Highlights
-
•
Clusters of decreased grey matter were associated with lateral flexion of the trunk.
-
•
Robust clusters were found in the posterior parietal cortex and the thalamus.
-
•
A tilt of the SVV correlated with the degree of trunk deviation.
-
•
Grey matter values from the thalamic cluster correlated with the SVV estimates.
Keywords: Parkinson’s disease, Subjective visual vertical, MRI, Lateral trunk flexion, Pisa syndrome
Abstract
Background
Disruption of central networks, particularly of those responsible for integrating multimodal afferents in a spatial reference frame, were proposed in the pathophysiology of lateral trunk flexion in Parkinson’s disease (PD). Knowledge about the underlying neuroanatomical structures is limited.
Objective
To investigate if decreased focal grey matter (GM) is associated with trunk flexion to the side and if the revealed GM clusters correlate with a disturbed perception of verticality in PD.
Methods
37 PD patients with and without lateral trunk flexion were recruited. Standardized photos were taken from each patient and trunk orientation was measured by a blinded rater. Voxel-based morphometry (VBM) was used to detect associated clusters of decreased GM. The subjective visual vertical (SVV) was assessed as a marker for perception of verticality and SVV estimates were correlated with GM clusters.
Results
VBM revealed clusters of decreased GM in the right posterior parietal cortex and in the right thalamus were associated with lateral trunk flexion. The SVV correlated with the extent of trunk flexion, and the side of the SVV tilt correlated with the side of trunk flexion. GM values from the thalamus correlated with the SVV estimates.
Conclusions
We report an association between neurodegenerative changes within the posterior parietal cortex and the thalamus and lateral trunk flexion in PD. These brain structures are part of a network proposed to be engaged in postural control and spatial self-perception. Disturbed perception of verticality points to a shifted egocentric spatial reference as an important pathophysiological feature.
1. Introduction
Mobile lateral trunk flexion (LTF) is one of the postural deformities in Parkinson’s disease (PD) that frequently occur as the disease progresses. At onset the degree of LTF is usually subtle, but LTF may get worse as the disease progresses. In its extreme form it results in the so-called Pisa syndrome (Tinazzi et al., 2016). LTF composes a relevant determinant of quality of life in PD patients, since it is associated with lower back pain and results in an increased imbalance (Doherty et al., 2011). PD patients with LTF and Pisa syndrome, respectively, are not always aware of their deviated body axis and do not adopt their head orientation to correct the alignment of visual input (Scocco et al., 2014), thus pointing to a profound disturbance of postural control in these patients. A previous study demonstrated that the subjective visual vertical (SVV) is shifted in PD patients with LTF (Scocco et al., 2014). The SVV is of great interest in this regard because it reflects the individual’s perception of verticality in relation to a gravitational reference for space (Barra et al., 2010). Knowledge about anatomical structures associated with LTF in PD, however, is still sparse and limited to a few lesion studies in animals and reports of patients who developed LTF after lesional and functional brain surgery within the basal ganglia (Doherty et al., 2011, Castrioto et al., 2014).
Currently, most evidence for the neuronal network involved in human postural control comes from stroke survivors who developed the so-called Pusher syndrome. This term refers to a flexion of the trunk to the side in these patients due to an active pushing away from the non-paretic side (Karnath and Broetz, 2003). The Pusher syndrome is often accompanied by a deviated perception of verticality (Baier et al., 2012). Although the underlying etiology is different in this syndrome and, in contrast to PD, the postural abnormality is due to a more active pushing, both syndromes share the lateral deviation of the trunk and a tilted SVV as common clinical features. Ischemic lesions within the brainstem, thalamus, cerebellum as well as the posterior insular and periinsular cortex, were found to result in an active trunk flexion to one side along with an erroneous perception of sitting upright in this position and/or a tilt of the SVV (Baier et al., 2012a, Baier et al., 2012b, Johannsen et al., 2006). Furthermore, the posterior parietal cortex was suggested to play an important role in postural control, since it integrates multimodal afferents and generates a mental allocentric and egocentric body representation in space (Sack, 2009). Accordingly disruption of this brain area results in a disturbed perception of body orientation including a tilted SVV (Rousseaux et al., 2013, Blanke et al., 2000). All of the above-mentioned nodes are tightly connected with the thalamus, which itself receives multiple afferents including afferents from the vestibular system. (Wijesinghe et al., 2015). Lesions to the thalamus themselves are also associated with a tilt of the SVV (Baier et al., 2016).
According to the Braak model for PD, most of the above-mentioned neuronal structures patients get affected by neurodegeneration as the disease progresses (Braak et al., 2004). Given the lack of knowledge about the neuroanatomical correlates associated with LTF in PD, we sought to reveal grey matter (GM) structures that are associated with this clinical sign. We assumed that this postural deformity might be related to focal clusters of decreased GM volume in the postural control network. Furthermore, we had the assumption that GM volume of areas associated with the occurrence of LTF correlated with a tilt of the SVV estimates.
2. Methods
2.1. Cohort and study design
37 patients with PD diagnosed according to the MDS diagnostic criteria (Postuma et al., 2015) with and without LTF were recruited. Patients were pre-screened in order to include a comparable number of PD patients with and without LTF. The presence of LTF was confirmed by a blinded rater as described below. Exclusion criteria were atypical Parkinsonism, the presence of any trunk deformity not associated with PD such as idiopathic scoliosis, dyskinesias severe enough to preclude standardized quantification of trunk position (as described below), major visual disturbances such as blindness or hemianopia, neglect, peripheral vestibular disorders, deep brain stimulation and presence of any other neurological disease that could interfere with postural control (e.g. stroke). All patients gave their written informed consent prior to study inclusion. The study was approved by the local ethic committee and was conducted according to the ICH-GCP guidelines.
2.2. Clinical assessment
A standardized interview for demographics and disease history was performed and the total levodopa equivalent dose (LED) and the LED for dopamine agonists were calculated for each participant (Tomlinson et al., 2010). All patients were screened clinically for the presence of relevant confounding disorders as defined by the exclusion criteria. Vestibular dysfunction was excluded by asking for indicative signs and by a focused clinical examination. The clinical assessment was performed in the on state and included the Unified Parkinson Disease Rating Scale (UPDRS) part I-III, Hoehn and Yahr stage and brief cognitive examination including the Mini Mental Status Examination (MMSE) and Frontal Assessment Battery (FAB).
2.3. Assessment of trunk deviation
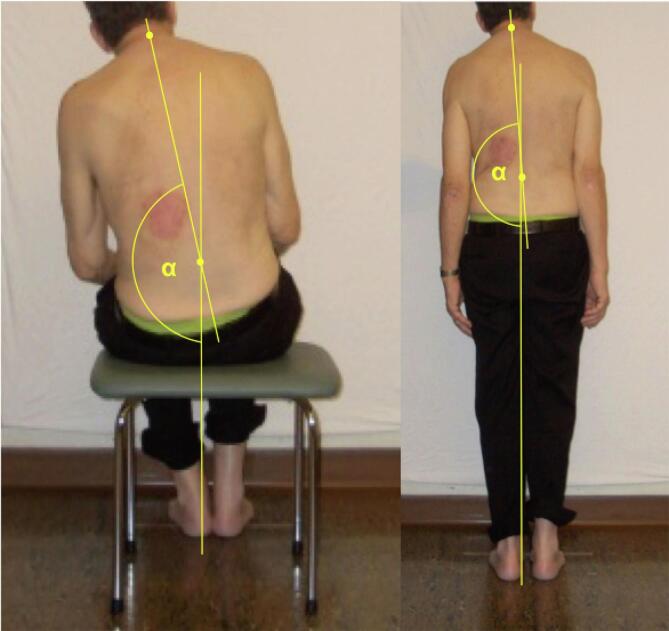
Patients were assessed while sitting and standing with their eyes open. In the sitting position, they were advised to sit comfortably on a stool with the hands on their thighs and their heels positioned close together. During stance they were asked to align their feet along a line drawn on the floor, again with their heels together. They were positioned in front of a white wall without any visual reference. The spinous processes of C7 and L4 were marked with a pen. A camera was placed 2.85 m behind the patients and three pictures were taken in each position. An investigator classified the patients as having normal or abnormal trunk orientation. A second investigator who was blinded to the clinical details reviewed all pictures and also judged if the patient’s trunk was tilted and, if so, recorded the side of the deviation. The same rater also measured the angle between the gravitational vertical and a virtual line drawn between the two markings (α) by the means of the MB Ruler® software in each patient. The difference between the optimal angle (i.e. 180°) and the observed angle value was calculated (delta(α); Fig. 1). Furthermore, the orientation of the head in relation to the horizontal plane was measured. The respective angle values are provided as difference between the actual angle and optimal angle of 90°. Judgments whether abnormal trunk orientation was present were congruent in 34/37 patients. Interrater reliability rate was calculated by the means of kappa statistics. Calculations yielded a kappa coefficient of 0.84, which was observed as proportion of maximum possible kappa thus indicating good interrater reliability. The three participants whose group allocation was not congruent between the two raters were excluded from subsequent analysis.
Fig. 1.
Illustration of the trunk measurement in the sitting and standing position.
2.4. Assessment of the subjective visual vertical
The SVV was estimated by the validated bucket test. This bedside test has been proven to have a good inter-test reliability compared to technically more elaborate methods of SVV estimation (Zwergal et al., 2009). Participants were asked to repeatedly orientate the reference line in the bucket along the vertical axis after it had been tilted clockwise and anticlockwise by 10°, 20°, 30° or 40°, respectively. In total, 16 measurements were done. The SVV was assessed with both eyes open. Upon completion, the intra-subject mean was calculated from all measured values. According to published reference values, a mean tilt > 2.0° was considered as abnormal (Sun et al., 2014).
2.5. Imaging technique
T1-weighted magnetization-prepared rapid acquisition gradient echo sequence was acquired in the same 3 T MRI system (Verio, Siemens, Erlangen, Germany) for all participants. We applied a voxel-based morphometry (VBM) pipeline to analyze GM morphometry, using the Computational Anatomy Toolbox 12 (http://dbm.neuro.uni-jena.de/) for SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) with default settings. The images were corrected for bias-field inhomogeneities, registered and normalized to Montreal National Institute (MNI) standard space by linear and non-linear transformation using Diffeomorphic Anatomical Registration Through Exponential Lie Algebra (DARTEL). All images were segmented into GM, white matter and cerebrospinal fluid using tissue probabilistic maps. All images were smoothed by an isotropic Gaussian kernel with 8 mm full-width at half maximum. In a second approach we accounted for the side of trunk deviation. For this purpose the scans from those patients who leant to the left were right-left flipped and pre-processed as described above. By flipping sides, it was assumed that all patients leant to the same side. The Anatomy toolbox and AAL3 toolbox were used to support the identification of the exact anatomical location of the clusters (Eickhoff et al., 2005, Rolls et al., 2020).
2.6. Statistics
SPSS 23 (IBM Corp., NY, United States) and MATLAB (Mathworks, Natick, US) were used for statistical analysis. Differences between two groups were calculated by the independent t-test or Mann-Whitney-U test as appropriate for their normal distribution. Likewise Pearson’s test or Spearman’s rank test were used for correlation analysis. Fisher’s exact test was applied for categorical variables. Values are provided as means +/- standard deviation.
A full factorial model with group information as binomial variable and degree of LTF (delta(a)) as covariate interacting with group information was set up for VBM. The model was corrected for age, disease duration and total intracranial volume (TIV). Family-wise error (FWE) correction was applied on the cluster-level and peak-level to correct for multiple testing. Since associated neuropathology in PD is diffuse and widespread already in the early phases of the disease (Braak et al., 2004) we applied a less conservative approach in which the cluster height threshold was set at p < 0.001 (uncorrected) and the clusters were corrected for multiple comparisons (FWE) only on the cluster-level. The brain regions detected by VBM were drawn as regions of interest (ROI) and GM values from each ROI were extracted by the virtue of the MarsBaR toolbox (http://marsbar.sourceforge.net/). The SVV estimates were subsequently correlated with the extracted GM values in a linear regression model together with age and TIV as further independent covariates.
3. Results
3.1. Clinical data
Demographics and disease characteristics of our cohort are summarized in table 1. 18/34 PD patients (52.9%) were classified by the raters as consistently leaning to the side. The measured angle was significantly larger in PD patients with LTF than in those without postural deformity (sitting position: 5.1 +/- 3.7° vs. 1.4 +/- 0.9°; t(32) = -4.109, p = 0.001; standing position: 3.4 +/- 2.8° vs. 1.5 +/- 1.0°; t(32) = -2.638, p = 0.015). 8/18 PD patients (44.4%) showed a trunk deviation to the left and 10/18 patients (55.6%) to the right. The degree of LTF did not correlate with the degree of head tilt (ρ = -0.079, p = 0.754). There was no significant association between the side of the body tilt and the PD dominant side (p = 0.520). The trunk deviation was larger in the sitting position than in the standing position (5.1 +/- 3.7 vs. 3.4 +/- 2.8; t(17) = 2.307, p = 0.034). We assumed that in our approach the sitting position provoked LTF better than stance, thus sitting being more sensitive in revealing this postural deformity. We therefore introduced only the angle values measured in the sitting position into our imaging analysis. There was a borderline significant correlation of the degree of trunk flexion with age (ρ = 0.334; p = 0.053), but with none of the other demographics and disease characteristics.
Table 1.
Demographics and clinical characteristics.
| PD-LTF | PD + LTF | p-value | |
|---|---|---|---|
| N subjects | 16 | 18 | |
| Demographics | |||
| Age (yrs) | 60.3 +/- 9.0 | 64.9 +/- 6.6 | 0.09 |
| Sex | |||
| male | 10 | 12 | 0.54 |
| female | 6 | 6 | |
| Disease specific data | |||
| Dominance of PD symptoms | |||
| left-sided | 11 | 8 | 0.10 |
| right-sided | 5 | 10 | |
| Duration of PD (yrs) | 8.3 +/- 3.7 | 11.1 +/- 5.3 | 0.10 |
| PD type | |||
| tremor-dominant type | 5 | 3 | 0.60 |
| akinetic-rigid type | 7 | 9 | |
| equivalence type | 4 | 6 | |
| Hoehn/Yahr stage | 2.1 +/- 0.4 | 2.3 +/- 0.4 | 0.35 |
| UPDRS I | 1.9 +/- 1.5 | 2.8 +/- 2.0 | 0.27 |
| UPDRS II | 11.5 +/- 5.2 | 15.8 +/- 5.0 | 0.08 |
| UPDRS III | 24.4 +/- 10.3 | 27.1 +/- 5.7 | 0.35 |
| FAB | 14.9 +/- 1.7 | 15.3 +/- 1.9 | 0.27 |
| MMSE Score | 28.9 +/- 1.3 | 28.1 +/- 1.5 | 0.12 |
| LED | |||
| Total | 972.2 +/- 557.8 | 918.0 +/- 492.6 | 0.65 |
| Dopamine agonists | 206.3 +/- 163.2 | 259.1 +/- 140.9 | 0.42 |
Legend: Mean values +/- standard deviation are provided. Abbreviations: FAB = Frontal Assessment Battery; LED = levodopa equivalent dose; LTF = lateral flexion of the trunk; MMSE = Mini Mental Status Examination; N = number; PD = Parkinson’s disease; UPDRS = Unified Parkinson’s Disease Rating Scale.
3.2. Subjective visual vertical
PD patients with LTF had significantly larger SVV tilts than those without (5.2° +/- 3.1° vs. 1.6° +/- 1.2°; t(32) = -4.386, p < 0.001). Within the whole cohort, 23/34 patients had formally pathological SVV estimates. 5/23 patients (21.7%) showed a SVV tilt to the right and 18/23 (78.3%) to the left. 17 of these 23 patients (73.9%) were classified as sitting abnormally. There was a significant relationship between the side of the SVV tilt and the side of trunk deviation (p = 0.001). 15/17 patients (88.2%) showed an ipsiversive and 2/17 (11.8%) a contraversive tilt of the SVV. There was no relationship between the side of the SVV tilt and the PD dominant side (p = 0.618). The SVV showed a correlation with the degree of LTF (ρ = 0.478, p = 0.004) in the sitting position. In those patients who leant to the side the degree of LTF did not correlate with the degree of head flexion to the side in the sitting position (ρ = 0.045, p = 0.858).
3.3. Imaging results
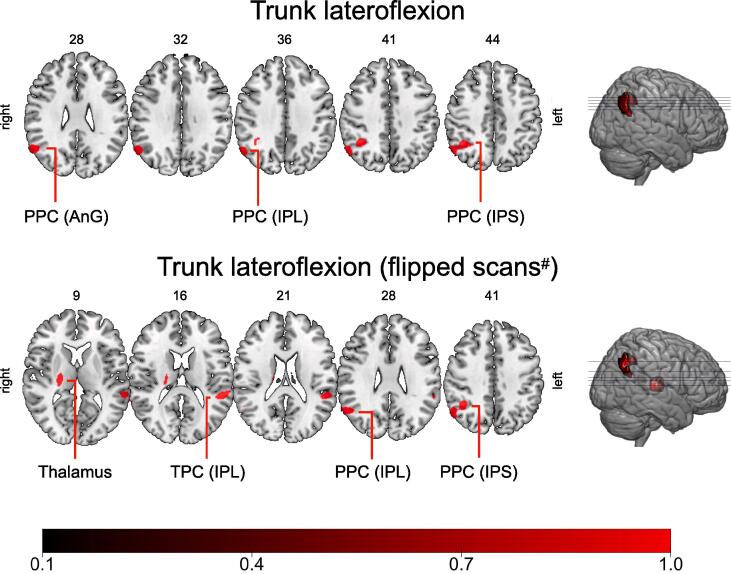
VBM revealed a significant cluster of decreased GM volume in the right inferior parietal lobule (MNI-coordinates: x = 56/y = -62/z = 32; p < 0.05, FWE corrected on the peak-level and cluster-level). In the less stringent approach the cluster extended into the posterior parietal cortex including the right inferior parietal lobule, the intraparietal sulcus and the angular gyrus (p < 0.001, FWE correction at cluster-level). Applying VBM after the scans from patients who leant to the left were right-left flipped, revealed a significant cluster in the ventral lateral nucleus of the right thalamus (MNI-coordinates: x = 20/y = -20/z = 18 ; p < 0.05, FWE correction on the peak-level and cluster-level). In the more lenient approach further clusters were detected in the right posterior parietal cortex (inferior parietal lobule and intraparietal sulcus) and the left temporoparietal cortex (inferior parietal lobule, superior and middle temporal gyrus). For details see Fig. 2 and table 2. GM values from the thalamus predicted SVV estimates (β = -0.372, p = 0.030; for further details see supplementary materials)
Fig. 2.
Clusters of decreased grey matter associated with lateral flexion of the trunk in PD. Multiple axial slices of a T2-weighted MRI scan of the brain with their respective z-coordinates are shown. The clusters (cluster-forming threshold p < 0.001, FWE correction at the cluster-level) that correlated with lateral flexion of the trunk in our voxel-based morphometry model are superimposed in red. The lower row of slices shows the results from the approach in which scans from patients leaning to the left were right-left flipped (#). A color bar with the coding of the p-values (1-p) is provided at the bottom of the figures. Abbreviations: AnG – angular gyrus; IPL – inferior lobule; IPS – intraparietal sulcus; PPC – posterior parietal cortex; TPC – temporoparietal cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Clusters of decreased grey matter associated with lateral flexion of the trunk in PD.
| Region | Side | cluster size | T-values | MNI coordinates | ||
|---|---|---|---|---|---|---|
| (voxels) | x | y | z | |||
| trunk flexion (no flipping of scans) | ||||||
| Posterior parietal cortex | right | 1130 | ||||
| - Inferior parietal lobule/angular gyrus* | 6.24 | 56 | −62 | 32 | ||
| - Intraparietal sulcus | 5.70 | 39 | −50 | 40 | ||
| - Inferior parietal lobule | 4.89 | 54 | −58 | 45 | ||
| trunk flexion (right-left flipping of scans#) | ||||||
| Thalamus (ventral lateral nucleus)* | right | 379 | 5.92 | 20 | −20 | 18 |
| Temporoparietal cortex | left | 740 | ||||
| - Superior temporal gyrus/inferior parietal lobule | 5.67 | −54 | −40 | 15 | ||
| - Middle temporal gyrus | 5.25 | −64 | −34 | 3 | ||
| Posterior parietal cortex | right | 1076 | ||||
| - Inferior parietal lobule | 5.14 | 51 | −57 | 26 | ||
| - Intraparietal sulcus | 5.08 | 40 | −57 | 45 | ||
Legend: Voxel-based-morphometry findings (cluster-forming threshold < 0.001, FWE correction at the cluster-level) that correlated with lateral flexion of the trunk are reported. The respective clusters including local maxima are listed. The asterisk (*) indicates clusters significant at p < 0.05 after FWE correction on the peak and cluster-level. #Scans from patients who leant to the left side were right-left flipped as described in the methods section. Abbreviations: MNI = Montreal National Institute.
4. Discussion
We found a focal cluster of decreased GM volume within the right posterior parietal cortex (including the inferior parietal lobule and the angular gyrus) to be associated with the occurrence of LTF in our cohort of PD patients. By right-left flipping the MRI scans from those patients who leant to the left side we assumed that all subjects had the same direction of LTF. We then found associated clusters in the right thalamus and, at a less stringent statistical threshold, in the right posterior parietal cortex and the left temporoparietal cortex. Differences in the detected clusters by the two approaches may indicate an asymmetry of GM decrease depending on the side of deviation and maybe also points to certain hemisphere dominance of the postural control network. Furthermore, the SVV estimates, a measurement for egocentric perception of verticality (Fetter, 2000), correlated with both the degree of LTF and GM values from the thalamus. Among those classified as having both an abnormal trunk orientation and SVV tilt, a relationship between the side of the SVV tilt and LTF was seen, thus being congruent with previous observations (Scocco et al., 2014). Interestingly, seven patients had an abnormal SVV, although they were classified as not leaning to the side. This observation could arguably indicate that disturbed perception of verticality might precede the occurrence of this postural deformity, but this needs to be confirmed in longitudinal observations. The deviation of the trunk was greater in the sitting than in the standing position. This observation is in line with that by Bonanni and colleagues (Bonanni et al., 2007). In our study the angles obtained during stance were measured within a few minutes upon standing up. The smaller angles in the standing position may be explained by a short-term compensation of the trunk deviation. Walking around, however, is considered as a further provoking factor of postural abnormalities and it may have increased the lateroflexion in the upright position (Bonanni et al., 2007). Interestingly, head orientation in the transversal plane did not correlate with that of the trunk suggesting independent control of these two body segments in the context of a pathological trunk lateroflexion in PD.
There was a trend towards a correlation of age with the degree of LTF, which was also described in previous observations (Tinazzi et al., 2015). In line with two previous studies, but in contrast to some others (reviewed in (Doherty et al., 2011), we did not find any association between the side of LTF and the dominant side of PD symptoms. We could not find any association with the intake of dopaminergic drugs either, particularly not with dopamine agonists. This finding thus contrasts observations of patients with Pisa syndrome in previous reports which proposed the occurrence of this sign in relation to the intake of these drugs (Castrioto et al., 2014, Tinazzi et al., 2015, Cannas et al., 2009, Galati et al., 2014). Taken together, we postulate that structural alterations within a network engaged in postural control lead to LTF in PD. One important prerequisite is a faulty perception of verticality which is tightly linked to the occurrence of this postural deformity. In this study we found some indications that the posterior parietal cortex and thalamus may be the main sites where the settings for an egocentric gravitational reference are flawed in PD.
The groups had comparable baseline characteristics, in particular with regard to parameters of motor progression (UPDRS III scores, Hoehn & Yahr) and cognitive decline, and the revealed GM clusters were significant after correcting for age-related confounders. Furthermore, we included the whole spectrum of this postural deformity, ranging from mild, but consistent, to severe LTF and accounted for the different degree of LTF in our VBM model. Hence we believe that the revealed clusters are specific for LTF and may not be ascribed to the impact of global disease progression or age-related processes.
The role of the brain regions, identified by our VBM approach, for postural control and spatial cognition has been well described in humans and animals: The posterior parietal cortex receives strong inputs from sensory, vestibular and visual afferents and is thus an important node for the processing of multimodal afferent information (Blanke and Arzy, 2005, Blanke et al., 2005, Bonda et al., 1995). Another important function of this network node is the mental representation of space (Sack, 2009). It is assumed that the posterior parietal cortex is the side where afferent information is integrated into an internal egocentric or allocentric body representation by modulating its gain (Blanke and Arzy, 2005, Naito et al., 2016, Whitlock, 2017). Likewise, the cluster in the temporoparietal cortex, also called temporoparietal junction, is considered to be a central node for multiafferent processing, particularly of vestibular and visual information (Blanke and Arzy, 2005, Whitlock, 2017). The thalamus can be regarded as a relay station for afferents projecting to the cortex and thus plays a central role in postural control. The ventral nuclear group receives strong projections from the vestibular nuclei and vestibulocerebellum as well as from the basal ganglia (Wijesinghe et al., 2015). It is, in turn, tightly interconnected with various cortical regions including the parieto-insular (vestibular) cortex and the inferior parietal lobule (Wijesinghe et al., 2015).
The relationship between the SVV estimates and the extent of LTF is of great interest, because the SVV reflects the individual’s perception of verticality in relation to a gravitational reference (Scocco et al., 2014). The SVV is organized by an internal model and is modulated by both bottom-up mechanisms including multisensory inputs and top down mechanisms such as spatial self-perception and body awareness (Barra et al., 2010, Barra et al., 2012). A disturbed SVV has already been demonstrated in association with postural disturbances in PD including Pisa syndrome and postural instability (Scocco et al., 2014, Pereira et al., 2014, Huh et al., 2018). An abnormal SVV as such, however, is not specific for a particular neuroanatomical structure, but is observed in different disorders of either the peripheral vestibular or the central nervous system (including lesions within the brainstem, thalamus and posterior insular cortex) (Baier et al., 2012a, Baier et al., 2012b). An adaptive deviation of upright perception can even be observed in healthy persons after tilting their body axis. These adaptive changes are referred to as Aubert(A)- or Müller(E)-effect depending if the tilt angle is smaller or>60° (Jaggi-Schwarz and Hess, 2003). Therefore, the question arises whether an abnormal SVV is cause or result of the postural abnormality. In our study the SVV estimates did not correlate with the head orientation. Furthermore, there were still a few patients who had an abnormal SVV, but a normal trunk orientation (and vice versa) and there was no fully consistent association between the side of the SVV tilt and the side of the body deviation. These observations render the A- or E-effect as an unlikely explanation for our results. Previous observations that PD patients with a Pisa syndrome still erroneously estimate the SVV or feel tilted after their body has been straightened are further evidence that the abnormal SVV is probably not secondary to LTF (Scocco et al., 2014). We believe that failure of vestibular afferents does not explain our results either. First, patients with clinical signs of peripheral vestibular disorders were excluded. Second, peripheral vestibular disorders result in a consistent ipsiversive SVV tilt, whereas we still found two patients with contraversive SVV tilts.
Our study has some limitations: First, although all patients were considered as consistently leaning to the side by two examiners, not all fulfilled the criterion of a Pisa syndrome requiring > 10° flexion angle as suggested by Doherty et al. (Doherty et al., 2011). However, this is an arbitrary cut-off, which has never been validated and we believe that this cut-off might miss mild forms within the spectrum of LTF. Furthermore, various methods of measuring trunk orientation are in use (Tinazzi et al., 2016), thus increasing heterogeneity of methodological approaches across studies on this topic. We used an approach which included assessments by two raters, one of them blinded to the patients’ background information, and we only included patients in whom the presence of LTF was judged consistently by both raters. LTF represents a clinical spectrum with Pisa syndrome at the extreme end and we therefore believe that our cohort is still representative for our research question. However, it may be discussed whether including exclusively patients with > 10° LTF may have yielded a better signal-to-noise-ratio of the statistical effects and may thus have resulted in a greater number of statistically more robust VBM clusters. Second, there was no systematic assessment of camptocormia in our study. Since camptocormia and LTF may co-occur in PD patients, future studies may address the question whether a comparable association between GM decrease and camptocormia exists. Third, since the head appears to be controlled independently from the trunk in PD patients with a pathological LTF, exploring the relationship between the head and trunk e.g. by using a kinematic analysis system may have provided further insights into postural control of the whole body. Future studies should pay more attention to this aspect. Fourth, GM pathology is considered to be rather diffuse in PD. This may result in a low signal-to-noise ratio for structural changes. Including a higher number of patients and patients with a more severe LTF may have led to the detection of more clusters and clusters of larger size associated with this postural deformity. Fifth, our study provides indications for a close relationship between the SVV and LTF, but we could not clarify how exactly the SVV is modulated by the different bottom-up and top-down inputs and how they eventually contribute to the trunk deviation. Therefore, further studies focusing on this aspect are needed.
5. Conclusion
In summary, our study provides evidence that there is an association between neurodegenerative changes within the network engaged in the integration of multimodal afferents and spatial self-representation, respectively, and LTF in PD. An abnormal perception of verticality may be a prerequisite that facilitates the occurrence of this postural deformity in PD.
CRediT authorship contribution statement
Florian Brugger: Conceptualization, Software, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization, Project administration. Julia Walch: Investigation, Data curation, Writing - review & editing. Stefan Hägele-Link: Resources, Writing - review & editing. Eugenio Abela: Methodology, Writing - review & editing. Marian Galovic: Methodology, Formal analysis, Writing - review & editing. Georg Kägi: Methodology, Resources, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102469.
Appendix.
A.1 Acknowledgment
We thank Anna Müller for proof reading of the manuscript.
A.2 Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
A3 Declaration of interests
F. Brugger: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: None; Employment: Kantonsspital St. Gallen, Switzerland; Partnerships: None; Contracts: None; Honoraria: Speaker’s honorarium from Zambon Switzerland; Royalties: Südwestdeutscher Verlag für Hochschulschriften; Grants: Baasch-Medicus stipend, research grant by the KSSG Forschungskommission, travel grant by Abbvie Switzerland; Other: None.
Conflict of interests: none
J. Walch: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: None; Employment: Kantonsspital St. Gallen, Switzerland; Partnerships: None; Contracts: None; Honoraria: None; Royalties: None; Grants: Travel grant by Abbvie Switzerland; Other: None.
Conflict of interests: none
S. Hägele-Link: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: none; Employment: Kantonsspital St. Gallen, Switzerland; Partnerships: None; Contracts: None; Honoraria: Speaker’s honorarium from Abbvie Switzerland; Royalties: None; Grants: None; Other: None.
Conflict of interests: none
E. Abela: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: None; Employment: Inselspital Berne; Partnerships: None; Contracts: None; Honoraria: None; Royalties: None; Grants: SNF 33CM30-124089, and NIHR BRC Preparatory Fellowship from King’s College London; Other: None. Conflict of interests: none
M. Galovic: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: None; Employment: University Hospital Zurich, Switzerland; Partnerships: None; Contracts: None; Honoraria: None; Royalties: None; Grants: Epilepsy Research UK; Other: None.
Conflict of interests: none
G. Kägi: Stock Ownership in medically-related fields: None; Intellectual Property Rights: None; Consultancies: None; Expert Testimony: None; Advisory Boards: Bayer, Zambon; Employment: Kantonsspital St. Gallen, Switzerland; Partnerships: None; Contracts: None; Honoraria: none; Royalties: None; Grants: Swiss Parkinson Association, Swiss Heart Foundation; Other: None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Baier B., Janzen J., Muller-Forell W., Fechir M., Muller N., Dieterich M. Pusher syndrome: its cortical correlate. J. Neurol. 2012;259(2):277–283. doi: 10.1007/s00415-011-6173-z. [DOI] [PubMed] [Google Scholar]
- Baier B., Suchan J., Karnath H.O., Dieterich M. Neural correlates of disturbed perception of verticality. Neurology. 2012;78(10):728–735. doi: 10.1212/WNL.0b013e318248e544. [DOI] [PubMed] [Google Scholar]
- Baier B., Thomke F., Wilting J., Heinze C., Geber C., Dieterich M. A pathway in the brainstem for roll-tilt of the subjective visual vertical: evidence from a lesion-behavior mapping study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(43):14854–14858. doi: 10.1523/JNEUROSCI.0770-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B., Conrad J., Stephan T., Kirsch V., Vogt T., Wilting J., Müller-Forell W., Dieterich M. Vestibular thalamus: Two distinct graviceptive pathways. Neurology. 2016;86(2):134–140. doi: 10.1212/WNL.0000000000002238. [DOI] [PubMed] [Google Scholar]
- Barra J., Marquer A., Joassin R., Reymond C., Metge L., Chauvineau V., Perennou D. Humans use internal models to construct and update a sense of verticality. Brain : a journal of neurology. 2010;133(Pt 12):3552–3563. doi: 10.1093/brain/awq311. [DOI] [PubMed] [Google Scholar]
- Barra J., Perennou D., Thilo K.V., Gresty M.A., Bronstein A.M. The awareness of body orientation modulates the perception of visual vertical. Neuropsychologia. 2012;50(10):2492–2498. doi: 10.1016/j.neuropsychologia.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Blanke O., Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2005;11(1):16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Blanke O., Perrig S., Thut G., Landis T., Seeck M. Simple and complex vestibular responses induced by electrical cortical stimulation of the parietal cortex in humans. J Neurol Neurosurg Psychiatry. 2000;69(4):553–556. doi: 10.1136/jnnp.69.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O., Mohr C., Michel C.M., Pascual-Leone A., Brugger P., Seeck M., Landis T., Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(3):550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni L., Thomas A., Varanese S., Scorrano V., Onofrj M. Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Movement disorders : official journal of the Movement Disorder Society. 2007;22(14):2097–2103. doi: 10.1002/mds.21694. [DOI] [PubMed] [Google Scholar]
- Bonda E., Petrides M., Frey S., Evans A. Neural correlates of mental transformations of the body-in-space. PNAS. 1995;92(24):11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rub U., Bratzke H., Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Cannas A., Solla P., Floris G., Tacconi P., Serra A., Piga M., Marrosu F., Marrosu M.G. Reversible Pisa syndrome in patients with Parkinson's disease on dopaminergic therapy. J. Neurol. 2009;256(3):390–395. doi: 10.1007/s00415-009-0072-6. [DOI] [PubMed] [Google Scholar]
- Castrioto A., Piscicelli C., Perennou D., Krack P., Debu B. The pathogenesis of Pisa syndrome in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2014;29(9):1100–1107. doi: 10.1002/mds.25925. [DOI] [PubMed] [Google Scholar]
- Doherty K.M., van de Warrenburg B.P., Peralta M.C., Silveira-Moriyama L., Azulay J.P., Gershanik O.S., Bloem B.R. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10(6):538–549. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fetter M. Assessing vestibular function: which tests, when? J. Neurol. 2000;247(5):335–342. doi: 10.1007/s004150050599. [DOI] [PubMed] [Google Scholar]
- Galati S., Moller J.C., Stadler C. Ropinirole-induced Pisa syndrome in Parkinson disease. Clin. Neuropharmacol. 2014;37(2):58–59. doi: 10.1097/WNF.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Huh Y.E., Kim K., Chung W.H., Youn J., Kim S., Cho J.W. Pisa Syndrome in Parkinson's Disease: Pathogenic Roles of Verticality Perception Deficits. Sci. Rep. 2018;8(1):1804. doi: 10.1038/s41598-018-20129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi-Schwarz K., Hess B.J. Common reference system for estimation of the postural and subjective visual vertical. Ann N Y Acad Sci. 2003;1004:516–520. doi: 10.1196/annals.1303.065. [DOI] [PubMed] [Google Scholar]
- Johannsen L., Broetz D., Naegele T., Karnath H.O. “Pusher syndrome” following cortical lesions that spare the thalamus. J. Neurol. 2006;253(4):455–463. doi: 10.1007/s00415-005-0025-7. [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Broetz D. Understanding and treating “pusher syndrome”. Phys. Ther. 2003;83(12):1119–1125. [PubMed] [Google Scholar]
- Naito E., Ota J., Murata A. Body representation in the brain. Neurosci Res. 2016;104:1–3. doi: 10.1016/j.neures.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Pereira J.B., Svenningsson P., Weintraub D., Bronnick K., Lebedev A., Westman E., Aarsland D. Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. 2014;82(22):2017–2025. doi: 10.1212/WNL.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., Halliday G., Goetz C.G., Gasser T., Dubois B., Chan P., Bloem B.R., Adler C.H., Deuschl G. MDS clinical diagnostic criteria for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- Rolls E., Huang C., Lin C., Feng J., Joliot M. Automated anatomical labelling atlas 3. NeuroImage. 2020;206(116189) doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- Rousseaux M., Honoré J., Vuilleumier P., Saj A. Neuroanatomy of space, body, and posture perception in patients with right hemisphere stroke. Neurology. 2013;81(15):1291–1297. doi: 10.1212/WNL.0b013e3182a823a7. [DOI] [PubMed] [Google Scholar]
- Sack A.T. Parietal cortex and spatial cognition. Behav. Brain Res. 2009;202(2):153–161. doi: 10.1016/j.bbr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Scocco D.H., Wagner J.N., Racosta J., Chade A., Gershanik O.S. Subjective visual vertical in Pisa syndrome. Parkinsonism Relat Disord. 2014;20(8):878–883. doi: 10.1016/j.parkreldis.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Sun D.Q., Zuniga M.G., Davalos-Bichara M., Carey J.P., Agrawal Y. Evaluation of a bedside test of utricular function - the bucket test - in older individuals. Acta Otolaryngol. 2014;134(4):382–389. doi: 10.3109/00016489.2013.867456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M., Fasano A., Geroin C., Morgante F., Ceravolo R., Rossi S., Thomas A., Fabbrini G., Bentivoglio A., Tamma F., Cossu G., Modugno N., Zappia M., Volonte M.A., Dallocchio C., Abbruzzese G., Pacchetti C., Marconi R., Defazio G., Canesi M., Cannas A., Pisani A., Mirandola R., Barone P., Vitale C., G. Italian Pisa Syndrome Study, Pisa syndrome in Parkinson disease: An observational multicenter Italian study. Neurology. 2015;85(20):1769–1779. doi: 10.1212/WNL.0000000000002122. [DOI] [PubMed] [Google Scholar]
- Tinazzi M., Geroin C., Gandolfi M., Smania N., Tamburin S., Morgante F., Fasano A. Pisa syndrome in Parkinson's disease: An integrated approach from pathophysiology to management. Movement disorders : official journal of the Movement Disorder Society. 2016;31(12):1785–1795. doi: 10.1002/mds.26829. [DOI] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Whitlock J.R. Posterior parietal cortex. Current biology : CB. 2017;27(14):R691–R695. doi: 10.1016/j.cub.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Wijesinghe R., Protti D.A., Camp A.J. Vestibular Interactions in the Thalamus. Front. Neural Circuits. 2015;9:79. doi: 10.3389/fncir.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwergal A., Rettinger N., Frenzel C., Dieterich M., Brandt T., Strupp M. A bucket of static vestibular function. Neurology. 2009;72(19):1689–1692. doi: 10.1212/WNL.0b013e3181a55ecf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.