Abstract
Background
Postoperative urinary retention (POUR) in total joint arthroplasty (TJA) is common. However, risk factors for POUR and its consequences, specifically on postoperative renal function, have not been well defined.
Methods
We performed a review of prospectively collected data on consecutive adult patients undergoing primary total joint arthroplasty from August 2014 to December 2015. Catheters were placed preoperatively and removed on the first or second postoperative day. The exclusion criterion was traumatic catheter insertion or the presence of fracture or neoplasm. Univariate and multiple logistic regression identified associations with POUR and its invasive therapies. Subgroup analysis of renal function by incidence of preoperative bladder outlet obstruction (BOO) and POUR was performed with nonparametric testing.
Results
A total of 591 operations met inclusion criteria. The incidence of POUR was 6.4% and was directly related to a positive history of BOO (odds ratio [OR]: 4.15) and increased the duration of urinary catheterization (OR: 1.04). These factors, in addition to preoperative incontinence (OR: 8.36, 28.69) and lengthier hospitalizations (OR: 1.37, 1.30), were significantly associated with intermittent straight catheterization and reinsertion of an indwelling catheter to treat POUR. Serum creatinine increased with combined preoperative BOO and POUR (+0.22 mg/dL) but was preserved in others (+0.02-0.04 mg/dL) (P < 0.01).
Conclusions
Preoperative BOO and longer catheterization increased the risk of POUR and were associated with the use of invasive modalities to treat POUR. POUR was associated with a longer hospitalization and impaired renal function in those with preoperative BOO; therefore, renal function should be monitored closely and nephrotoxic medications used cautiously when using urinary catheters in this patient population.
Level of Evidence
Retrospective Analysis, Level IV.
Keywords: Total hip arthroplasty, Total knee arthroplasty, Urinary catheterization, Postoperative urinary retention, Bladder outlet obstruction, Renal function
Introduction
The overall incidence of postoperative urinary retention (POUR) has been shown to be as high as 75% after lower limb arthroplasty and occurs approximately 20 times more often than in the general surgical population [1]. Demographic information such as the patient sex and medical comorbidities along with physician-controlled factors such as the type of anesthesia used have been shown to affect the incidence of POUR [2]. As one of the more common complications after total hip arthroplasty (THA) and total knee arthroplasty (TKA), POUR has been linked to delayed mobilization, prolonged hospitalization, and increased readmission rates [3,4]. Prophylaxis against POUR can be accomplished with a perioperative indwelling urinary catheter. A recent meta-analysis demonstrated that indwelling urinary catheterization with removal 24-48 hours postoperatively was superior to intermittent catheterization in preventing POUR and that placement of an indwelling urinary catheter is a reasonable option for patients with multiple risk factors for developing POUR [4]. It is known that bladder overdistention inhibits detrusor function, with bladder volumes as low as 270 mL representing a risk factor for POUR [5]. If undiagnosed, bladder overdistension can lead to permanent bladder dysfunction and secondary urinary tract infections [5].
Given the increased risk of urinary tract infection and subsequent PJI in the early postoperative setting [6], the risk factors associated with the development of POUR after prophylactic indwelling catheterization should be well understood by orthopaedic surgeons conducting total joint arthroplasty (TJA). POUR has been defined as the inability to void spontaneously after surgery despite the presence of elevated bladder volumes, ranging from 400 to 800 mL within the literature [7,8]. Other definitions of POUR also include the inability of the patient to urinate spontaneously in the presence of abdominal distension and discomfort [7].
With the development of fast-track protocols after TJAs, it is imperative that POUR can be quickly identified and managed to reduce the development of subsequent complications. Previous studies have used routine postoperative bladder scans, stating that bladder volumes of greater than 200 mL significantly increases the risk of developing POUR [9]. On review of the literature, the risk factors associated with the development of POUR after prophylactic indwelling catheterization have been sparingly defined and their consequential effect on renal function has been understudied.
The purpose of this study was to elucidate the risk factors and consequences of POUR in the early postoperative setting after TJA with regard to the duration of hospitalization, mobilization, disposition, and renal function.
Material and methods
This study was approved by our institutional review board before implementation of the study protocol. We performed a retrospective review of prospectively collected data at our urban academic medical center comprising 2 board-certified TJA surgeons from August 1, 2014, to December 31, 2015. Consecutive adult patients (aged >18 years) undergoing primary THA and TKA for degenerative or inflammatory arthritis were identified via a search of Current Procedural Terminology codes 27447 and 27130 (primary knee and hip arthroplasties, respectively). Those who underwent THA or TKA as definitive treatment for fracture or neoplasm, those who did not receive urinary catheterization, those with traumatic or multiple catheter insertion attempts, and those with chronic kidney disease were excluded. All patients received prophylactic indwelling urinary catheter immediately preoperatively. Surgeon preference dictated catheter removal on the first or second postoperative day, depending on the type of anesthesia used.
All patients were admitted for a minimum of 2 overnights. Physical and occupational therapy were initiated on the afternoon of the day of surgery or the morning of postoperative day one, depending on the time of arrival to the floor and the patient’s physical ability to participate. Urinary catheter insertion and removal time, bladder scan volumes, utilization of intermittent urinary catheterization to relieve urinary obstruction, and exact ambulation distances postoperatively were documented prospectively. Patients were discharged to their home or a rehabilitation facility after recommendations from physicians and physical and occupational therapists.
The anesthesiologist made the final determination for either general or spinal anesthesia with a surgeon preference of spinal anesthesia for THA with the addition of a local peripheral nerve block for TKA. Postoperative analgesia was standardized and included oxycodone or hydrocodone, acetaminophen, celecoxib, gabapentin or pregabalin, and breakthrough intravenous narcotic with either hydromorphone or morphine. In addition to the analgesia regimen listed previously, one of the 2 arthroplasty surgeons also used an epidural catheter with 0.1% bupivacaine, which was protocoled to be discontinued on the second postoperative day at 12:01 AM.
Retrospective review of physician, nursing, and therapy notes identified the patient demographics and comorbidities, history of preoperative bladder outlet obstruction (BOO) as defined by their primary care physician or urologist, type of anesthesia, preoperative and inpatient serum creatinine, inpatient ambulation distance, weight-bearing status, outpatient disposition, urinary catheter duration, and incidence of POUR. Per our medical center’s protocol, POUR was defined as the absence of spontaneous micturition with (1) a bladder ultrasound demonstrating greater than 300 mL 4 hours after urinary catheter removal or (2) the use of intermittent straight catheterization to relieve urinary obstruction [10]. If the 4-hour bladder scan demonstrated 300-500 mL of urine within the bladder, then the bladder scan was repeated in 1 hour. If the bladder scan demonstrated greater than 500 mL of urine within the bladder, then intermittent straight catheterization was performed followed by a 4-hour trial of void. This was repeated one additional time before reinsertion of an indwelling urinary catheter.
Statistical analysis
Data were split into 3 groups—(1) POUR in all forms, (2) POUR managed by intermittent straight catheterization, and (3) POUR managed by reinsertion of an indwelling Foley catheter. Descriptive statistics were initially calculated followed by univariate analysis by Student's t-test to compare means or the χ2 test to compare categorical values. A backward-elimination variable selection method based on logistic regression was applied to identify key predictors that associate with the odds of POUR with stepwise removal of variables until a statistically significant loss of fit was observed. The result yielded significant associations with POUR, P < 0.05. The subgroup analysis of multiple means was conducted with analysis of variance with a Bonferroni correction for multiple comparison. The analysis was conducted using SPSS Statistics, version 27.0 (IBM, Armonk, NY).
Results
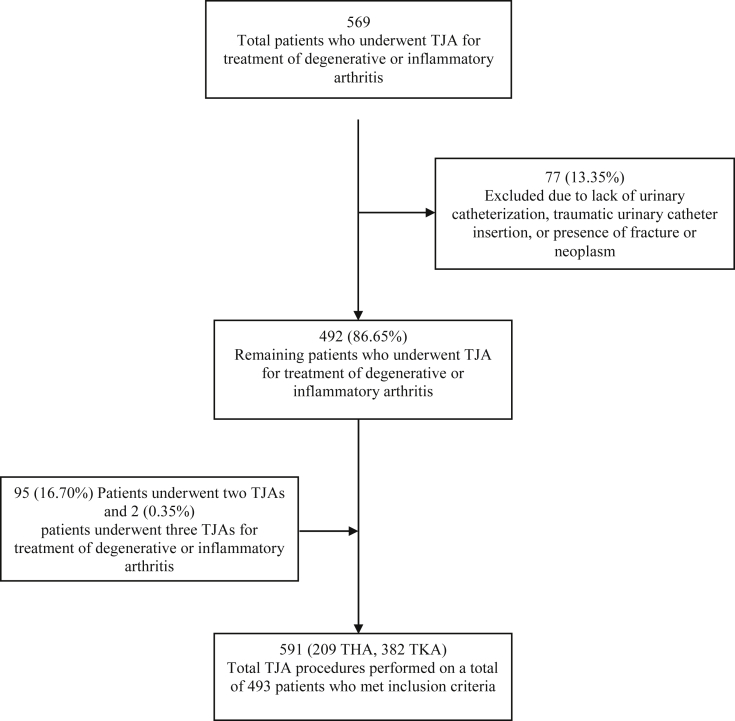
Five hundred sixty-nine individuals met screening criteria. Sixteen were excluded for missing either the urinary catheter duration or ambulation distance, 4 were excluded for traumatic urinary catheter insertion or multiple attempts, one was excluded for refusal of a urinary catheter, and 56 were excluded for treatment for fracture or neoplasm. Of included individuals, 95 individuals underwent 2 primary THAs or TKAs and 2 individuals underwent 3 primary THAs or TKAs, resulting in 591 primary THAs and TKAs included in the analysis (Fig. 1).
Figure 1.
Flowchart diagram demonstrating patients who met inclusion criteria and the number of total TJA procedures included within the study analysis.
There were 330 (55.8%) women and 261 men (44.2%). The mean age was 61.7 ± 10.9 years (range: 24-92), and the mean body mass index (BMI) was 31.7 ± 6.3 kg/m2. Thirty (5.1%) were diagnosed with preoperative BOO by their primary care physician or urologist, and 9 (1.5%), with preoperative incontinence. One (0.17%) individual had both BOO and incontinence. Two hundred nine underwent THA (35.4%), whereas 382 underwent TKA (64.6%). Five hundred thirty-four (90.4%) were allowed to weight-bear as tolerated, whereas 57 (9.6%) were made partial weight-bearing. Four hundred eighty-six (82.2%) underwent spinal anesthesia, whereas 105 (17.8%) underwent general anesthesia. Four hundred thirty-four (73.4%) were discharged home, whereas 157 (26.6%) were discharged to a rehabilitation facility. The perioperative urinary catheter duration averaged 32.1 ± 10.9 hours (range: 13.9-81.6). Demographic and clinical data for all study participants are presented in Table 1.
Table 1.
Summary of demographic and clinical data for all participants with POUR vs no POUR.
| Factor | POUR (n = 38) | No POUR (n = 553) | Univariate |
Multivariate |
|
|---|---|---|---|---|---|
| P-value | Odds ratio [95% CI] | P-value | |||
| Age, y, mean (SD) | 64.1 (10.9) | 61.5 (10.9) | .156 | R | .943 |
| Sex, n (%) | .283 | R | .924 | ||
| Male | 20 (52.6) | 241 (43.6) | |||
| Female | 18 (47.4) | 312 (56.4) | |||
| BMI, kg/m2, mean (SD) | 31.6 (6.2) | 31.7 (6.3) | .925 | R | .814 |
| Preop. BOO, n (%) | 6 (15.8) | 31 (5.6) | .012∗ | 4.15 [1.46-11.76] | .010∗ |
| Preop. incontinence, n (%) | 2 (5.3) | 24 (4.3) | .788 | 6.41 [0.96-43.48] | .060 |
| Δ sCr, mg/dL, mean (SD) | 0.06 (0.14) | 0.02 (0.18) | .180 | R | .459 |
| Foley, hours, mean (SD) | 34.3 (12.8) | 32.0 (10.8) | .210 | 1.04 [1.01-1.08] | .020∗ |
| Anesthesia, n (%) | .028∗ | R | .613 | ||
| General | 12 (31.0) | 96 (17.4) | |||
| Spinal | 26 (69.0) | 457 (82.6) | |||
| Postop epidural, n (%) | .461 | R | .634 | ||
| Epidural | 14 (36.8) | 172 (31.1) | |||
| No epidural | 24 (63.2) | 381 (68.9) | |||
| PT day 1, feet, mean (SD) | 80.7 (73.6) | 86.8 (105.1) | .725 | R | .514 |
| PT day 2, feet, mean (SD) | 162.1 (113.2) | 136.9 (101.5) | .196 | R | .107 |
| Admission length, days, mean (SD) | 3.13 (1.51) | 2.61 (1.08) | .006∗ | 1.23 [0.99-1.53] | .070 |
| Disposition, n (%) | .138 | R | .376 | ||
| Home | 24 (63.2) | 410 (74.1) | |||
| Rehab | 14 (36.8) | 143 (25.9) | |||
| Weight-bearing, n (%) | .849 | R | .397 | ||
| WBAT | 34 (89.5) | 500 (90.4) | |||
| PWB | 4 (10.5) | 53 (9.6) | |||
R, removed; Preop., preoperative; sCr, serum creatinine; Postop., postoperative; PT, physical therapy; WBAT, weight-bearing as tolerated; PWB, partial weight-bearing; CI, confidence interval.
Depicted above are the results of the univariate analysis and multivariate regression model.
P < 0.05.
The frequency of POUR was 6.4% (n = 38). The mean duration of catheterization for those who developed POUR was 34.3 (range: 19.5-81.6) hours compared with 32.0 (range: 13.9-70.7) hours for those who did not. Ultimately, 2 (5.3%/0.3%) were managed with an adjuvant alpha antagonist, 28 (73.7%/4.7%) required intermittent straight catheterization, and 14 (36.8%/2.3%) were discharged with a urinary catheter.
The univariate analysis for all participants with POUR vs all without POUR identified significantly higher prevalence of preoperative BOO, use of general anesthesia, and longer admission in those with POUR. The logistic regression identified patients with a history of preoperative BOO (odds ratio [OR] = 4.15) and increasing hours of catheter duration (OR = 1.04) as statistically significant associations with the development of POUR (Table 1).
The univariate analysis for all participants requiring intermittent straight catheterization vs all who did not identified significantly higher prevalence of preoperative BOO and preoperative urinary incontinence as well as longer admission in those who required intermittent straight catheterization to relieve POUR (P < 0.05). The logistic regression identified patients with a history of preoperative BOO (OR = 4.91), a history of preoperative incontinence (OR = 8.36), increasing hours of catheter duration (OR = 1.06), and increasing days of hospitalization (OR = 1.37) as statistically significant associations with requirement for straight catheterization to relieve POUR (Table 2).
Table 2.
The results of the univariate analysis and multivariate regression model for patients requiring intermittent straight catheterization vs those not requiring intermittent straight catheterization.
| Factor | Straight catheter (n = 28) | Not required (n = 563) | Univariate |
Multivariate |
|
|---|---|---|---|---|---|
| P-value | Odds ratio [95% CI] | P-value | |||
| Age, y, mean (SD) | 64.7 (11.7) | 61.5 (10.9) | .131 | R | .580 |
| Sex, n (%) | .156 | R | .441 | ||
| Male | 16 (57.1) | 245 (43.5) | |||
| Female | 12 (42.9) | 318 (56.5) | |||
| BMI, kg/m2, mean (SD) | 31.4 (6.0) | 31.7 (6.3) | .816 | R | .674 |
| Preop. BOO, n (%) | 5 (17.9) | 25 (4.4) | .002∗ | 4.91 [1.49-16.12] | .009∗ |
| Preop. incontinence, n (%) | 2 (7.1) | 7 (1.2) | .013∗ | 8.36 [1.06-65.71] | .043∗ |
| Δ sCr, mg/dL, mean (SD) | 0.06 (0.14) | 0.02 (0.18) | .247 | R | .330 |
| Foley, h, mean (SD) | 35.4 (13.7) | 32.0 (10.8) | .109 | 1.06 [1.02-1.10] | .006∗ |
| Anesthesia, n (%) | .125 | R | .324 | ||
| General | 8 (28.6) | 97 (17.2) | |||
| Spinal | 20 (71.4) | 466 (82.8) | |||
| Postop. epidural, n (%) | .362 | R | .198 | ||
| Epidural | 11 (39.3) | 175 (31.1) | |||
| No epidural | 17 (60.7) | 388 (68.9) | |||
| PT day 1, feet, mean (SD) | 62.3 (57.6) | 87.6 (104.9) | .206 | 0.99 [0.99-1.00] | .105 |
| PT day 2, feet, mean (SD) | 160.0 (121.6) | 137.4 (101.3) | .254 | 1.00 [1.00-1.01] | .054 |
| Admission length, days, mean (SD) | 3.4 (1.6) | 2.6 (1.1) | <.001∗ | 1.37 [1.08-1.74] | .008∗ |
| Disposition, n (%) | .118 | R | .532 | ||
| Home | 17 (60.7) | 417 (74.1) | |||
| Rehab | 11 (39.3) | 146 (25.9) | |||
| Weight-bearing, n (%) | .844 | R | .518 | ||
| WBAT | 25 (89.3) | 509 (90.4) | |||
| PWB | 3 (10.7) | 54 (9.6) | |||
R, removed; Preop., preoperative; sCr, serum creatinine; Postop., postoperative; PT, physical therapy; WBAT, weight-bearing as tolerated; PWB, partial weight-bearing; CI, confidence interval.
P < 0.05.
The univariate analysis of all participants demonstrated a statistically significant association between required reinsertion of an indwelling urinary catheter to relieve POUR and older age, diagnosis of preoperative BOO and preoperative urinary incontinence, longer postoperative catheter duration, longer admission, and higher rate of discharge to a rehabilitation facility when compared to those who did not require reinsertion of an indwelling urinary catheter (P < 0.05). The logistic regression identified patients with a history of preoperative BOO (OR = 8.74), a history of preoperative incontinence (OR = 28.69), increasing hours of catheter duration (OR = 1.09), and increasing days of hospitalization (OR = 1.30) as statistically significant associations with requirement for reinsertion of an indwelling urinary catheter to relieve POUR (Table 3).
Table 3.
The results of the univariate analysis and multivariate regression model for patients requiring indwelling catheter reinsertion vs those not requiring indwelling catheter reinsertion.
| Factor | Foley reinserted (n = 14) | Not required (n = 577) | Univariate |
Multivariate |
|
|---|---|---|---|---|---|
| P-value | Odds ratio [95% CI] | P-value | |||
| Age, y, mean (SD) | 67.6 (10.3) | 61.6 (10.9) | .042∗ | R | .797 |
| Sex, n (%) | .125 | R | .242 | ||
| Male | 9 (64.3) | 252 (43.7) | |||
| Female | 5 (35.7) | 325 (56.3) | |||
| BMI, kg/m2, mean (SD) | 30.7 (5.3) | 31.7 (6.3) | .556 | R | .792 |
| Preop. BOO, n (%) | 4 (28.6) | 26 (4.5%) | <.001∗ | 8.74 [2.04-37.55] | .004∗ |
| Preop. incontinence, n (%) | 2 (14.3) | 7 (1.2) | <.001∗ | 28.69 [3.11-264.27] | .003∗ |
| Δ sCr, mg/dL, mean (SD) | 0.09 (0.17) | 0.02 (0.18) | .151 | R | .366 |
| Foley, hours, mean (SD) | 38.4 (15.9) | 32.0 (10.7) | .029∗ | 1.09 [1.04-1.14] | <.001∗ |
| Anesthesia, n (%) | .076 | R | .562 | ||
| General | 5 (35.7) | 100 (17.3) | |||
| Spinal | 9 (64.3) | 477 (82.7) | |||
| Postop. epidural, n (%) | .131 | R | .281 | ||
| Epidural | 7 (50.0) | 179 (31.0) | |||
| No epidural | 7 (50.0) | 398 (69.0) | |||
| PT day 1, feet, mean (SD) | 56.8 (41.9) | 87.1 (104.2) | .278 | R | .114 |
| PT day 2, feet, mean (SD) | 160.6 (127.8) | 138.0 (101.7) | .415 | R | .238 |
| Admission length, days, mean (SD) | 3.4 (1.2) | 2.6 (1.1) | .007∗ | 1.30 [1.03-1.65] | .022∗ |
| Disposition, n (%) | .009∗ | R | .150 | ||
| Home | 6 (42.9) | 428 (74.2) | |||
| Rehab | 8 (57.1) | 149 (25.8) | |||
| Weight-bearing, n (%) | .131 | R | .968 | ||
| WBAT | 11 (78.6) | 523 (90.6) | |||
| PWB | 3 (21.4) | 54 (9.4) | |||
R, removed; Preop., preoperative; sCr, serum creatinine; Postop., postoperative; PT, physical therapy; WBAT, weight-bearing as tolerated; PWB, partial weight-bearing; CI, confidence interval.
P < 0.05.
A subgroup analysis on perioperative renal function as a function of preoperative BOO and POUR was performed. No difference was observed in preoperative creatinine; however, the group with both preoperative BOO and POUR demonstrated a significant increase in serum creatinine by discharge compared with the other cohorts (Table 4). A post hoc power analysis demonstrated our sample size was adequate to detect the differences seen at α = 0.05, 1-β = 0.80.
Table 4.
Analysis of serum creatinine preoperatively and at discharge in those with known history of BOO, POUR, or both BOO and POUR.
| Serum creatinine level | Preoperative diagnosis of BOO and/or POUR |
|||
|---|---|---|---|---|
| BOO and POUR | BOO only | POUR only | None | |
| Preoperative sCr, mean (SD) | 0.83 (0.03) | 0.85 (0.16) | 0.80 (0.28) | 0.81 (0.22) |
| Discharge sCr, mean (SD) | 1.05 (0.12)A | 0.89 (0.14)A,B | 0.83 (0.22)A,B | 0.84 (0.24)B |
| Δ sCr, mean (SD) | 0.22 (0.10)A | 0.04 (0.09)B | 0.03 (0.26)B | 0.03 (0.18)B |
sCr, serum creatinine; Δ sCr, change in serum creatinine.
No difference was observed in preoperative creatinine among the 4 groups (P = .68). Discharge sCr was higher in combined BOO and POUR than in none (P = .025) but similar to POUR only (P = .07) and BOO only (P = .876). Δ sCr was higher in combined BOO and POUR than in BOO only (P = .010), POUR only (P = .009), and none (P = .003). Δ sCr was similar among BOO only, POUR only, and none (P = .99).
Superscript lettering denotes statistically similar groups, where P > .05 within the group.
Discussion
POUR is not a benign process, and after evaluation of the risk factors and consequences associated with POUR in the early postoperative setting after TJA, our analysis identified that when a urinary catheter is used as prophylaxis, the risk of retention is higher in those with preoperative BOO and increasing hours of urinary catheter duration regardless of the age, sex, BMI, postoperative weight-bearing, anesthetic used, or use of epidural analgesia. In addition, both the requirement for straight catheterization and reinsertion of an indwelling urinary catheter to relieve POUR were associated with preoperative BOO and urinary incontinence, increasing hours of urinary catheter duration, and increasing days of hospitalization. Although the univariate analysis identified potential associations with POUR and its invasive management with the use of a general anesthetic, older age, and discharge disposition to a rehabilitation facility, these associations were not confirmed in the multivariate regression. Furthermore, our analysis identified BOO as a primary predictor of POUR, which is consistent with prior studies [[11], [12], [13]].
In our study, a small difference was observed in the occurrence of POUR based on the duration of indwelling catheterization where the odds of POUR increased by 1%-8% per hour of catheterization, the odds of requiring intermittent straight catheterization increased by 2%-10% per hour of catheterization, and the odds of requiring reinsertion of an indwelling urinary catheter increased by 4%-14% per hour of catheterization. Furthermore, the mean initial catheter duration was observed to be higher in those requiring reinsertion of an indwelling catheter (38.4 hours) than in those requiring intermittent straight catheterization (35.4 hours), all participants diagnosed with POUR (34.3 hours), and all who did not suffer from POUR (32.0 hours), suggesting that this small clinical difference may indeed be meaningful. However, a shorter duration did not eliminate POUR entirely; wide ranges of catheter duration were observed in all cohorts. In support of this, the authors of a recent meta-analysis [4] found that the duration of catheterization may influence POUR and stated that a duration of 24-48 hours in those with risk factors for POUR is a reasonable option to reduce its occurrence. Furthermore, the anesthetic type and use of continuous epidural analgesia did not influence POUR, which is similar to prior results [14], but overall controversial [12,15]. Bladder hypotonia is expected to resolve within 8 hours after discontinuation of spinal anesthesia [5], so it is not surprising that no difference in observed urinary retention was observed in our study, which had a minimum of 13.9 hours of urinary catheterization.
Importantly, patients with BOO who developed POUR demonstrated an acute increase in serum creatinine. Although the rise in creatinine that was seen is not diagnostic for acute kidney injury [16,17], it should not be ignored as outpatient postoperative renal function surveillance is not routine after THA and TKA. Creatinine levels that continue to rise after 48 hours from the initial insult portend a higher risk of developing chronic renal impairment, and a consensus report of the Acute Disease Quality Initiative 16 Workgroup recommended avoidance of nephrotoxins and nephrotoxic medications in conjunction with patient education and follow-up with a primary care provider or nephrologist when this is observed [18]. Consequently, this finding should heighten awareness for underlying renal parenchymal disease that, although not clinically apparent, renders this population at risk for chronic worsening of renal function especially with repetitive incidences of POUR or exposure to nephrotoxic medications including commonly prescribed nonsteroidal anti-inflammatory drugs and prophylactic antimicrobials [16].
In addition to impairing postoperative renal function, a longer hospitalization was associated with invasive management for POUR despite no difference observed in postoperative mobilization, weight-bearing limitation, or discharge disposition. Although this is not a new finding [4,19], it and other complications of POUR including the effect on renal function affect patient health and wellness as well as outcome-based physician reimbursement [20], and all efforts should be made to reduce its occurrence.
There were limitations to our study. The study design is retrospective in nature, which introduces the potential for bias related to patient selection and measurement of outcomes. Furthermore, the criteria used by primary care physicians or urologists to diagnose patients with preoperative BOO are not known and may differ among practitioners. In addition, posthospital surveillance of serum creatinine was not available, and further studies will be necessary to corroborate the perioperative impact of POUR on chronic renal function; however, given the present results, the authors argue that there is benefit in withholding nephrotoxic agents in patients with combined preoperative BOO and POUR.
With the results of the present study, our institution has adopted a change to our urinary management protocol. We continue to use urinary catheters ubiquitously but now counsel those with a history of BOO regarding a higher risk of POUR and all patients on a possible delay in discharge if POUR were to occur. Furthermore, although the present data support expeditious removal of indwelling catheters, the surgeon who uses the bupivacaine epidural believes that the benefit of analgesia outweighs the small increased risk of urinary retention with prolonged catheterization. In addition, shortly after data collection, one surgeon stopped the routine collection of postoperative renal function labs; the results changed this practice such that if urinary retention were to occur, renal function testing is performed and nephrotoxic medications are withheld appropriately.
Conclusion
Preoperative BOO as diagnosed per primary care physicians or urologists and longer duration of postoperative catheterization increased the risk of POUR regardless of the age, sex, BMI, or anesthetic or perioperative analgesic used. The use of invasive modalities to treat POUR was associated with a longer hospitalization despite no differences observed in postoperative mobilization, weight-bearing restriction, or discharge disposition. Furthermore, in those with preoperative BOO, renal function should be monitored closely when POUR occurs and nephrotoxic medications used cautiously. Special attention should be given to patient risk factors for POUR to decrease the risk of its occurrence, worsening renal function, and lengthier hospitalizations.
Conflict of interests
J.A. Shapiro is a board or committee member of the Renal Physicians Association; D.J. Del Gaizo is a member of the speakers' bureau for Pacira Pharmaceuticals, is a paid consultant for DePuy, OrthAlign, Pacira Pharmaceuticals, and SPR Therapeutics, receives research support from Biom'Up, ConforMIS, DePuy, Pacira, Reflexion Health, Stryker, and Zimmer, and is a member of the editorial or governing board of the Journal of Arthroplasty; all other authors declare no potential conflicts of interest.
Appendix A. Supplementary data
References
- 1.Balderi T., Carli F. Urinary retention after total hip and knee arthroplasty. Minerva Anestesiol. 2010;76:120. [PubMed] [Google Scholar]
- 2.Griesdale D.E., Neufeld J., Dhillon D. Risk factors for urinary retention after hip or knee replacement: a cohort study. Can J Anesth. 2011;58:1097. doi: 10.1007/s12630-011-9595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karason S., Olafsson T.A. Avoiding bladder catheterisation in total knee arthroplasty: patient selection criteria and low-dose spinal anaesthesia. Acta Anaesthesiol Scand. 2013;57:639. doi: 10.1111/aas.12089. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W., Liu A., Hu D. Indwelling versus intermittent urinary catheterization following total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2015;10:e0130636. doi: 10.1371/journal.pone.0130636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldini G., Hema B., Armen A., Carli F., Phil M. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 6.Wroblewski B.M., del Sel H.J. Urethral instrumentation and deep sepsis in total hip replacement. Clin Orthop Relat Res. 1980:209. [PubMed] [Google Scholar]
- 7.Bjerregaard L.S., Bogo S., Raaschou S. Incidence of and risk factors for postoperative urinary retention in fast-track hip and knee arthroplasty. Acta Orthop. 2015;86:183. doi: 10.3109/17453674.2014.972262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerregaard L.S., Hornum U., Troldborg C., Bogoe S., Bagi P., Kehlet H. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast-track total hip and knee arthroplasty: a randomized, open-label, controlled trial. Anesthesiology. 2016;124:1256. doi: 10.1097/ALN.0000000000001112. [DOI] [PubMed] [Google Scholar]
- 9.Kort N.P., Bemelmans Y., Vos R., Schotanus M.G. Low incidence of postoperative urinary retention with the use of a nurse-led bladder scan protocol after hip and knee arthroplasty: a retrospective cohort study. Eur J Orthop Surg Traumatol. 2018;28:282. doi: 10.1007/s00590-017-2042-5. [DOI] [PubMed] [Google Scholar]
- 10.Negro C.L.A., Muir G.H. Chronic urinary retention in men: how we define it, and how does it affect treatment outcome. BJU Int. 2012;110:1590. doi: 10.1111/j.1464-410X.2012.11101.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawrie C.M., Ong A.C., Hernandez V.H., Rosas S., Post Z.D., Orozco F.R. Incidence and risk factors for postoperative urinary retention in total hip arthroplasty performed under spinal anesthesia. J Arthroplasty. 2017;32:3748. doi: 10.1016/j.arth.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Kotwal R.S., Hodgson P.H., Carpenter C.E. Urinary retention following lower limb arthroplasty: analysis of predictive factors and review of literature. Acta Orthop Belg. 2008;74:332. [PubMed] [Google Scholar]
- 13.Scholten R., Kremers K., van de Groes S.A.W., Somford D.M., Koëter S. Incidence and risk factors of postoperative urinary retention and bladder catheterization in patients undergoing fast-track total joint arthroplasty: a prospective observational study on 371 patients. J Arthroplasty. 2018;33:1546. doi: 10.1016/j.arth.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Halawi M.J., Caminiti N., Cote M.P., Lindsay A.D., Williams V.J. The most significant risk factors for urinary retention in fast-track total joint arthroplasty are iatrogenic. J Arthroplasty. 2019;34:136. doi: 10.1016/j.arth.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Niazi A.A., Taha M.A. Postoperative urinary retention after general and spinal anesthesia in orthopedic surgical patients. Egypt J Anaesth. 2015;31:65. [Google Scholar]
- 16.Ostermann M., Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20:299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 19.Jackson J., Davies P., Leggett N. Systematic review of interventions for the prevention and treatment of postoperative urinary retention: prevention and treatment of postoperative urinary retention. BJS Open. 2019;3:11. doi: 10.1002/bjs5.50114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services (CMS) HHS. Medicare Program; Merit-based Incentive Payment System (MIPS) and Alternative Payment model (APM) incentive under the physician fee schedule, and criteria for physician-focused payment models. Final rule with comment period. Fed Regist. 2016;81:77008. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.