Abstract
Objective
This study aimed to investigate a suitable risk assessment model to predict deep vein thrombosis (DVT) in patients with gynecological cancer.
Methods
Data from 212 patients with gynecological cancer in the Affiliated Tumor Hospital of Guangxi Medical University were retrospectively analyzed. Patients were risk-stratified with three different risk assessment models individually, including the Caprini model, Wells DVT model, and Khorana model.
Results
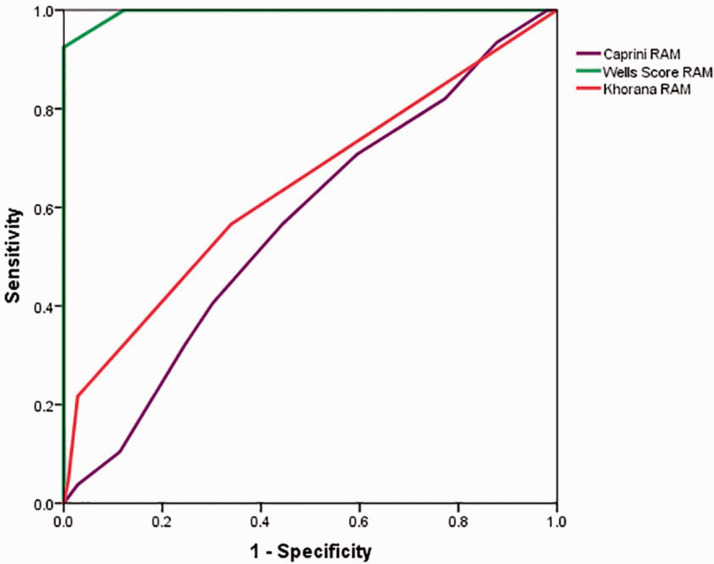
The difference in risk level evaluated by the Caprini model was not different between the DVT and control groups. However, the DVT group had a significantly higher risk level than the control group with the Wells DVT or Khorana model. The Wells DVT model was more effective for stratifying patients in the DVT group into the higher risk level and for stratifying those in the control group into the lower risk level. Receiver operating curve analysis showed that the area under the curve of the Wells DVT, Khorana, and Caprini models was 0.995 ± 0.002, 0.642 ± 0.038, and 0.567 ± 0.039, respectively.
Conclusion
The Wells DVT model is the most suitable risk assessment model for predicting DVT. Clinicians could also combine the Caprini and Wells DVT models to effectively identify high-risk patients and eliminate patients without DVT.
Keywords: Gynecological cancer, deep vein thrombosis, risk assessment, survival, thromboembolism, Wells model
Introduction
Venous thromboembolism (VTE), including deep venous thrombosis and pulmonary embolism, is a common complication in patients with cancer and is associated with a poor prognosis. The annual incidence of VTE is approximately 13.9% in patients with cancer, but only 3.0% in patients without cancer. Furthermore, the proportion of patients with gynecological malignancy is 26.8% (accounting for 13% of ovarian cancer, 9.8% of cervical cancer, and 4% of uterine corpus cancer) and the incidence is increasing yearly.1 Once thrombosis occurs, it seriously threatens the life of patients, adversely influences the quality of life, and delays the standard treatment of tumors. Even though there are effective treatments, patients with cancer-associated thromboembolism still have a high recurrence rate and are associated with a poor prognosis.2,3 The median survival time or overall survival of patients with cancer-related thrombosis is significantly lower than that in those without thromboembolism in patients with cancer, particularly in patients with ovarian cancer.4,5 Therefore, prevention, early detection, early diagnosis, and early treatment are important for reducing the incidence of VTE, to eventually improve the quality of life and prognosis of patients with gynecological cancer. Identification of risk factors and establishment of a protocol for risk assessment are crucial issues. However, optimizing prevention and the treatment regimen for gynecological oncology-related thromboembolism have no unified standard at present. Therefore this study aimed to identify risk factors, to compare the effectiveness of identifying patients at higher risk of VTE among three risk assessment models, and to assess the prognosis of patients with gynecological cancer with deep vein thrombosis (DVT).
Materials and methods
Study design and patients
We performed a retrospective study of female patients with VTE who were diagnosed with gynecological cancer during hospitalization at the Affiliated Tumor Hospital of Guangxi Medical University between January 1994 and September 2014. The patients were divided into the DVT group or the control group. The inclusion criteria of patients in the DVT group included the following: diagnosis was confirmed by ultrasound and/or venography; and gynecological cancer was definitely diagnosed by biopsy, puncture, surgery, cytology, or histopathology. Exclusion criteria of the study were as follows: catheter-related DVT; only having clinical manifestations of DVT, but without the support of ultrasonography and/or venography; benign gynecological diseases; and hematological disorders or coagulation disorders. The inclusion criteria of patients in the control group included the following: gynecological cancer was definitely diagnosed by biopsy, puncture, surgery, cytology, or histopathology; and without DVT and pulmonary embolism (PE). The exclusion criteria of patients in the control group included the following: benign gynecological diseases; and hematological disorders or coagulation disorders. We used the computer random method according to a 1:1 proportion to select patients with gynecological cancer in the diagnosis of DVT as cases and those with gynecological cancer without DVT as controls.
The study protocol was approved by the Ethics Committee of the Affiliated Tumor Hospital of Guangxi Medical University (LW2018024). All patients received an explanation concerning the aims of the study and provided signed informed consent.
Risk factors
Potential risk factors were analyzed, including age, body mass index (BMI), history, complications, tumor type, therapeutic schedule, blood transfusion, central venous catheter use, colony-stimulating factor, and the coagulation index, including the white blood cell count, platelets, prothrombin time (PT), and fibrinogen (FIB).
Risk assessment models
Patients were risk-stratified using three risk assessment models individually, including the Caprini model, the Wells DVT model, and the Khorana model. We referred to the 2012 edition of the 9th American College of Chest Physicians’ antithrombotic therapy and thrombosis prophylaxis clinical practice guidelines for the Caprini model.6 This model included approximately 40 risk factors, which were marked by scores of 1 to 5 according to the degree of danger. Based on the cumulative score, patients were classified as low risk (0–1 point), moderate risk (2 points), high risk (3–4 points), and very high risk (≥5 points) levels. The Wells DVT risk assessment model (RAM) was from the modified version of the 2003 wells DVT risk assessment model.7 This model encompassed 10 risk factors, such as signs and symptoms of DVT, treatment-related risk factors, diagnosis, and history. Positive predictive factors were allocated 1 point and negative predictive factors were allocated −2 points. According to the cumulative scores, the patients were divided into low risk (≤0 points), moderate risk (1–2 points), and high risk (≥3 points) levels. The Khorana RAM was derived from the American Society of Clinical Oncology Clinical Practice Guideline.8 This model included five risk factors, including the original tumor site (gynecological malignancies: 1 point), platelet count ≥350 × 109/L, white blood cell count >11 × 109/L, hemoglobin levels ≤100 g/L or the use of erythropoietin, and BMI ≥35 kg/m2. According to the rating scale, patients were divided into the low risk group (0 points), moderate risk group (1–2 points), and high risk group (≥3 points).
Prognosis
Patients were followed up throughout the duration of the hospital stay, and for out-patients, letters and telephone calls were used to determine the condition of the patients. The follow-up indices included the present situation, relapse and recurrence times, and whether the patients were alive or dead by 31 March 2015. The follow-up deadline was 31 March 2015. The potential risk factors that may affect prognosis were analyzed, including age, BMI, tumor type, tumor stage, and scope of DVT (whether DVT was combined with PE).
Statistical analysis
All statistical analyses were performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Univariate analysis was performed by the two-independent samples t test or the χ2 test. Multivariate analysis was performed by logistic regression analysis. The predictive values of the models were compared with a nonparametric test and the receiver operating curve (ROC). We used the Kaplan–Meier method to conduct survival analysis, including the log-rank test and Cox regression model. A p value <0.05 was considered significant.
Results
Patients
Our study included 106 patients in the DVT group and 106 patients in the control group.
Analysis of risk factors
In univariate analysis, BMI, hypertension, diabetes mellitus, a history of thrombosis, staging of the tumor, blood transfusion, use of colony-stimulating factor, the white blood cell count, the platelet count, PT, and FIB levels were significantly different between the DVT and control groups (all p < 0.05) (Table 1). Risk factors that were significant in univariate analysis were analyzed by multivariate analysis. Logistic regression analysis showed that staging of cancer, use of colony-stimulating factor, the white blood cell count, PT, and FIB levels were independent risk factors for DVT (all p < 0.05).
Table 1.
Univariate analysis of risk factors of DVT.
| Clinical risk factors |
DVT group |
Control group |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | t or χ2 | p | |
| Age (years) | 0.494 | 0.781 | ||||
| <41 | 19 | 17.9 | 18 | 17.0 | ||
| 41–61 | 69 | 65.1 | 66 | 62.3 | ||
| ≥61 | 18 | 17.0 | 22 | 20.8 | ||
| Other conditions | ||||||
| hypertension | 25 | 23.6 | 9 | 8.5 | 8.968 | 0.003 |
| Diabetes | 23 | 21.7 | 7 | 6.6 | 9.940 | 0.002 |
| History of thrombosis | 4 | 3.8 | 0 | 0 | 4.077 | 0.043 |
| Type of tumor | 1.354 | 0.716 | ||||
| Cervical cancer | 51 | 48.1 | 52 | 49.1 | ||
| Endometrial cancer | 14 | 13.2 | 17 | 16.0 | ||
| Ovarian cancer | 38 | 35.8 | 36 | 34.0 | ||
| Others | 3 | 2.8 | 1 | 0.9 | ||
| Staging of the tumor | 16.926 | 0.001 | ||||
| I–II | 41 | 38.7 | 64 | 60.4 | ||
| III | 42 | 39.6 | 37 | 34.9 | ||
| IV | 23 | 21.7 | 5 | 4.7 | ||
| Treatment of the tumor | 3.289 | 0.193 | ||||
| Simple surgery | 21 | 19.8 | 18 | 17.0 | ||
| Surgery + radi-otherapy/chemotherapy | 42 | 39.6 | 55 | 50.0 | ||
| Radiotherapy/chemotherapy | 43 | 40.6 | 33 | 33.0 | ||
| Transfusion | 43 | 40.6 | 29 | 27.4 | 4.122 | 0.042 |
| Central venous catheter | 66 | 62.3 | 62 | 58.5 | 0.315 | 0.574 |
| Hematological indicators | ||||||
| WBC count (109/L) | 10.841 | 0.001 | ||||
| <10 | 79 | 74.5 | 97 | 91.5 | ||
| ≥10 | 27 | 15.1 | 9 | 4.7 | ||
| Platelet count (109/L) | 9.989 | 0.002 | ||||
| <300 | 43 | 40.6 | 66 | 62.3 | ||
| ≥300 | 63 | 55.6 | 40 | 35.8 | ||
| PT (seconds) | 10.203 | 0.001 | ||||
| <14 | 86 | 81.1 | 10 | 95.3 | ||
| ≥14 | 20 | 18.9 | 5 | 4.7 | ||
| FIB (g/L) | 23.016 | <0.001 | ||||
| <4 | 54 | 50.9 | 87 | 82.1 | ||
| ≥4 | 52 | 7.6 | 19 | 1.9 | ||
| Types of operation | 3.558 | 0.059 | ||||
| Laparotomy | 52 | 50 | ||||
| Laparoscopy | 11 | 23 | ||||
| Operation time (minutes) | 307 ± 76 | 276 ± 73 | 2.453 | 0.015 | ||
| Blood loss (mL) | 408 ± 269 | 304 ± 171 | 2.760 | 0.007 | ||
| Transfusion (n) | 29 | 20 | 5.095 | 0.024 | ||
| Vascular injury (n) | 2 | 1 | 0.511 | 0.475 | ||
Values are mean ± standard deviation or n (%). DVT: deep vein thrombosis; WBC: white blood cell; PT: prothrombin time; FIB: fibrinogen.
Comparison of perioperative indicators
Patients were divided into those who had surgery and those without surgery. In the DVT group, 63 (59.4%, 63/106) patients had surgery, while in the control group, 73 (68.9%, 73/106) had surgery. The operation was carried out according to the different types of cancer and by following the standard procedure for surgery, including endometrial cancer staging surgery, cervical cancer radical surgery, and ovary tumor debulking surgery. The operation time was longer, intraoperative blood loss was greater, and intraoperative or postoperative blood transfusion was greater in the DVT group compared with the control group (all p < 0.05). However, the rate of vascular injury and the operation method were not significantly different between the groups (Table 1).
Comparison of risk assessment models
Patients were graded and risk stratified with three RAMs. The distribution of patients by the cumulative risk score and risk level in the Caprini, Wells DVT, and Khorana RAMs is shown in Table 2. In the Caprini RAM, patients with a very low risk or low risk level in the DVT and control groups had a low risk of DVT. However, when the accumulated score was >5, the risk of DVT significantly increased with an increase in score (p = 0.006, p = 0.001, and p = 0.029 for low, moderate, and high risk, respectively). In the Wells DVT RAM, the average accumulated score of the DVT group was higher than that of the control group. The factor of a high risk in the DVT group was 10.728 times higher than that of a moderate risk. In the Khorana RAM, the average accumulated score in the DVT group was also higher than that in the control group. The factor of a high risk in the DVT group was 10.597 times higher than that of a moderate risk.
Table 2.
Comparison of the three models.
| Risk group | Score | DVT group (n) | Control group (n) | OR (95% CI) | p |
|---|---|---|---|---|---|
| Caprini model | |||||
| Very low risk | 5–6 | 25 | 46 | – | – |
| Low risk | 7–8 | 26 | 16 | 2.990 (1.356, 6.592) | 0.006 |
| Moderate risk | 9–10 | 30 | 18 | 3.067 (1.433, 6.562) | 0.001 |
| High risk | ≥11 | 6 | 2 | 4.774 (1.036, 29.404) | 0.029 |
| Wells DVT model | |||||
| Low risk | 0 | 0 | 92 | – | – |
| Moderate risk | 1–2 | 27 | 11 | – | – |
| High risk | ≥3 | 79 | 3 | 10.728 (2.783, 40.355) | <0.001 |
| 3–4 | 60 | 3 | 8.148 (2.102, 31.588) | 0.001 | |
| ≥5 | 19 | 0 | – | – | |
| Khorona model | |||||
| Low risk | 0 | 0 | 0 | – | – |
| Moderate risk | 1–2 | 81 | 103 | 1 | – |
| High risk | ≥3 | 25 | 3 | 10.597 (3.090, 36.342) | <0.001 |
DVT: deep vein thrombosis; OR: odds ratio; CI: confidence interval.
Comparison between the Wells DVT RAM and Khorana RAM with the Wilcoxon rank test showed that the Wells DVT RAM divided more patients with DVT into a higher level and divided more patients without DVT into a lower level than the Khorana RAM. The ROC was plotted and is shown in Figure 1. The ROC of the Caprini RAM was close to the chance line and the area under the curve was 0.567 ± 0.039 (p = 0.091). The diagnostic value of thrombosis with the Caprini RAM was low. The area under the curve of the Wells DVT RAM was 0.995 ± 0.002 (p < 0.001) and that of the Khorana RAM was 0.642 ± 0.038 (p < 0.001). The area under the curve was not significantly different between the Caprini RAM and the Khorana RAM (Z=9.289, p = 1). However, because the area under the curve of the Wells DVT RAM was approximately 1, it might have higher clinical predictive value than the Khorana RAM.
Figure 1.
Receiver operating curve of deep vein thrombosis in three risk assessment models. RAM: risk assessment model.
None of the patients experienced any of the following complications: varicosity, a minor operation, sepsis (<1 month), severe lung disease, including pneumonia (<1 month), chronic obstructive pulmonary disease, acute myocardial infarction, congestive heart failure, a history of inflammatory bowel disease, arthroscopic surgery, being confined to bed (>72 hours), immobilization in a plaster cast (<1 month), acute spinal cord injury (paralysis) (<1 month), and hip, pelvis, or leg fracture (<1 month).
Prognosis
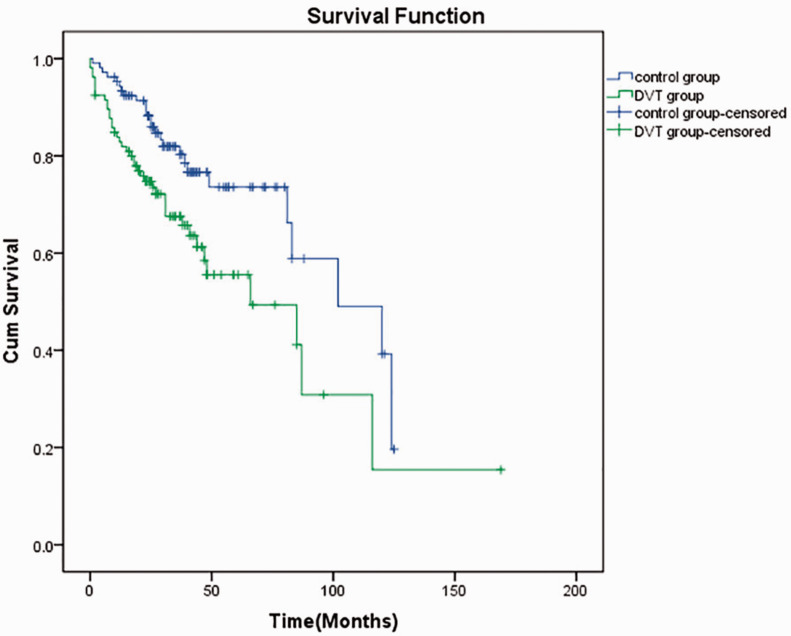
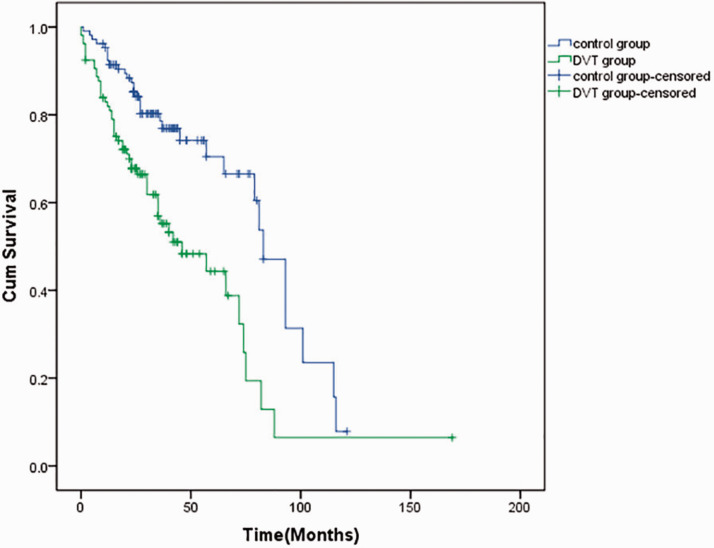
None of the 106 patients with DVT had vaginal hemorrhage, bloody stools, or ecchymosis. After treatment, thrombus was thrombolysis in 77 patients. The vascular recanalization rate was 72.6%. There was no difference in the cancer recurrence rate between the DVT and control groups. A total of 40 patients died in the DVT group (7 patients died within 1 month) and the median survival time was 66 months. In the control group, 26 patients died and the median survival time was 102 months. In the DVT group, the 3-year rates of overall survival (OS) and progression-free survival (PFS) were 70.8% and 61.3%, respectively. The 5-year rates of OS and PFS were 66.0% and 57.6%, respectively. In the control group, the 3-year rates of OS and PFS were 84.0% and 81.1%, respectively. The 5-year rates of OS and PFS were 80.2% and 78.3%, respectively (Figure 2 and Figure 3). The 3-year and 5-year OS and PFS rates were significantly lower in the DVT group than in the control group (all p < 0.05).
Figure 2.
Total survival curves of the two groups. DVT: deep vein thrombosis; cum: cumulative.
Figure 3.
Progression-free survival curves of the two groups. DVT: deep vein thrombosis; cum: cumulative.
Univariate analysis by the Kaplan–Meier log-rank test showed that the stage of the tumor (p = 0.041), DVT with PE (p < 0.001), and treatment of DVT (p < 0.001) were prognostic factors of patients with gynecological malignancy complicated by DVT (Table 3). Multivariate survival analysis by the Cox regression model showed that DVT with PE and the stage of the tumor (both p < 0.001) were independent risk factors.
Table 3.
Factors affecting the prognosis of patients with DVT.
| Factors | n | Death (n) | OS (%) | p |
|---|---|---|---|---|
| Age (years) | 0.111 | |||
| <40 | 19 | 4 | 79.0 | |
| 41–61 | 69 | 26 | 62.3 | |
| ≥61 | 18 | 10 | 44.4 | |
| BMI (kg/m2) | 0.279 | |||
| <25 | 63 | 27 | 57.1 | |
| ≥25 | 43 | 13 | 69.8 | |
| Type of tumor | 0.393 | |||
| Cervical cancer | 51 | 20 | 60.8 | |
| Ovarian cancer | 38 | 16 | 57.9 | |
| Endometrial cancer | 14 | 4 | 71.4 | |
| Others | 3 | 0 | 100.0 | |
| Staging of the tumor | 0.041 | |||
| I | 22 | 3 | 86.4 | |
| II | 19 | 5 | 73.7 | |
| III | 42 | 19 | 54.8 | |
| IV | 23 | 13 | 43.5 | |
| Presence of PE | <0.001 | |||
| Only DVT | 96 | 32 | 66.7 | |
| DVT + PE | 10 | 8 | 20.0 | |
| Treatment of DVT | <0.001 | |||
| Combined therapy | 72 | 23 | 68.1 | |
| Single therapy | 29 | 12 | 58.6 | |
| Untreated | 5 | 5 | 0 | |
| Hypertension | 0.553 | |||
| Yes | 25 | 64 | ||
| No | 81 | 61.73 | ||
| Diabetes | 0.229 | |||
| Yes | 23 | 12 | 47.83 | |
| No | 83 | 28 | 66.27 |
DVT: deep vein thrombosis; OS: overall survival; BMI: body mass index; PE: pulmonary embolism.
Discussion
The incidence of thrombosis is 13.9% in patients with cancer, and 26.8% of them have gynecological malignancy, while only 3.0% of patients have thrombosis without cancer.1 Gynecology oncology patients with thromboembolism have the characteristics of difficulty in treatment, a high recurrence rate, poor prognosis, and poor quality of life. Identification of risk factors for this condition can guide clinicians in implementing effective preventive methods as soon as possible, which has clinical significance in reducing the incidence of thrombosis and improving the prognosis of patients.
Risk factors
Age, BMI, diabetes, and hypertension are closely related to the occurrence of DVT. With an increase in age and BMI, the risk of DVT increases.9,10 In our study, BMI of patients in the DVT group was higher than that in the control group, and patients with hypertension and diabetes were more prone to having DVT than those in the control group. Therefore, patients with advanced age, obesity, hypertension, and diabetes should increase their amount of exercise, have a bland diet, control BMI, and achieve positive control of blood pressure and blood sugar levels.
The site, type, and stage of tumor are closely related to the occurrence and development of DVT. With progress of a malignant tumor, coagulants in the serum, such as D-dimer, fibrinopeptide A, and von-Willebrand factor are increased, and the risk of DVT is significantly increased.11,12 The incidence of DVT can be increased from 1.5% in the early stage of malignant tumors to 10.5% in the late stage. Additionally, the incidence of DVT in patients with ovarian cancer is as high as 50%.12 In our study, 65 patients had stages III–IV in the DVT group, which was higher than that in the control group (n=42).
The type of therapy for gynecological malignancy, such as surgery, radiotherapy, chemotherapy, and hormone therapy, might affect the occurrence of DVT. Without any preventive measures, the incidence of DVT is as high as 15% to 40% in patients with gynecological cancer undergoing major surgery.13 Our study showed that extension of the operation time, the amount of bleeding, and blood transfusion increased the risk of DVT. Despite the minimal invasiveness of laparoscopic surgery, there is considerable debate about whether it increases or decreases the risk of DVT. Some scholars believe that because the abdominal and lower extremity veins are under pressure by pneumoperitoneum and a long time is spent in the lithotomy position, laparoscopic surgery can increase the risk of DVT. However, Sandadi et al14 and Bouchard-Fortier et al15 showed that patients with gynecological cancer undergoing laparoscopic surgery without any preventive measures had an incidence of DVT of only 0.5% to 0.6%. Our study could not verify a significant effect of laparoscopy. Therefore, more studies are required to determine the effect of laparoscopy on DVT. Radiotherapy and chemotherapy can also increase the risk of occurrence of DVT, and its incidence is as high as 7% to 21% 12 months from starting the first chemotherapy session.16–18 Jacobson et al19 showed that the incidence of DVT in patients with radiation therapy for cervical cancer was 17%. However, in our study, neither chemotherapy nor radiotherapy increased the risk of DVT, which might be due to improvement of radiation technology and hemodilution by adequate transfusion.
In recent years, peripheral central venous catheters have been widely used, but the incidence of DVT in patients with cancer and a central venous catheter could be as high as 50%.20 In our study, 66 patients in the DVT group and 62 in the control group had a central venous catheter, with no difference between the two groups. However, a central venous catheter can increase the risk of DVT. Therefore, clinicians should control the indications for a central venous catheter and reduce the puncture rate.
DVT is the outcome of coagulation and anticoagulation system disorders. A hypercoagulable state can be reflected by hematological indicators, and is also called a prethrombotic state. In our study, the white blood cell count, platelet count, PT, and FIB levels in the DVT group were significantly higher than those in the control group. Therefore, vigilance should be practiced while hematological indicators are increased. D-dimer is also an important indicator for screening, diagnosing, and observing the clinical effect. The risk of DVT in patients with high D-dimer levels is three times higher than that in patients with low D-dimer levels. D-dimer can also be used to assess the risk of malignant tumors in patients with thrombosis. More than 58% of patients with both thrombosis and high D-dimer levels have malignant tumors.21,22 However, we did not measure D-dimer levels, and thus cannot support this conclusion.
Risk assessment model
An ideal DVT risk assessment tool should have the following characteristics: (1) it can screen for low-risk patients to implement prevention and treatment, and conserve medical resources; and (2) it can identify high-risk patients at an early stage for providing anticoagulant prophylaxis treatment, and to strengthen follow-up monitoring. The current study evaluated the Caprini model, the Wells DVT assessment model, and the Khorana assessment model in 212 patients. Our goal was to investigate the practicability, including simplicity, promptness, and efficiency, of these RAMs and risk stratification standard, for improving treatment of patients with gynecological cancer. The Caprini model is widely used, but its application to study patients with gynecological malignancies is still limited. The Caprini model score was not significantly different between the DVT and control groups in our study. However, most of the patients in this model were in the high risk group. Further, stratification of patients in the high risk group showed that with an increase in cumulative scores, the risk of DVT was 2.5 to 5-fold higher than that of patients with a score of 5 to 6. A large number of studies have confirmed the effectiveness and feasibility of the Caprini model. Patients with a higher score and a higher grade in the Caprini model have a higher incidence of DVT.23–26 In 2014, Stroud et al27 performed a retrospective analysis on 1123 patients with gynecological cancer and showed that, although 92% of these patients were in the very high risk group, the cumulative score of patients with thrombosis was higher than that in those without thrombosis. The American College of Chest Physicians guideline recommends that patients with a low risk (0–1) should get out of bed as early as possible, those with a moderate risk (2) should have mechanical prophylaxis or unfractionated heparin prophylaxis, those with a high risk (3–4) should take heparin or drugs combined with mechanical prevention, and those with a very high risk (>5) should take unfractionated heparin or enoxaparin combined with mechanical prevention.8 However, our study and Stroud et al's27 studies showed that as many as 90% of patients with gynecological cancer were stratified to high risk and very high risk. Therefore, patients in both of these groups should participate in a prevention program. However, a nonstandard prevention program may lead to a waste of medical resources and increase in the financial burden of patients. Therefore, clinicians should be more aware of this very high risk group and guide individualized prevention stratification programs for these patients.
Our study showed that the Wells DVT RAM allowed more patients with DVT to be classified into the high risk group, while more patients without DVT were in the low risk group. This could help clinicians to better identify high-risk patients and to exclude low-risk patients, and thus reduce excessive treatment and medical resource waste. High-risk patients who were stratified by the Wells DVT RMA had an approximately 8 to 10 times greater risk of DVT than that in the moderate risk group. Ljungqvis et al28 showed that a combination of assessment of the Wells DVT model and normal levels of D-dimer excluded patients without DVT. Most scholars consider that the value of the Wells DVT RAM is to exclude patients with suspected DVT. When the Wells DVT score indicates a low risk and D-dimer levels are negative, clinicians can rule out the possibility of thrombosis and these patients do not need to have any other tests and preventive anticoagulant therapy. Therefore, the Wells DVT RAM combined with D-dimer levels and vascular ultrasound is a simple, safe, economical, and efficient diagnostic strategy.
The Khorana model includes five indicators: the tumor type, BMI, platelet count, white blood cell count, and hemoglobin levels. Our study showed that the Khorana model could classify more patients with DVT to a higher risk level than those without DVT. In 2010, Ay et al.29 demonstrated the sensitivity of the Khorana model for predicting VTE in a study of 819 patients with cancer. This previous study also extended the Khorana evaluation model and showed that when D-dimer and P-selectin levels were increased, the cumulative incidence of thrombosis was as high as 35.0% in patients with a score of ≥5, while it was only 10.3% for a score of 3 and 1.0% for a score of 2. Therefore, to guide an individualized VTE prevention program, assess the prognosis of patients, and perform anti-tumor treatment, clinical researchers need to appropriately add more indicators and a re-planning group to the Khorana assessment model.
Prognosis
DVT promotes migration, proliferation, and metastasis of tumor cells,30 resulting in a shortened survival time and increased risk of death. In patients with ovarian cancer with DVT, OS and PFS is significantly shortened. In our study, the median survival time was 102 months in the control group, while it was only 66 months in the DVT group. OS and PFS of patients with DVT were shorter than those of patients without DVT. Tumor staging, PE, and treatment methods of DVT were important factors that affected the prognosis of patients with DVT. Additionally, tumor staging and PE were independent risk factors. Therefore, once patients are diagnosed with DVT, they should receive timely and early standardized treatment.
Limitations
There are some limitations in this study. First, this was a retrospective analysis and it may not have been able to reflect risk factors because of the different methods of medical records and missing information. Second, this was a single-center study with a limited sample size. Therefore, a large-sample multicenter study with high quality is required in the future.
Summary
DVT in patients with gynecological malignant tumors is multifarious, has a wide range, and is caused by many types of risk factors. The Caprini RAM, Wells DVT RAM, and Khorana RAM have some ability to predict the occurrence of DVT in patients with gynecological malignancies. Clinicians can combine the Caprini RAM with the Wells DVT RAM to efficiently identify high-risk patients and guide individualized prevention programs. This could lead to rationalization of the use of medical resources, a reduction in the incidence of thrombosis in patients with gynecological cancer and DVT, and an improvement in the prognosis of patients.
Highlights
Patients were graded and risk-stratified by analysis of risk factors.
Three risk assessment models were compared to predict thromboembolism in patients with gynecological cancer.
Combining the Caprini RAM and Wells DVT RAM can efficiently identify high-risk patients and guide individualized treatment.
Staging, PE, and treatment methods of DVT are vital prognostic factors.
Author contributions
Study conception and design: Xindan Wang, Jing Huang, LI Li
Performance of the research and acquisition of data: Xindan Wang, Jing Huang, Zhao Bingbing, Shape Li, LI Li
Analysis and interpretation of data: Xindan Wang, Jing Huang, Zhao Bingbing, Shape Li
Drafting of manuscript: Xindan Wang, Jing Huang, LI Li
Critical revision: Xindan Wang, Jing Huang, LI Li
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xindan Wang https://orcid.org/0000-0003-0672-4133
References
- 1.Walker AJ, Card TR, West Jet al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer 2013; 49: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res 2010; 125: 490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandala M, Clerici M, Corradino Iet al. Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: the ‘SENDO experience'. Ann Oncol 2012; 23: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 4.Abu Saadeh F, Norris L, O'Toole Set al. Venous thromboembolism in ovarian cancer: incidence, risk factors and impact on survival. Eur J Obstet Gynecol Reprod Biol 2013; 170: 214–218. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson CC, Thomas ED, Slaughter KNet al. The survival detriment of venous thromboembolism with epithelial ovarian cancer. Gynecol Oncol 2014; 134: 73–77. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Garcia DA, Wren SMet al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells PS, Anderson DR, Rodger Met al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Khorana AA, Kuderer NMet al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013; 31: 2189–2204. [DOI] [PubMed] [Google Scholar]
- 9.Montagnana M, Favaloro EJ, Franchini Met al. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis 2010; 29: 489–496. [DOI] [PubMed] [Google Scholar]
- 10.Bakirhan K, Strakhan M. Pharmacologic prevention of venous thromboembolism in obese patients. J Thromb Thrombolysis 2013; 36: 247–257. [DOI] [PubMed] [Google Scholar]
- 11.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005; 6: 401–410. [DOI] [PubMed] [Google Scholar]
- 12.Petterson TM, Marks RS, Ashrani AAet al. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res 2015; 135: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geerts WH, Bergqvist D, Pineo GFet al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest 2008; 133: 381S–453S. [DOI] [PubMed] [Google Scholar]
- 14.Sandadi S, Lee S, Walter Aet al. Incidence of venous thromboembolism after minimally invasive surgery in patients with newly diagnosed endometrial cancer. Obstet Gynecol 2012; 120: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard-Fortier G, Geerts WH, Covens Aet al. Is venous thromboprophylaxis necessary in patients undergoing minimally invasive surgery for a gynecologic malignancy? Gynecol Oncol 2014; 134: 228–232. [DOI] [PubMed] [Google Scholar]
- 16.Lyman GH, Eckert L, Wang Yet al. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 2013; 18: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khorana AA, Dalal M, Lin Jet al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013; 119: 648–655. [DOI] [PubMed] [Google Scholar]
- 18.Pant A, Liu D, Schink Jet al. Venous thromboembolism in advanced ovarian cancer patients undergoing frontline adjuvant chemotherapy. Int J Gynecol Cancer 2014; 24: 997–1002. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson GM, Kamath RS, Smith BJet al. Thromboembolic events in patients treated with definitive chemotherapy and radiation therapy for invasive cervical cancer. Gynecol Oncol 2005; 96: 470–474. [DOI] [PubMed] [Google Scholar]
- 20.Rosovsky RP, Kuter DJ: Catheter-related thrombosis in cancer patients: pathophysiology, diagnosis, and management. Hematol Oncol Clin North Am 2005; 19: 183–202, vii. [DOI] [PubMed] [Google Scholar]
- 21.Chevallier-Grenot M, Bulabois B, Seinturier Cet al. [Identification of patients at high risk of cancer after a venous thromboembolic disease]. J Mal Vasc 2013; 38: 172–177. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Li G, Liu YDet al. A new D-dimer cutoff value to improve the exclusion of deep vein thrombosis in cancer patients. Asian Pac J Cancer Prev 2014; 15: 1655–1658. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Wang L, Wu Xet al. Validation of a venous thromboembolism risk assessment model in hospitalized Chinese patients: a case-control study. J Atheroscler Thromb 2014; 21: 261–272. [DOI] [PubMed] [Google Scholar]
- 24.Bahl V, Hu HM, Henke PKet al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010; 251: 344–350. [DOI] [PubMed] [Google Scholar]
- 25.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol 2001; 38: 12–19. [DOI] [PubMed] [Google Scholar]
- 26.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost 2004; 2: 2156–2161. [DOI] [PubMed] [Google Scholar]
- 27.Stroud W, Whitworth JM, Miklic Met al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol 2014; 134: 160–163. [DOI] [PubMed] [Google Scholar]
- 28.Ljungqvist M, Soderberg M, Moritz Pet al. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19: 285–288. [DOI] [PubMed] [Google Scholar]
- 29.Ay C, Dunkler D, Marosi Cet al. Prediction of venous thromboembolism in cancer patients. Blood 2010; 116: 5377–5382. [DOI] [PubMed] [Google Scholar]
- 30.Donnellan E, Kevane B, Bird BRet al. Cancer and venous thromboembolic disease: from molecular mechanisms to clinical management. Curr Oncol 2014; 21: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]