Abstract
Objective
To investigate the in vitro cytocompatibility and osteogenic potential of an experimental calcium silicate-based cement and the inflammatory response in human periodontal ligament stem cells (hPDLSCs).
Methods
Cellular responses, osteogenic-related gene expression, and the production of inflammatory cytokines including interleukin (IL)-6 and IL-8 were studied in hPDLSCs exposed to the experimental root canal-filling material C-Root, the commercial tricalcium silicate-based material BioRoot RCS, and the epoxy resin-based material AH Plus. Differences were analyzed using one-way ANOVA with Bonferroni-adjusted pairwise comparison.
Results
Exposure to BioRoot and C-Root caused time-dependent increases in cell proliferation. Significantly more mineralized nodules were formed in cells exposed to AH Plus and BioRoot compared with the negative control. Alkaline phosphatase (ALP) activity was significantly lower in AH Plus cells compared with negative control, BioRoot, and C-Root cells. ALP, osteocalcin (OCN), and runt-related transcription factor2 (RUNX2) mRNA expression levels were all significantly higher in C-Root compared with AH Plus cells at day 7. IL-6 and IL-8 levels differed significantly among the experimental groups, with the highest IL-8 levels in BioRoot cells at days 7 and 14.
Conclusion
The experimental root canal-filling material C-Root has similar in vitro cytocompatibility to BioRoot and better osteogenic potential than AH Plus.
Keywords: Cytocompatibility, periodontal ligament stem cell, root canal, calcium silicate, osteogenic, inflammatory cytokine
Introduction
A root canal sealer in combination with a core material are used to seal voids and canal spaces in dentistry, to prevent coronal and apical penetration of fluids and microorganisms. However, the sealers may be extruded out via the apical foramen during root canal treatment, or toxic compounds may leach out of them causing irritation and delaying the healing of periodontal tissue and alveolar bone.1 It has therefore been recommended that endodontic sealers should be non-cytotoxic and biocompatible, and be well tolerated by periapical tissues without causing severe inflammatory reactions.2
Various endodontic sealers have been examined in preclinical studies and commercialized for clinical applications. These sealers are mainly classified based on their composition, including zinc oxide eugenol, glass ionomer, resin-based, calcium hydroxide, and calcium silicate-based materials. AH Plus (AH-P) is an epoxy resin-based sealer that has gained popularity in clinics due to its high radio-opacity and antimicrobial properties.3–5 However, AH-P tends to shrink during setting, resulting in bacterial or fluid microleakage as well as early debonding from the root canal wall.3,6
Substantial developments in materials science have led to the introduction of calcium silicate-based root canal sealers. Calcium silicate-based materials have demonstrated superior characteristics in terms of biocompatibility and bioactivity,7 and the tricalcium silicate-based sealer BioRoot RCS (BIO-R) has been shown to be bioactive in inducing the mineralization of dentinal structures.8 BIO-R induces the production of osteogenic growth factors by human periodontal ligament cells9 and creates a favorable microenvironment for periradicular tissue repair.7 A novel material using a calcium silcate-based setting system, C-Root (C-R), has recently been developed by Beijing C-root Dental Medical Devices Co. Ltd. (Beijing, China) and has gained increasing interest. It is mainly composed of strontium silicate, calcium dihydrogen phosphate, potassium dihydrogen phosphate, zirconium dioxide fillers, and a glycol-based gel. As a hydrophilic sealer, C-R uses moisture within the canal to complete the setting reaction and does not shrink on setting. BIO-R and the novel material C-R have been recommended as root canal sealers, in contrast to traditional mineral trioxide aggregate (MTA), which was introduced as a root repair material and was later incorporated into root canal sealers. It is therefore important to test the cytocompatibility and mineralization potential of C-R prior to its introduction for commercial use.
In this study, we examined the in vitro cytocompatibility, osteogenic potential, and inflammatory response of the experimental bioactive material C-R and compared it with BIO-R and AH-P, using human periodontal ligament stem cells (hPDLSCs).
Materials and methods
Selection and preparation of specimens
Single rooted maxillary premolars (n = 74) from an existing pool of collected teeth were used in this study. The teeth had been extracted from patients with periodontitis or undergoing orthodontic treatment, after obtaining written informed consent for extraction of the teeth and their use in experimental studies. The crowns were sectioned and root lengths were standardized at 12 mm. The root canals were shaped using ProTaper nickel titanium rotary instruments to size F5 (Dentsply Sirona Endodontics, York, PA, USA) and modified with Gates Glidden drills (sizes 2 and 3) to achieve an apical diameter of 0.8 mm. The canals were irrigated with 5 mL of 5.25% sodium hypochlorite solution during instrumentation. Once instrumentation was completed, the root canals were rinsed with 2 mL of 17% ethylenediaminetetraacetic acid (EDTA) for 2 minutes. All canals were then rinsed with 5 mL of sterile saline, dried with absorbent paper points (Dentsply Sirona Endodontics), and autoclave sterilized at 135°C for 35 minutes. The study protocol was approved by the Institutional Review Board of The University of Hong Kong (UW13‐120).
Root canal filling
All sealers were mixed according to the manufacturers’ instructions. C-R powder (Beijing C-root Dental Medical Devices Co. Ltd., Beijing, China) was first mixed with polyethylene glycol-based gel and then homogenized with a LR-5 homogenizer (Yekeey, Wuxi, China) for 15 minutes at 3500 ×g. All procedures were performed by the same operator and the root canals were filled under sterile conditions in a laminar flow hood using BIO-R (Septodont, Saint-Maur-des-Fosses, France), C-R, or AH-P (Dentsply De Trey, Konstanz, Germany) (n = 28 per group). The compositions of the materials are given in Table 1. Excess filling materials were removed with a sterile scalpel. Teeth with unfilled root canals (n = 10) served as a negative control. For sterilization, the teeth were irradiated with ultraviolet light for 4 hours and stored in an incubator at 37°C and 100% humidity for 24 hours to achieve complete setting.
Table 1.
Composition of materials used in this study.
| Material | Acronym | Manufacturer | Lot no. | Components | |
|---|---|---|---|---|---|
| AH Plus Jet™ Mixing Syringe | AH-P | Dentsply DeTrey GmbH, Konstanz, Germany | 1504000319, 03‐17 | Paste A: bisphenol-A epoxy resin, bisphenol-F epoxy resin, calcium tungstate, zirconium oxide, silica, iron oxide pigments | Paste B: dibenzyldiamine, aminoadamantane tricyclodecane-diamine, calcium tungstate, zirconium oxide, silica silicone oil |
| Bio-Root RCS powder | BIO-R | Septodont, Saint Maur‐des‐Fosses, France | B15847, 06‐17 | Powder: tricalcium silicate, zirconium oxide and povidoneLiquid: aqueous solution of calcium chloride and polycarboxylate | |
| C-Root (experimental material) | C-R | Beijing C-Root Dental Medical Devices Co. Ltd, China | Experimental | Powder: calcium silicate compound, strontium silicate, calcium dihydrogen phosphate, potassium dihydrogen phosphate, magnesium oxide, zirconium dioxideLiquid: polyethylene glycol, water-free liquid | |
Cell culture
The hPDLSCs used in this study were a kind gift from Dr. Chongshan Liao (The University of Hong Kong), and have been previously characterized by our research group.10 All experimental protocols involving the use of hPDLSCs were approved by the Institutional Review Board of The University of Hong Kong (UW13‐120). hPDLSCs at passage 3–7 were used for all experiments. hPDLSCs were cultured in alpha minimum essential medium (α-MEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin antibiotic solution at 37°C in a humidified 5% CO2 incubator. The culture medium was replaced every 3 to 4 days, and confluent monolayers were dissociated with 0.5% (w/v) trypsin/EDTA for routine cell passage. For osteogenic/odontogenic induction, hPDLSCs were seeded at a density of 1 × 105 or 2 × 104 cells in 6-well plates and cultured in α-MEM, 10% (v/v) FBS, and 1% (v/v) penicillin–streptomycin supplemented with 10−8 M dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid for 21 days. The induction medium was replaced every 3 days. Negative controls were established with hPDLSCs cultured for 21 days in the absence of test materials under non-osteogenic-inducing conditions in normal culture medium, to ensure methodological validity.
Root canal-filling model apparatus
A root canal-filling model apparatus was fabricated based on previously published studies.11,12 Briefly, the lower 3- to 5-mm proportion of polypropylene Eppendorf tubes (ExtraGene, Taichung City, Taiwan) (volume 1.5 mL) was cut to allow 5 mm of the apical third of the root to protrude into a 6-well cell culture plate. A rubber O-ring was used to adjust the position of the tooth and the extent of immersion into the cell culture.
Alizarin red S assay
Calcium mineralization in the cell cultures was measured by Alizarin red S assay after 14 days of osteogenic/odontogenic induction. Briefly, the culture medium was removed from each well and the hPDLSCs were washed three times with 1× phosphate-buffered saline, and then fixed with 4% (w/v) paraformaldehyde for 15 minutes at room temperature. The fixative was removed and the specimens were washed with deionized water, cleaned, and stained with 1% (w/v) Alizarin red S (pH 4.1–4.2) at room temperature.
After additional rinsing in deionized water, the cells were observed under a phase contrast microscope. Calcium mineralization in the cultured cells was quantified by the addition of 10% (w/v) acetic acid to each well, followed by incubation for around 30 minutes at room temperature with gentle shaking. The cytolysates were collected in a microcentrifuge tube and vortexed for 30 seconds. Samples were heated to 85°C for 10 minutes and then incubated on ice for 5 minutes. The slurry was centrifuged at 2000 × g for 15 minutes and the absorbance was measured at 405 nm using a SpectraMAX 340® microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Alkaline phosphatase (ALP) staining assay
ALP activity was detected using a SensoLyte® pNPP Alkaline Phosphatase Assay Kit (AnaSpec, Inc., Fremont, CA, USA). Briefly, hPDLSCs were cultured in osteogenic/odontogenic induction medium for 14 days and then washed twice with 1× assay buffer. Triton X-100 (20 μL) was added into 1× assay buffer (20 mL) and 0.15 mL of 1× assay buffer was additionally added to the cells. Adherent cells were scraped off and the cell suspensions were collected into microcentrifuge tubes. Upon dephosphorylation, the para-nitrophenylphosphate turned yellow and was detected at an absorbance of 405 nm using a SpectraMAX 340® microplate reader. The protein concentration of the cell suspensions was measured by bicinchoninic acid assay (Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. ALP activity was expressed in units per mg protein.
Cell proliferation assay
The proliferation of hPDLSCs was evaluated after 24 hours of exposure to the test materials using a Cell Counting Kit-8 (CCK-8; Dojindo, Japan) assay according to the manufacturer’s protocol, after 1, 3, 5, and 7 days of culture. Briefly, hPDLSCs were seeded at a density of 2 × 104 cells per well into 6-well plates and maintained under normal culture conditions from days 1 to 7. For each sample, 50 µL CCK 8 was mixed with 500 µL culture medium. After incubation at 37°C for 4 hours, three 110-μL aliquots of the solution were transferred from each well to a 96-well plate and the absorbance was measured at 450 nm using a SpectraMAX 340® microplate reader.
Quantitative real time polymerase chain reaction (qRT-PCR)
hPDLSCs were seeded at a density of 1 × 105 cells per well into 6-well plates and incubated with the test materials in osteogenic/odontogenic induction medium for 7 and 14 days. Total RNA was isolated using an RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) and then reverse transcribed into cDNA using Super Script VILO Master Mix (Life Technologies, Grand Island, NY, USA). qRT-PCR was performed using SYBR Select Master Mix (Applied Biosystems, Grand Island, NY, USA) on a Step One Real-Time PCR System (Applied Biosystems). The following marker genes were analyzed: ALP, osteocalcin (OCN), runt-related transcription factor 2 (RUNX2), and dentin matrix protein 1 (DMP1), and the internal control housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The corresponding primer sequences for these genes are listed in Table 2. The amplification parameters for qRT-PCR were as follows: 2 minutes at 50°C, 20 seconds at 95°C, and 40 cycles of 3 seconds at 95°C followed by 30 seconds at 60°C. The relative cycle threshold was determined using the 2−ΔΔ cycle threshold method and normalized against the endogenous GAPDH gene.
Table 2.
Primer sequences of gene markers used in this study.
| Gene | Primer sequence |
|---|---|
| ALP | Forward: 5′-CCTCGTTGACACCTGGAAGAG-3′Reverse: 5′-TTCCGTGCGGTTCCAGA-3′ |
| OCN | Forward: 5′-CACTCCTCGCCCTATTGGC-3′Reverse: 5′-CCCTCCTGCTTGGACACAAAG-3 |
| RUNX2 | Forward: 5′-TCTTAGAACAAATTCTGCCCTTT-3′Reverse: 5′-TGCTTTGGTCTTGAAATCACA-3 |
| DMP1 | Forward: 5′-CTCCGAGTTGGACGATGAGG-3′Reverse: 5′-TCATGCCTGCACTGTTCATTC-3′ |
| GAPDH | Forward: 5′-GGCATGGACTGTGGTCATGAG-3′Reverse: 5′-TGCACCACCAACTGCTTAGC-3′ |
Enzyme-linked immunosorbent assay (ELISA)
Serum concentrations of the inflammatory cytokines interleukin (IL)-6 and IL-8 were quantified using commercial ELISA kits (Human IL-6 Duo Set and Human IL-8/CXCL8 Duo Set, respectively; R&D Systems Inc., Minneapolis, MN, USA), according to the manufacturer’s instructions. The reactions were read at 540 nm or 570 nm using a SpectraMAX 340® microplate reader.
Statistical analyses
All data were normally distributed and were analyzed using parametric tests. Statistical analyses were performed using one-way ANOVA with Bonferroni-adjusted pairwise comparisons for each time-point. A value of P < 0.05 was considered significant.
Results
Cell proliferation
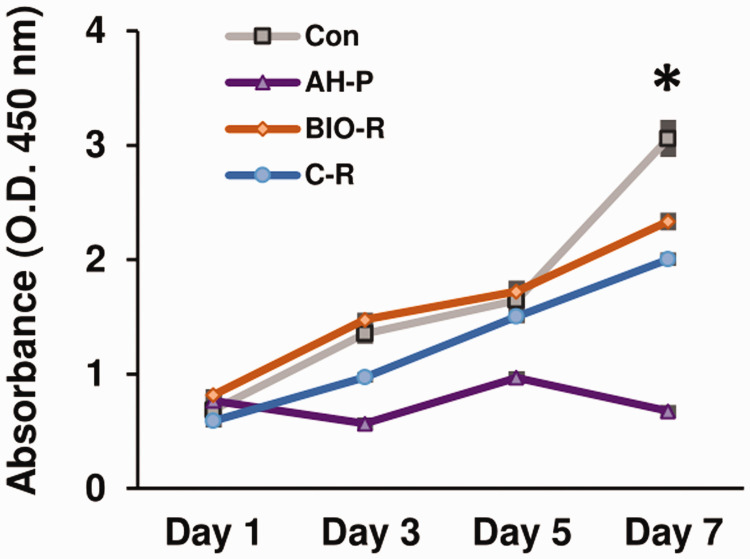
Cell proliferation varied with time and among the different materials (Figure 1). There were significant differences in cell proliferation among the groups from days 1 to 7 (P < 0.05). Cells exposed to BIO-R showed the highest proliferation rate from days 1 to 5 (P < 0.05), whereas cells exposed to AH-P showed the lowest proliferation rate (P < 0.05) from days 3 to 7. The proliferation of cells exposed to BIO-R was comparable to that in the negative control group before day 5, but was significantly lower at day 7 (P < 0.05).
Figure 1.
Cell proliferation assay using Cell Counting Kit (CCK)-8. *P < 0.05 among the groups. O.D., optical density; Con, negative control; AH-P, AH Plus; Bio-R, BioRoot RCS; C-R, C-Root.
qRT-PCR
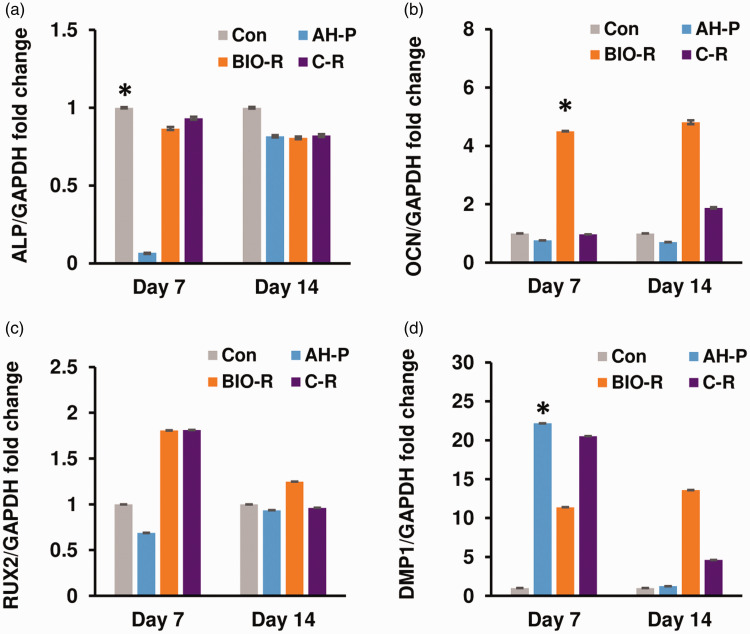
mRNA expression levels of ALP, OCN, and DMP1 in hPDLSCs different significantly among the groups after 7 days of osteogenic induction (P < 0.05) (Figure 2). Among the different materials, AH-P-exposed cells exhibited the lowest expression of ALP and OCN (P < 0.05) at day 7, while BIO-R-exposed cells exhibited the highest expression of OCN (P < 0.05). Compared with BIO-R, C-R-exposed cells displayed significantly higher ALP and DMP1 mRNA levels at day 7 (P < 0.05). RUNX2 mRNA levels were about two-fold higher in BIO-R- and C-R-exposed compared with AH-P-exposed cells, but the difference was not significant. At day 14, OCN and DMP1 mRNA levels were higher in the BIO-R and C-R groups compared with the AH-P and negative control groups, but the differences among the groups for all four osteogenic-related genes were not significant.
Figure 2.
Gene expression profiles of ALP, OCN, RUNX2, and DMP1 in human periodontal ligament stem cells treated with the test materials, analyzed using qRT-PCR. *P < 0.05 among the groups. ALP, alkaline phosphatase; OCN, osteocalcin; RUNX2, runt-related transcription factor 2; DMP1, dentin matrix protein 1, DMP1; glyceraldehyde 3-phosphate dehydrogenase; Con, negative control; AH-P, AH Plus; Bio-R, BioRoot RCS; C-R, C-Root.
Alizarin red S assay
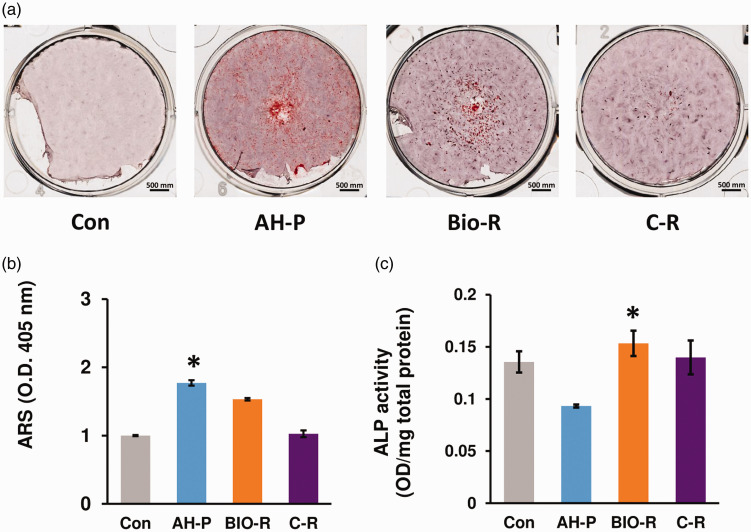
Alizarin red S staining revealed significant differences among the groups (P < 0.05) (Figure 3). Compared with the negative control group, all three experimental groups showed significantly more mineralized nodules (P < 0.05). In addition, AH-P-exposed cells showed significantly more mineralized nodules than the BIO-R and C-R groups (P < 0.05).
Figure 3.
(a, b) Alizarin red S and (c) alkaline phosphatase assays demonstrating the osteogenic differentiation potentials of the materials evaluated in this study. *P < 0.05 among the groups. ARS, Alizarin red S; ALP, alkaline phosphatase; O.D., optical density; Con, negative control; AH-P, AH Plus; Bio-R, BioRoot RCS; C-R, C-Root.
ALP activity
ALP activity differed significantly among the groups (P < 0.05) (Figure 4). ALP activity was significantly higher in the BIO-R and C-R groups compared with the AH-P group (P < 0.05).
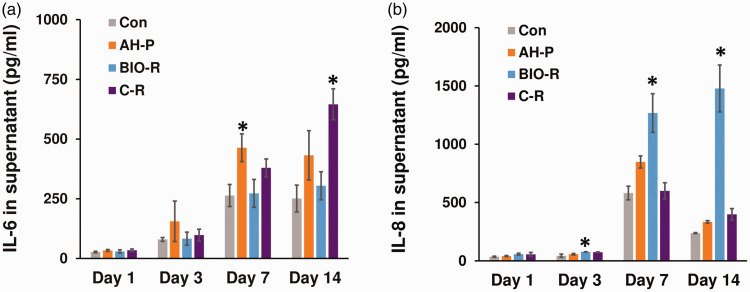
Figure 4.
Immunomodulatory potential of the tested materials evaluated using ELISA. *P < 0.05 among the groups. Con, negative control; AH-P, AH Plus; Bio-R, BioRoot RCS; C-R, C-Root; IL, interleukin.
ELISA
There were no significant differences in IL-6 secretion among the groups at days 1 and 3. However, IL-6 production differed significantly at days 7 and 14. IL-6 levels were highest in the AH-P group at day 7 and in the C-R group at day 14 (P < 0.05). IL-8 levels also differed significantly among the groups at days 7 and 14, with the highest levels in the BIO-R group (P < 0.05).
Discussion
A good apical seal is key to the long-term clinical success of root canal therapy. However, sealers or their eluents in contact with the periapical tissues may release toxic substances, resulting in severe inflammation or even cell necrosis.1 In vitro testing of the cell–material interaction is therefore an indispensable precursor to understanding the cytocompatibility of new root canal sealers. In this study, we assessed the biological responses of hPDLSCs to three different endodontic materials: BIO-R, AH-P, and C-R. We showed that the new experimental root canal filling material, C-R, had comparable in vitro cytocompatibility to BIO-R and higher osteogenic potential than AH-P. We used hPDLSCs in this study because these are the predominant cell types present in the periodontal ligament and may have direct contact with the sealers during apical sealing. hPDLSCs are also multipotent cells able to differentiate into collagen-forming and cementoblast cells,13–15 and are thus suitable for evaluating the osteogenic/odontogenic potentials of the sealing materials.
The present study also used an in vitro root canal filling model for cell culture adapted from previous studies, which was superior to the conventional 2-D culture system for mimicking the in vivo cellular microenvironment.11,12 However, further improvements are needed, given that the hPDLSCs in the current root canal model still formed a monolayer, potentially resulting in contact inhibition. The combination of a 3-D scaffold together with a root canal model may therefore be required to support cell growth and biological cell functionality.11,12
In this study, we evaluated cell proliferation by CCK-8 assay, which is considered to have better detection sensitivity and accuracy than 3-[4, 5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay.16 AH-P resulted in the lowest cell proliferation rate among the three types of endodontic sealers. Notably, the number of viable cells decreased significantly from days 1 to 3 and from days 5 to day 7, indicating a potential cytotoxic effect of AH-P on the hPDLSCs. Indeed, the cytotoxicity of AH-P has been well documented in different cell populations.6,17–19 AH-P was previously shown to be cytotoxic to primary human oral fibroblasts17 and inhibited the growth of hPDLCs from days 1 to 7.18 This may be due to the release of formaldehyde from AH-P during setting20 and to the epoxy resin component, which is a mutagen potentially able to damage the cellular DNA.21 In contrast however, other studies have suggested that AH-P has little or no cytotoxicity.22 AH-P was initially moderately cytotoxic, mildly cytotoxic at day 7, and nontoxic after 14 days in an in vitro model using L929 and HeLa cells.23,24 Our current results indicated that hPDLSCs exposed to C-R and BIO-R showed significantly higher rates of cell proliferation than those exposed to AH-P from days 1 to 7. This may be attributed to the lower toxicity of the BIO-R and C-R eluents and to the constant release of calcium ions from the materials.25
Notably, in vitro cytotoxicity tests are not necessarily effective indicators of the performance of sealing materials in vivo,26 and the concentration of toxic substances is likely to be decreased by tissue fluids under clinical situations.27 Additionally, wide variations in experimental methods and conditions may produce conflicting results. The root canal model used herein had a much smaller contact area with the cells than the 2-D normal culture system, and may thus reduce the apparent toxic effects of the root canal sealers.
We assessed four osteogenic-associated markers, ALP, OCN, DMP1, and RUNX2, to investigate the mineralization potentials of the three sealers. ALP is an early marker of osteoblast differentiation,28,29 while OCN is regarded as a critical marker of mature osteoblasts.29 In the present study, hPDLSCs exposed to BIO-R and C-R showed significantly higher expression levels of ALP and OCN mRNA than cells exposed to AH-P after 7 days of osteogenic induction. ALP activity results were consistent with the gene expression results for AH-P, BIO-R, and C-R, further suggesting that BIO-R and C-R exhibited higher osteogenic differentiation potentials than AH-P. Unlike the bioinert AH-P, BIO-R and C-R are bioactive and release calcium ions, which may diffuse into periapical tissues, thereby inducing the mineralization of PDLCs30 and mesenchymal stem cells.31 BIO-R also simulated hPDLCs to secret bone morphogenetic protein 2,32 a potent growth factor inducing the differentiation of mesenchymal stem cells and bone formation.33 Both BIO-R and C-R significantly increased DMP1 expression at 14 days, while C-R significantly increased DMP1 expression in the short term (7 days) compared with BIO-R. This result is clinically relevant, given that DMP1 plays important roles in dentin bridges and periapical barriers induced by cements.34
An ideal root canal sealer should not cause severe inflammation in living tissue or impair the healing process.18 However, emerging evidence suggest that eluates from sealers in contact with periapical cells induce production of the inflammatory mediators IL-6 and IL-8.12,35,36 IL-6 is produced by osteoblasts as a pro-inflammatory cytokine to simulate bone resorption,37,38 while IL-8 plays an critical role in recruiting neutrophils to induce acute inflammation39 and promotes the formation of new blood vessels.40 Consistently, IL-6 and IL-8 production were detected following exposure to all three types of sealers used in this study. IL-6 secretion levels were similar in BIO-R and negative control cells but relatively high in AH-P-exposed cells at days 7 and 14, and IL-8 levels were highest in BIO-R-exposed cells. However, most in vitro studies evaluating the cytocompatibility of root canal sealers have been conducted over a short period (usually from freshly mixed to 14 days), and this interval may be too short to predict the inflammatory and biologic responses of cells to extruded sealers that remain in contact with periradicular tissues for decades.
The solubility and setting time of a sealer critically determines the long-term outcome of apical sealing. A long setting time would lead to dissolution of sealer in contact with tissue fluids, creating gaps within and between the material and the root dentin and thereby leading to leakage, bacterial colonization, and reinfection.41–43 Calcium silicate-based sealers have displayed high solubility.44,45 The solubilities of iRoot-SP, EndoSequence, Smartpastebio, and MTA-Fillapex increased significantly over time, and exceeded the American National Standards Institute/American Dental Association tolerance of 3% by mass.44,46 However, calcium silicate sealers have been claimed to provide good apical sealing as a result of their setting expansion.24 The high hydrophilicity of calcium silicate promotes water ingress and subsequent expansion of the materials, thus improving the seal along the interface between the materials and dentin. However, high water sorption is likely to increase porosity, making the sealer more susceptible to microleakage.24,41 Limited evidence is currently available regarding to the evidence-based clinical outcomes of calcium silicate-based sealers. Despite their favorable properties, these materials therefore need more investigation before their routine use as root canal fillings, and further studies are required to clarify the physiochemical behaviors of bioactive sealing materials.
Conclusions
This in vitro study concluded that BIO-R and C-R were more cytocompatible in terms of cell proliferation than AH-P. Importantly, C-R showed a comparable osteogenic differentiation capacity to BIO-R, and may thus be a promising alternative for future root canal treatments.
Acknowledgements
The authors sincerely thank Beijing Dental Medical Devices Co. Ltd., China for donating the experimental material (C-Root) for this study.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Yan Jing https://orcid.org/0000-0001-9584-0587
Huan Wang https://orcid.org/0000-0001-7249-9830
Prasanna Neelakantan https://orcid.org/0000-0003-3025-7598
References
- 1.Tepel J, Darwisch el Sawaf M, Hoppe W. Reaction of inflamed periapical tissue to intracanal medicaments and root canal sealers. Endod Dent Traumatol 1994; 10: 233–238. [DOI] [PubMed] [Google Scholar]
- 2.Grossman LI, Oliet S, Del Rio CE. Endodontic Practice. 12th ed. New Delhi: Walter Kluwer Pvt. Ltd., 2010. Chapter 9, Obturation of Radicular Space 2010: 278–309.
- 3.Pawar SS, Pujar MA, Makandar SD. Evaluation of the apical sealing ability of calcium silicate-based materials sealer, AH plus & epiphany: an in vitro study. J Conserv Dent: JCD 2014; 17: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradhan PK, Das S, Patri Get al. Evaluation of sealing ability of five different root end filling material: an in vitro study. J Int Oral Health 2015; 7: 11. [PMC free article] [PubMed] [Google Scholar]
- 5.Shakya VK, Gupta P, Tikku APet al. An invitro evaluation of antimicrobial efficacy and flow characteristics for AH Plus, MTA Fillapex, CRCS and Gutta Flow 2 root canal sealer. J Clin Diagn Res 2016; 10: ZC104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miletić I, Prpić-Mehičić G, Maršan Tet al. Bacterial and fungal microleakage of AH26 and AH Plus root canal sealers. Int Endod J 2002; 35: 428–432. [DOI] [PubMed] [Google Scholar]
- 7.Simon S, Flouriot AC. BioRoot™ RCS a new biomaterial for root canal filling. J Case Studies Collection 2016; 13: 4–11. [Google Scholar]
- 8.Dimitrova-Nakov S, Uzunoglu E, Ardila-Osorio Het al. In vitro bioactivity of Bioroot™ RCS, via A4 mouse pulpal stem cells. Dent Mater 2015; 31: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 9.Camps J, Jeanneau C, El Ayachi Iet al. Bioactivity of a calcium silicate–based endodontic cement (BioRoot RCS): interactions with human periodontal ligament cells in vitro. J Endod 2015; 41: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 10.Zhu SY, Wang PL, Liao CSet al. Transgenic expression of ephrinB2 in periodontal ligament stem cells (PDLSCs) modulates osteogenic differentiation via signaling crosstalk between ephrinB2 and EphB4 in PDLSCs and between PDLSCs and pre-osteoblasts within co-culture. J Periodontal Res 2017; 52: 562–573. [DOI] [PubMed] [Google Scholar]
- 11.Silva EJ, Senna PM, De-Deus Get al. Cytocompatibility of Biodentine using a three-dimensional cell culture model. Int Endod J 2016; 49: 574–580. [DOI] [PubMed] [Google Scholar]
- 12.da Silva EJNL, Zaia AA, Peters OA. Cytocompatibility of calcium silicate-based sealers in a three-dimensional cell culture model. Clin Oral Investig 2017; 21: 1531–1536. [DOI] [PubMed] [Google Scholar]
- 13.Seo BM, Miura M, Gronthos Set al. Investigation of multipotent postnatal stem cells from human periodontal ligament. The Lancet 2004; 364: 149–155. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Yu F, Sun Yet al. Concise reviews: characteristics and potential applications of human dental tissue derived mesenchymal stem cells. Stem Cells 2015; 33: 627–638. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Papagerakis S, Faulk Det al. Extracellular matrix membrane induces cementoblastic/osteogenic properties of human periodontal ligament stem cells. Front Psychol 2018; 9: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai L, Qin X, Xu Zet al. Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method. ACS Omega 2019; 11: 12036–12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai KW, Huang FM, Chang YC. Cytotoxic evaluation of root canal filling materials on primary human oral fibroblast cultures and a permanent hamster cell line. J Endod 2001; 27: 571–573. [DOI] [PubMed] [Google Scholar]
- 18.Huang FM, Tai KW, Chou MYet al. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J 2002; 35: 153–158. [DOI] [PubMed] [Google Scholar]
- 19.Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez REet al. Cytotoxicity of Guttaflow Bioseal, Guttaflow2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod 2017; 43: 816–822. [DOI] [PubMed] [Google Scholar]
- 20.Garza EG, Wadajkar A, Ahn Cet al. Cytotoxicity evaluation of methacrylate-based resins for clinical endodontics in vitro. J Oral Sci 2012; 54: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweikl H, Schmalz G, Federlin M. Mutagenicity of the root canal sealer AH Plus in the Ames test. Clin Oral Investig 1998; 2: 125–129. [DOI] [PubMed] [Google Scholar]
- 22.Leyhausen G, Heil J, Reifferscheid Get al. Genotoxicity and cytotoxicity of the epoxy resin-based root canal sealer AH plus. J Endod 1999; 25: 109–113. [DOI] [PubMed] [Google Scholar]
- 23.Miletić I, Devčić N, Anić Iet al. The cytotoxicity of RoekoSeal and AH plus compared during different setting periods. J Endod 2005; 31: 307–309. [DOI] [PubMed] [Google Scholar]
- 24.Silva EJ, Rosa TP, Herrera DRet al. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod 2013; 39: 274–277. [DOI] [PubMed] [Google Scholar]
- 25.Takita T, Hayashi M, Takeichi Oet al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J 2006; 39: 415–422. [DOI] [PubMed] [Google Scholar]
- 26.Wataha JC, Hanks CT, Strawn SEet al. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil 1994; 21: 453–462. [DOI] [PubMed] [Google Scholar]
- 27.Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent 2009; 2009: 464280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda M, Kikushima K, Kawanobe Yet al. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J Mater Sci: Mater Med 2012; 23: 2923–2932. [DOI] [PubMed] [Google Scholar]
- 29.Tabatabaei FS, Torshabi M, Nasab MMet al. Effect of low-level diode laser on proliferation and osteogenic differentiation of dental pulp stem cells. Laser Physics 2015; 25: 095602. [Google Scholar]
- 30.Rodríguez-Lozano FJ, Collado-González M, Tomás-Catalá CJet al. GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent Mater 2019; 35: 114–124. [DOI] [PubMed] [Google Scholar]
- 31.Gandolfi MG, Shah SN, Feng Ret al. Biomimetic calcium-silicate cements support differentiation of human orofacial mesenchymal stem cells. J Endod 2011; 37: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camps J, Jeanneau C, El Ayachi Iet al. Bioactivity of a calcium silicate-based endodontic cement (BioRoot RCS): interaction with human periodontal ligament cells in vitro. J Endod 2015; 41: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Vepari C, Jin HJet al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006; 27: 3115–3124. [DOI] [PubMed] [Google Scholar]
- 34.Chaussain C, Eapen AS, Huet Eet al. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur Cell Mater 2009; 18: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouillaguet S, Wataha JC, Tay FRet al. Initial in vitro biological response to contemporary endodontic sealers. J Endod 2006; 32: 989–992. [DOI] [PubMed] [Google Scholar]
- 36.Diomede F, Caputi S, Merciaro Iet al. Pro‐inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. Inter Endo J 2014; 47: 864–872. [DOI] [PubMed] [Google Scholar]
- 37.Hwang YS, Lee SK, Park KKet al. Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral Oncol 2012; 48: 40–48. [DOI] [PubMed] [Google Scholar]
- 38.Apaydin ES, Shabahang S, Torabinejad M. Hard-tissue healing after application of fresh or set MTA as root-end-filling material. J Endod 2004; 30: 21–24. [DOI] [PubMed] [Google Scholar]
- 39.Harada A, Sekido N, Akahoshi Tet al. Essential involvement of interleukin‐8 (IL‐8) in acute inflammation. J Leukoc Biol 1994; 56: 559–564. [PubMed] [Google Scholar]
- 40.Li A, Dubey S, Varney MLet al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 2003; 170: 3369–3376. [DOI] [PubMed] [Google Scholar]
- 41.Ersahan S, Aydin C. Solubility and apical sealing characteristics of a new calcium silicate based root canal sealer in comparison to calcium hydroxide-, methacrylate resin- and epoxy resin-based sealers. Acta Odontol Scand 2013; 71: 857–862. [DOI] [PubMed] [Google Scholar]
- 42.Harel A. Comparison of 3 different types of root canal sealers to the most commonly used sealer AH-Plus (Doctoral dissertation, Lithuanian University of Health Sciences) 2017.
- 43.Vertuan GC, Duarte MA, de Moraes IGet al. Evaluation of physicochemical properties of a new root canal sealer. J Endod 2018; 44: 501–505. [DOI] [PubMed] [Google Scholar]
- 44.Abu Zeid ST, Mokeem Saleh AA. Solubility, pH Changes and releasing elements of different calcium silicate-based materials and mineral trioxide aggregate root canal sealers comparative study. J Trauma Treat 2015; 4: 2167–1222. [Google Scholar]
- 45.Prüllage RK, Urban K, Schäfer Eet al. Material properties of a tricalcium silicate-containing, a mineral trioxide aggregate-containing and an epoxy resin-based root canal sealer. J Endod 2016; 42: 1784–1788. [DOI] [PubMed] [Google Scholar]
- 46.Borges RP, Sousa-Neto MD, Versiani MAet al. Changes in the surface of four calcium silicate‐containing endodontic materials and an epoxy resin‐based sealer after a solubility test. Int Endod J 2012; 45: 419–428. [DOI] [PubMed] [Google Scholar]