exhalation of respiratory viruses by breathing, coughing, and talking. J. Med. Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- Swearengen J.R. CRC Press; 2012. Biodefense Research Methodology and Animal Models. [Google Scholar]
- Tan J., Mu L., Huang J., Yu S., Chen B., Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol. Community Health. 2005;59:186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.-J., Pui D.Y.H. Numerical study of particle deposition in bends of a circular cross-section-laminar flow regime. Aerosol. Sci. Technol. 1990;12:813–831. [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright C.E., France M.W., O’Rourke P., Anuj S., Kidd T.J., Nissen M.D., Sloots T.P., Coulter C., Ristovski Z., Hargreaves M., Rose B.R., Harbour C., Bell S.C., Fennelly K.P. Cough-generated aerosols of Pseudomonas aeruginosa and other Gram-negative bacteria from patients with cystic fibrosis. Thorax. 2009;64:926–931. doi: 10.1136/thx.2008.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S.J. Factors affecting the viability of air-borne bacteria. III. The role of bonded water and protein structure in the death of air-borne cells. Can J Microbiol. 1960;6:89–105. doi: 10.1139/m60-011. [DOI] [PubMed] [Google Scholar]
- Welch D., Buonanno M., Grilj V., Shuryak I., Crickmore C., Bigelow A.W., Randers-Pehrson G., Johnson G.W., Brenner D.J. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8:2752. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W.F. ON AIR-borne infection∗: study II. Droplets and droplet nuclei. Am. J. Epidemiol. 1934;20:611–618. [Google Scholar]
- WHO . 2009. WHO Guidline for Natural Ventilation for Infection Control in Health-Care Settings. [PubMed] [Google Scholar]
- WHO . WHO; 2014. WHO Guideline for Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. [PubMed] [Google Scholar]
- WHO . 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. [Google Scholar]
- Wong T.W., Lee C.K., Tam W., Lau J.T., Yu T.S., Lui S.F., Chan P.K., Li Y., Bresee J.S., Sung J.J., Parashar U.D. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li Y., Sun H., Liu L.J.S.S.L., ISI . JR Soc. Interface; 2009. Exhaled Droplets Due to Talking and Coughing; p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M., Nishimura H., Kalonji N.L., Deng X., Momma H., Shimotai Y., Nagatomi R. Effects of high temperature on pandemic and seasonal human influenza viral replication and infection-induced damage in primary human tracheal epithelial cell cultures. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Lee G.W., Chen C.M., Wu C.C., Yu K.P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 2007;20:484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- Yang W., Elankumaran S., Marr L.C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J. R. Soc. Interface. 2011;8:1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Elankumaran S., Marr L.C. Relationship between humidity and influenza A viability in droplets and implications for influenza’s seasonality. PloS One. 2012;7 doi: 10.1371/journal.pone.0046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Marr L.C. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PloS One. 2011;6 doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Marr L.C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl. Environ. Microbiol. 2012;78:6781–6788. doi: 10.1128/AEM.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Liu Z., Meng X., Wang W., Kan H., Wang W. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H., Leung D.Y., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Yuan J., Yun H., Lan W., Wang W., Sullivan S.G., Jia S., Bittles A.H. A climatologic investigation of the SARS-CoV outbreak in Beijing, China. Am. J. Infect. Contr. 2006;34:234–236. doi: 10.1016/j.ajic.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A.J., Dijkman R., Fabri T., de Jong M.C., Koerkamp P.W.G. Effects of temperature, relative humidity, absolute humidity, and evaporation potential on survival of airborne Gumboro vaccine virus. Appl. Environ. Microbiol. 2012;78:1048–1054. doi: 10.1128/AEM.06477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamenskaya Y., Sotres J., Engblom J., Arnebrant T., Kocherbitov V. Effect of hydration on structural and thermodynamic properties of pig gastric and bovine submaxillary gland mucins. J. Phys. Chem. B. 2012;116:5047–5055. doi: 10.1021/jp212495t. [DOI] [PubMed] [Google Scholar]