Abstract
Objectives
Coronavirus disease 2019 (COVID-19) carries a high risk for malnutrition owing to the state of debilitation that results from acute respiratory failure symptoms. The aim of this study was to provide an approach to reduce the risk for malnutrition and improve patients’ clinical outcomes.
Methods
Short age-adjusted Nutritional Risk Screening was performed with 94 non-intensive care unit (ICU) patients admitted to the Giovanni Borea Civil Hospital in Sanremo. Forty-nine patients in the ICU were considered at risk for malnutrition without screening and were fed with enteral nutrition plus supplemental parenteral nutrition. In the non-ICU setting, patients underwent a personalized nutritional protocol, considering their conditions, which consisted of a high-protein and high-calorie pureed diet, oral nutritional supplements, and/or artificial nutrition or other personalized nutritional path.
Results
The nutritional treatment was well tolerated by the patients. Of the non-ICU patients, 19.1% died. They were mainly women, with higher body mass indices and older in age. Of the patients in the ICU, 53.1% died. Of the 94 non-ICU patients, 72 scored positive on at least one nutritional risk screening item (excluding age). Of the 94 non-ICU patients, 68 were >70 y of age. Non-ICU patients whose energy and protein needs were not met were older (P = 0.01) and had a higher death rate than patients whose needs were met (P < 0.001).
Conclusions
This protocol should not be considered as a guideline; rather, it is intended to report the clinical experience of a nutrition team in an Italian reference center for the treatment of patients with COVID-19. Nutritional strategies should be implemented to prevent worsening of clinical outcomes.
Keywords: COVID-19, Nutritional therapy, Malnutrition, Oral nutritional supplement, Dysphagia, SARS-CoV-2
Highlights
-
•
Hospitalized patients affected by coronavirus disease 2019 (COVID-19) are at high risk for malnutrition.
-
•
Nutritional therapy is essential to avoid deteriorating health conditions and worsening prognosis, even for patients who are non-critically ill.
-
•
The management of patients with COVID-19 was personalized by taking into consideration their conditions and the specific device used for the respiratory support.
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19), was identified for the first time in humans at the end of 2019 in Wuhan, China [1]. Since then, it has gradually spread worldwide, to the point that in March 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic [2,3].
Patients with COVID-19 can develop interstitial pneumonia with acute respiratory failure symptoms [4] and, in severe cases, subintensive or intensive care is requested [5]. Hospitalized patients with COVID-19 are characterized by a hyperinflammatory condition [6] and severe oxidative stress [7], which often is associated with a polymorbidity status, elderly age [8], and hypoalbuminemia [9], independent of body mass index (BMI) [10,11]. This carries a high risk for malnutrition with poor clinical outcomes [12,13]. Additionally, weight loss and low total protein and vitamin D levels [14] are frequently reported, as well as loss of taste and smell and nausea and diarrhea, all of which further worsen nutritional status [15].
The close connection between nutritional status and the evolution of COVID-19 is easily appreciated, considering that prealbumin is an important marker of malnutrition [16], and at the same time it seems to be a good predictor of the progression of acute respiratory distress syndrome (ARDS) [17]. The role of nutrition on immune function is well established, in fact, an inadequate intake of vitamins A, C, D, E, B2, B6, B12, folic acid, and trace elements such as iron, zinc, copper, and selenium compromises the immune system and predisposes the patient to infections [18,19]. Therefore, managing the nutritional status of hospitalized patients with COVID-19 is a pivotal point in reducing complications and improving clinical outcomes [20]. The role of nutrition is further enhanced in patients admitted to the intensive care unit (ICU) for the severity of respiratory failure. In this population, nutritional intake often is compromised due to the high incidence of swallowing problems after extubation [21] or to the presence of a temporary tracheostomy leading to dysphagia in 11% to 93% of cases [22].
Expert statements from the European Society for Clinical Nutrition and Metabolism (ESPEN) on the nutritional management of patients who are positive for SARS-CoV-2 suggest evaluating the nutritional status of all patients (as soon as they are hospitalized) through the Nutrition Risk Screening 2002 (NRS-2002), especially those at high risk (such as elderly patients with chronic or acute disease), to identify their risk for malnutrition sooner [20] and to develop a personalized diet.
The practical protocol presented in this study aims to provide a management approach for reducing the risk for malnutrition and to improve the clinical outcomes of these patients.
Methods
Protocol description
Baseline screening
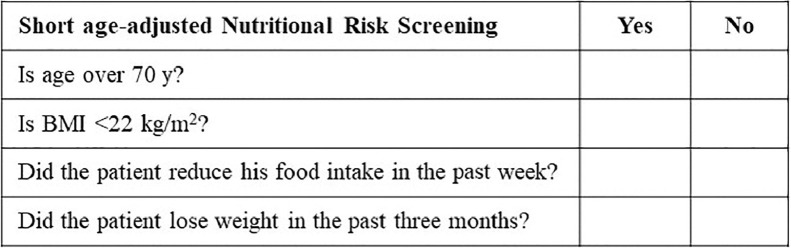
A short age-adjusted NRS was performed (Fig. 1 ) for all new non-ICU patients admitted to the Giovanni Borea Civil Hospital in Sanremo by adapting the NRS-2002 prescreening tool [23], adding the age question and removing the acute illness question. We used this modified NRS to enable immediate management, given the hospital emergency due to COVID-19. Weight and height were estimated or reported by the patients. Non-ICU patients who scored positive in at least one area of the short age-adjusted NRS were considered at risk for malnutrition and a nutrition expert physician administered nutritional counseling. ICU patients were considered at risk for malnutrition regardless, without performing the short age-adjusted NRS. ICU and non-ICU patients were also valued according to the Global Leadership Initiative on Malnutrition (GLIM) malnutrition tool. The latter contemplates the presence of at least one phenotypic criteria, which is low BMI and/or non-volitional weight loss (note that we have considered weight loss in the previous week and not in the previous 6 mo because we observed rapid deterioration in patients’ conditions) and one etiologic criteria, represented by the inflammatory condition.
Fig. 1.
Short age-adjusted Nutritional Risk Screening to identify the risk for malnutrition at admission to the hospital. BMI, body mass index.
Energy needs were estimated using the Harris–Benedict equation multiplied by a correction factor of 1.2 or 1.3 for the presence of the acute illness. Additionally, the following nutrition-related laboratory parameters were collected: complete blood count, blood glucose, creatinine, C-reactive protein (CRP), albumin, prealbumin, total proteins, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, magnesium, potassium, chloride, calcium, phosphorus, sodium, 25-hydroxyvitamin D (25[OH]D), interleukin (IL)-6, and fibrinogen.
Management of non-critically ill patients
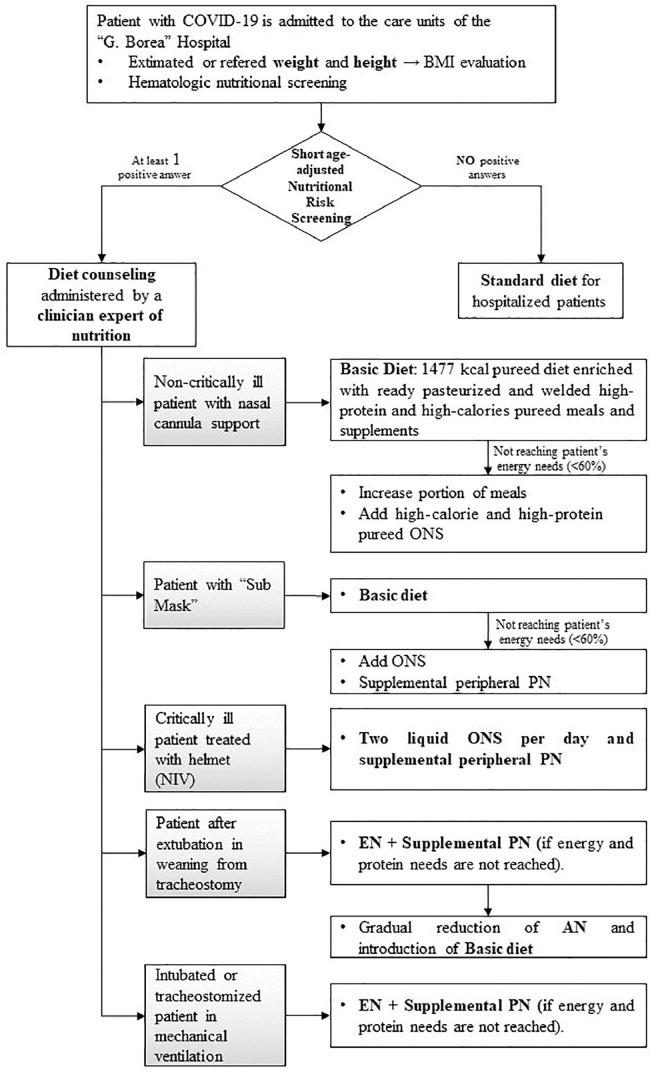
All COVID-19 non-critically ill patients with nasal cannula and simple oxygen face masks were subjected to an assessment of nutritional status and nutritional needs and were subsequently treated with a personalized nutritional protocol, which consisted of a fractionated pureed diet (basic diet; 1477 kcal - percentage of proteins: 19% of total kcal; percentage of lipids: 45% of total kcal; percentage of carbohydrates: 36% of total kcal) with modified-texture food, enriched with commercially available products designed for dysphagia. We used these commercial products due to their peculiar characteristics to improve adherence to the diet. These products take the form of natural pureed single-portion meals, which are ready to use, pasteurized, and supply high protein and high calories (average calorie content: 387 kcal; average protein content: 26.6 g). Furthermore, they are isolated from the external environment to ensure bacteriologic safety without affecting the organoleptic qualities. We also used a high-protein soluble powdered supplement for breakfast. The detailed composition of the basic diet, pureed meals, and high-protein food in soluble powder are described in the Supplementary Material. When patients needed more energy than the basic diet alone, they were administered increased portions of meals and/or high-calorie and high-protein pureed tasteless oral nutritional supplement (ONS) (125 g; 200 kcal; 12.5 g of proteins) to achieve nutritional targets.
Management of critically ill patients
The management of critically ill patients was carried out considering the specific device used for their respiratory support:
-
•
Patients in sub-ICU with the “Sub Mask” (a specially modified snorkeling mask connected to a ventilator), with an oxygen saturation level that can allow the detachment from the “Sub Mask” and the temporary positioning of the nasal cannula with high oxygen flow, for 10 to 15 min, in which the patients ate, were fed the basic diet and supplemental peripheral parenteral nutrition (PN).
-
•
Patients treated with a non-invasive ventilation (NIV) through the helmet, received two liquid high-calorie and high-protein tasteless ONS (200 mL; 320 kcal; 20 g of proteins) per day in bolus with a 500-mL syringe and supplemental peripheral PN, if tolerated.
-
•
Patients after extubation or in weaning from tracheostomy were fed enteral nutrition (EN) through nasogastric tube (NGT) and supplemental PN. When they were able to eat, they progressed to a gradual reduction in artificial nutrition and the introduction of basic diet.
-
•
In the ICU setting, intubated and tracheostomized patients in mechanical ventilation, both EN, via NGT and PN were administered. EN blends were low in carbohydrates (composition from 29% to 39% of carbohydrates, from 18% to 37% of proteins, from 500 to 750 kcal, in 500 mL).
We evaluated glycemia, arterial blood gas (ABG), lactates every 6 h, while pre-albumin, CRP, creatine phosphokinase, lactate dehydrogenase, liver and kidney function, electrolytes, procalcitonin were monitored every 48 to 72 h in all critically ill patients taking EN.
Figure 2 schematically shows nutritional management based on severity of the patient's illness.
Fig. 2.
Nutritional practical protocol based on patient severity. AN, artificial nutrition; EN, enteral nutrition; ONS, oral nutritional supplement; NIV, non-invasive ventilation; PN, parenteral nutrition.
Statistical analysis
Statistical analysis were performed using SPSS verison 25 (SPSS, IncChicago, IL, USA). The assumption of normality was verified with Kolmogorov–Smirnov test. Results of continuous variables were expressed as median and interquartile range (IQR) and mean plus SD, as appropriate. For ordinal and nominal variables, contingency tables indicating frequency and percentage in the population were used. For the comparison of continuous variables between different groups of patients non-parametric tests of Kruskal–Wallis, or Mann–Whitney when appropriate, were used. Categorical variables were examined with Fisher exact test.
Preliminary results
The Nutrition Department of Giovanni Borea Civil Hospital managed the diet of 143 patients:
-
•
In the ICU setting, 49 intubated or tracheostomy patients were fed with EN plus supplemental PN for the entire duration of mechanical ventilation. The mean age of the ICU patients was 69 ± 10 y (median 70; IQR, 65–76). Twenty-three ICU patients (46.9%) were discharged from the hospital, whereas 26 ICU patients (53.1%) died.
-
•
In the non-ICU setting, 35 of the 94 patients were given the basic diet alone. None of the 94 patients received ONS alone. However, to meet energy needs, 18 of the 94 patients received the basic diet integrated with ONS; 7 of the 94 patients received supplementary peripheral PN to the basic diet or ONS; 12 of the 94 patients were treated with EN or PN alone. Of the 94 patients, 13 were managed with other personalized nutritional paths due to the presence of comorbidities (i.e., kidney disease in which a low-protein diet is required). Characteristics of the 94 non-ICU patients are shown in Table 1 .
Table 1.
Non-ICU patient characteristics*
| Non-ICU setting (n = 94) | |
|---|---|
| Age (y) | 74 ± 15; 77 (68–85) |
| Women/Men | 37/57 |
| BMI (kg/m2) | 25.2 ± 4.4; 25.5 (22–28) |
| Albumin (g/dL) | 2.81 ± 0.53; 2.87 (2.5–3.2) |
| Total proteins (g/dL) | 5.8 ± 0.9; 5.9 (5.5–6.3) |
| Hemoglobin (g/dL) | 10.9 ± 1.7; 10.9 (9.7–12) |
| Polymorbidity, n (%) | |
| None | 17 (18.1) |
| ≥1 | 77 (81.9) |
| Therapy, n (%) | |
| Oxygen support | 68 (72.3) |
| Sub Mask | 15 (16) |
| NIV | 11 (11.7) |
| IMV | 0 |
| Nutritional support, n (%) | |
| Basic diet | 35 (37.2) |
| ONS | 9 (9.6) |
| Basic diet + ONS | 18 (19.1) |
| EN | 7 (7.4) |
| PN | 5 (5.3) |
| Basic diet/ONS + PN | 7 (7.4) |
| Personalized diet | 13 (13.8) |
| Outcome, n (%) | |
| Discharged | 76 (80.9) |
| Deceased | 18 (19.1) |
BMI, body mass index; EN, enteral nutrition; ICU, intensive care unit; IMV, intensive mechanical ventilation; ONS, oral nutritional supplement; NIV, non-invasive ventilation; PN, parenteral nutrition.
Data are mean ± SD; median (IQR) unless otherwise noted.
The prescribed nutritional treatment was well tolerated and the incidence of complications, in particular gastrointestinal complications (early satiety, diarrhea, abdominal distension), was low (only 5.3% of non-ICU patients were unable to tolerate the nutritional support). Nearly 81% of non-ICU patients and 46.9% of ICU patients were discharged from hospital; whereas 19.1% of non-ICU patients and 53.1% of ICU patients died. In the non-ICU group, the deceased patients were mainly women (n. 4/57, 7% versus 14/37, 37.8% deceased men and women, respectively, P < 0.001 at Fisher exact test), had higher BMI (median 24.9; IQR, 21.7–26.9 versus median 31.2; IQR, 28.3–31.4 kg/m2 in discharged and deceased patients, respectively; P < 0.001 at Mann–Whitney U test) and were older at hospitalization (median 75; IQR, 66–83 versus median 83; IQR, 78–87 y of age in discharged and deceased patients, respectively; P < 0.001 at Mann–Whitney U test; Table 2 ). On the other hand, hospitalized patients in ICU units were mainly men (42 men versus 7 women).
Table 2.
Classification of non-ICU patients based on the clinical outcome*
| Non-ICU patients (n = 94) |
P-value | ||
|---|---|---|---|
| Discharged (n = 76) | Deceased (n = 18) | ||
| Age (y) | 75, 66–83 | 83, 78–7 | ≤0.001† |
| Men (n = 57)/Women (n = 37) | 53 vs 23 | 4 vs 14 | ≤0.001‡ |
| BMI (kg/m2) | 24.9, 21.7–26.9 | 31.2, 28.3–31.4 | ≤0.001† |
BMI, body mass index.
Data are mean ± SD; median (IQR).
Mann–Whitney U test.
Fisher exact test.
The classification of non-ICU patients according to the short age-adjusted NRS is shown in Table 3 . Seventy-two of the 94 non-ICU patients scored positively on at least one malnutrition screening tool item (excluding age). Of the non-ICU 94 patients, 68 were >70 y of age.
Table 3.
Classification of non-ICU patients according to the short age-adjusted Nutritional Risk Screening and ICU and non-ICU patients according to GLIM malnutrition tool
| Short age-adjusted Nutritional Risk Screening for non-ICU patients | Non-ICU patients (n = 94) positive answer, n (%) | |
|---|---|---|
| BMI <22 kg/m2 | 20 (21.3) | |
| Food intake reduction in past week | 60 (63.8) | |
| Weight loss in past 3 mo | 29 (30.9) | |
| Age >70 y | 68 (72.3) | |
| GLIM for ICU and non-ICU patients | Non-ICU patients (n = 94) positive answer, n (%) | ICU patients (n = 49) positive answer, n (%) |
|---|---|---|
| Phenotypic criteria | ||
| Low BMI (<22 kg/m2) | 20 (21.2) | NA |
| Non-volitional weight loss in past week | 90 (95.7) | 49 (100) |
| Reduced muscle mass | NA | NA |
| Etiological criteria | ||
| Reduced food intake in past week | 59 (62.8) | 49 (100) |
| Disease burden/Inflammatory condition | 94 (100) | 49 (100) |
BMI, body mass index; ICU, intensive care unit; GLIM, Global Leadership Initiative on Malnutrition.
ICU and non-ICU patients were further classified according to GLIM malnutrition tool (Table 3). Patients presented at least one phenotypic criterion (between non-volitional weight loss, low BMI, low muscle mass) and one etiologic criterion (between reduced food intake, state of inflammation, comorbidity status).
Table 4 reported that of the 94 non-ICU patients, 56 reached their energy and protein needs with the personalized nutritional management. They reached their nutritional needs in a median of 14 d (IQR, 7–27). They were younger than the outstanding individuals who did not reach protein and energy targets (median 75; IQR, 64–82 versus median 80; IQR, 74–87 y of age, respectively; P = 0.01). Non-ICU patients meeting nutritional targets were more frequently discharged than those not meeting nutritional targets. In fact, 52 of 56 patients who met nutritional targets were discharged from the hospital. On the other hand, 14 of 38 patients (36.8%) who did not reach nutritional targets died. Conversely only 4 of 56 patients (7.1%) at target died (P < 0.001).
Table 4.
Classification of non-ICU patients based on the achievement of energy and protein needs*
| Non-ICU patients who met their energy and protein needs |
P-value | ||
|---|---|---|---|
| Yes (n = 56) | No (n = 38) | ||
| Age (y) | 70 ± 16, 75 (64-82) | 79 ± 11, 80 (74-87) | 0.011† |
| Women/Men | 20/36 | 17/21 | 0.380‡ |
| Polymorbidity, n (%) | |||
| None | 11 (19.6) | 6 (15.8) | 0.634‡ |
| ≥1 | 45 (80.4) | 32 (84.2) | |
| Outcome, n (%) | |||
| Discharged | 52 (92.9) | 24 (63.2) | ≤0.001‡ |
| Deceased | 4 (7.1) | 14 (36.8) | |
ICU, intensive care unit.
Data are mean ± SD; median (IQR), unless otherwise noted.
Mann–Whitney U test.
Fisher exact test.
Discussion
The present study aimed to provide practical indications for the nutritional management of patients with COVID-19. It is based on the experience of a single hospital, reference center for the SARS-CoV-2 outbreak, in a province, Imperia (Liguria, Italy), with a 39.2% prevalence of infected individuals of 215 800 inhabitants [24].
Nutritional therapy is essential to avoid deterioration of health conditions and worsening of prognosis, even for non-critically ill patients [25]. To our knowledge, there is still no guideline for the nutritional management of patients with COVID-19, although the use of the NRS reported in the present study is consistent with ESPEN expert recommendations [20]. The NRS was integrated with an age question as patients with COVID-19 often are elderly and have poor nutritional status [26]. The rationale of our short age-adjusted NRS was the early identification of those patients who may benefit from personalized nutritional intervention. Of the 94 non-ICU patients, 68 were >70 y of age. Using the age adjustment tool, an additional 22 patients were considered at risk for malnutrition because of their advanced age and therefore received nutritional support (Table 3). Moreover, according to the GLIM criteria for the diagnosis of malnutrition, all patients with COVID-19 presented with at least one phenotypical and one etiologic criteria, thus providing further evidence to validate the importance of nutritional intervention [27].
In the non-ICU group, patients who died were mainly women; to date the mortality from COVID-19 seems to be higher in men, although sex-related death mechanisms are not yet well known [28]. Furthermore, it would be useful to carry out a multivariate analysis to consider the influence of variables other than sex that, due to the state of emergency, we were unable to collect.
Non-ICU patients who died had a higher BMI than those who were discharged; in literature it emerges that overweight patients have a higher risk for mortality than normal weight patients [29], [30], [31] and this is in line with the present findings.
A crucial evidence of our preliminary results was the importance of reaching nutritional targets in non-ICU patients. In fact, deaths among the 38 patients who did not reach their energy and protein needs were reported more frequently than the 56 patients who reached their targets. This result is consistent with recently published reviews that highlighted the pivotal role of nutritional status in influencing the clinical outcomes of patients infected by SARS-CoV-2 [32,33]. Moreover, as reported in the ESPEN guidelines on clinical nutrition and hydration in geriatrics [12], even advanced age can influence the achievement of nutritional needs. In fact, in our sample, the patients who did not reach their nutritional needs were older than the outstanding patients.
Non-critically ill patients were fed a fractional pureed diet with high-protein and high-energy content to reduce the duration and volume of the meal. This basic diet had a soft consistency and was also chosen with the aim of reducing energy expenditure during meals, in patients already made fatigued and weakened from the respiratory effort associated with ARDS [34].
Critically ill patients treated with NIV were mostly fed by PN to limit gastrointestinal symptoms associated with EN, and to avoid NGT positioning, which can compromise NIV and increase the risk for aspiration [35]. However, liquid ONS (or basic diet for patients treated with Sub Mask) were also administered to increase nutrient intake in an effort to meet energy needs when these could not be satisfied with PN alone, and to preserve intestinal mucosal trophism [36].
We opted for the use of tasteless ONS, as many SARS-CoV-2–positive patients reported dysgeusia and/or anosmia at the time of admission. Subsequently, several studies confirmed this observation; and a recent meta-analysis has estimated that there is a prevalence of dysgeusia of 43.9% (95% confidence interval [CI], 20.5–69%) and olfactory dysfunction of 52.7% (95% CI, 29.6–75.2%) in patients with COVID-19 [37].
The extubation and/or weaning phase of the tracheostomy represented an important challenge of nutritional management, due to the almost constant presence in patients of a certain degree of dysphagia. In intubated patients, endotracheal tubes for mechanical ventilation can cause mucosal ulcerations and/or inflammation of the pharyngeal and laryngeal district; moreover, if the endotracheal intubation lasts >48 h, and therefore can be defined as prolonged oral endotracheal intubation, it tends to influence neuromuscular weakness of oropharyngeal structures. All this means that after extubation, patients often have problems swallowing, from odynophagia to aspiration [20]. In patients with tracheotomy, according to some authors, dysphagia could be explained by a reduction in sensory intake and subglottic air pressure, by the disuse of the laryngeal structure and its consequent slight atrophy; others, however, assert that swallowing impairment is partly due to the limitations that the tracheal tube gives to movement of the hyoid bone and laryngeal excursion [38]. These clinical categories of patients were therefore initially supported with EN via NGT and supplemental PN, then gradually the PN was suspended and the EN was integrated with the resumption of the basic diet orally.
Finally, in both critically and non-critically ill patients, carbohydrate intake was reduced, as the high-carbohydrate content in the diet has been associated with worsening of ARDS due to the increase in carbon dioxide production and consequent hypercapnia [25]. Additionally, ESPEN guidelines on clinical nutrition in the ICU suggest using low-carbohydrate formulas to avoid insulin resistance and hyperglycemia, which are frequently seen in critically ill patients [36].
The main limitation of the present protocol was that it was not possible to validate it due to the lack of measurements during hospitalization and at discharge. In fact, in our center, as in many others in Italy, the hospital directives advocated to avoid excessive contact with patients with COVID-19, due to the high risk for contagion of health personnel. Moreover, it was not possible to collect data from all patients admitted to the ICU due to their critical condition. Furthermore, as the COVID-19 health emergency remains ongoing in our hospital, we only have partial preliminary data on patient adherence to the protocol and its effectiveness.
Another important limitation of the present study was the lack of sample size calculation due to the evolving trend of the COVID-19 pandemic that characterized the period of patient hospitalization; therefore, the essential data for the calculation of the sample size, such as a real and certain data of the infected population and locoregional outcome data (i.e., mortality), were not available.
Conclusions
This protocol should not be considered as a guideline but is intended to report the clinical experience of an Italian hospital heavily affected by the pandemic emergency of COVID-19. The early implementation of appropriate nutritional strategies in these patients could improve the evolution and clinical outcome of the disease.
Statement of authorship
S. Demontis, C. Ivaldi contributed to conception/design of the nutritional protocol; L. Arieta, S. Bongiovanni, L. Panizzi, E. Valentino, S. Demontis implemented the nutritional protocol; E. Formisano, P. Di Maio, S. Demontis, E. Sferrazzo, M. Giudice drafted the manuscript; A. Pasta conducted the statistical analysis. All authors read and approved the final manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2020.111048.
Appendix. Supplementary materials
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Aziz TMA, Stockand JD. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) – an update on the status. Infect Genet Evol. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djalante R, Lassa J, Setiamarga D, Sudjatma A, Indrawan M, Haryanto B. Review and analysis of current responses to COVID-19 in Indonesia: period of January to March 2020. Prog Disaster Sci. 2020;6 doi: 10.1016/j.pdisas.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Végh T, László I, Juhász M, Berhés M, Fábián A, Koszta G. Practical aspects of intensive care for critically ill COVID-19 patients requiring respiratory support. Orv Hetil. 2020;161:678–684. doi: 10.1556/650.2020.31810. [DOI] [PubMed] [Google Scholar]
- 6.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity [Epub ahead of print] J Med Virol. 2020 doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugliera L, Spina A, Castellazzi P, Cimino P, Arcuri P, Negro A. Nutritional management of COVID-19 patients in a rehabilitation unit. Eur J Clin Nutr. 2020;74:8603–8609. doi: 10.1038/s41430-020-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19. Obes (Silver Spring) 2020;28:1175. doi: 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Cabrerizo S, Cuadras D, Gomez-Busto F, Artaza-Artabe I, Marín-Ciancas F, Malafarina V. Serum albumin and health in older people: review and meta-analysis. Maturitas. 2015;81:17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 14.D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handu D, Moloney L, Rozga M, Cheng F. Malnutrition care during the COVID-19 pandemic: considerations for registered dietitian nutritionists evidence analysis center [Epub ahead of print] J Acad Nutr Diet. 2020 doi: 10.1016/j.jand.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bharadwaj S, Ginoya S, Tandon P, Gohel TD, Guirguis J, Vallabh H. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep. 2016;4:272–280. doi: 10.1093/gastro/gow013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(suppl 1):S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 19.Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D. endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WJ, Park E, Min YS, Huh JW, Kim AR, Oh HM. Association between clinical risk factors and severity of dysphagia after extubation based on a videofluoroscopic swallowing study. Korean J Intern Med. 2020;35:79–87. doi: 10.3904/kjim.2018.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skoretz SA, Anger N, Wellman L, Takai O, Empey A. A systematic review of tracheostomy modifications and swallowing in adults [Epub ahead of print] Dysphagia. 2020 doi: 10.1007/s00455-020-10115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 24.ISS per COVID-19. Available at: https://www.iss.it/coronavirus. Accessed May 27, 2020.
- 25.Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol [Epub ahead of print] Nutrition. 2020 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cintoni M, Rinninella E, Annetta MG, Mele MC. Nutritional management in hospital setting during SARS-CoV-2 pandemic: a real-life experience. Eur J Clin Nutr. 2020;74:846–847. doi: 10.1038/s41430-020-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, GLIM Core Leadership Committee, GLIM Working Group GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busetto L, Bettini S, Fabris R, Serra R, Dal Pra’ C, Maffei P. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring) 2020;28:1600–1605. doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, LI G. Obesity predisposes to the risk of higher mortality in young COVID-19 patients [Epub ahead of print] J Med Virol. 2020 doi: 10.1002/jmv.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laviano A, Koverech A, Manetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruet M. Fatigue in chronic respiratory diseases: theoretical framework and implications for real-life performance and rehabilitation. Front Physiol. 2018;9:1285. doi: 10.3389/fphys.2018.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkin N, Krishnan K, Chang MG, Bittner EA. Nutrition in critically ill patients with COVID-19: challenges and special considerations. Clin Nutr. 2020;39:2327–2328. doi: 10.1016/j.clnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 38.Terk AR, Leder SB, Burrell MI. Hyoid bone and laryngeal movement dependent upon presence of a tracheotomy tube. Dysphagia. 2007;22:89–93. doi: 10.1007/s00455-006-9057-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.