Abstract
Objective
Chronic hepatitis B (CHB) is a worldwide disease and the most common cause of liver cancer. This study aimed to identify specific areas of research activity concerning CHB treatment between 1973 and 2018 and to aid in identifying new areas for future development.
Methods
The literature was searched from the GoPubMed and Web of Science databases using terms related to CHB treatment, analyzed with bibliometric methods and visualized using VOSviewer.
Results
A total of 9486 and 5883 papers were collected from PubMed and Web of science, respectively. The studies focused on two clusters of topics: antiviral therapy for CHB and progressive diseases, and drug resistance. Studies related to antiviral drugs concentrated on lamivudine (n = 788), entecavir (n = 390), and adefovir dipivoxil (n = 376). Studies addressing conditions developing from CHB highlighted hepatocellular carcinoma (n = 403) and cirrhosis (n = 223). China (n = 1978) contributed the most publications. The 10 most quantitatively prolific organizations were in France. All 20 of the most cited papers investigated antiviral treatments for CHB or CHB-associated cirrhosis.
Conclusions
Research on CHB treatment over the past 45 years has concentrated on antiviral therapy, CHB-associated progressive conditions, drug resistance and immunization. Although work on CHB treatment has made considerable progress, new approaches must be explored.
Keywords: Chronic hepatitis B, treatment, trend, literature, bibliometric analysis, cirrhosis, antiviral drugs
Introduction
CHB is a worldwide disease caused by hepatitis B virus, despite the implementation of preventive vaccines.1,2 Schweitzer and colleagues reported that up to 248 million individuals were seropositive against hepatitis B surface antigen (HBsAg) in 2010, by reviewing and analyzing the literature from 1965 to 2013 on the prevalence of CHB virus from databases such as MEDLINE and Web of Science.3 Uncontrolled CHB contributes to cirrhosis and even cancer.4 This disease was the major cause of liver cancer deaths (265,000 individuals [33%]) globally between 1990 and 2015.5
By 2030, the World Health Assembly’s goal is decrease new viral hepatitis infections by 90% and decrease the number of deaths from viral hepatitis by 65% globally.6 Extensive work on therapeutic strategies for CHB has been performed. A growing body of literature on treatment of CHB has been collected in PubMed, a free resource provided by the National Center for Biotechnology Information in the United States for readers to explore, as well as the Web of Science database, including the Science Citation Index expanded database. Nevertheless, studies on the status quo, focus areas, and future prospects of research toward CHB treatment are still absent.
Bibliometric methods have expanded the focus of topics, publications, countries, authors, institutions and journals in many research fields.7–11 A large number of studies have applied bibliometric methods to identify research trends in various fields.7,10,12–18 Although a few articles related to hepatitis have been bibliometrically analyzed,19 there is a lack of comprehensive bibliometrics for CHB treatment. The aims of this study were to apply bibliometric methods to identify specific areas of research activity concerning CHB treatment and to facilitate the identification of new areas for future development.
Materials & methods
Using GoPubMed as a retrieval tool,20 we searched PubMed for literature on the treatment of CHB. The search strategy in GoPubMed (www.gopubmed.org) was as follows: “Hepatitis B, Chronic” [mesh] AND Therapeutics [mesh]. The earliest available literature was from 1973. Relevant literature was also mined from the Web of Science database using the following search strategy: TI = (“Chronic hepatitis B” AND (therap* OR treat* OR cure* OR remedy*)) from 1973 to 2018. The search was conducted on October 29, 2018.
A network visualization map based on data searched from the Web of Science Core Collection database was created using VOSviewer (www.vosviewer.com) to analyze all keywords, as well as collaborations between countries, organizations, and authors, related to research on CHB treatment. The search strategy for the Web of Science Core Collection database was as described above.
All data were analyzed using Microsoft Excel 2003 (Microsoft Corp., Redmond, WA, USA). Bar graphs were prepared using OriginPro 2018 (OriginLab, Northampton, MA, USA).
Results
Annual publications on CHB treatment
From 1973 to 2018, PubMed indexed 9486 papers on the treatment of CHB, while Web of Science indexed 5883 papers. The results from GoPubMed showed that the number of published papers on treatment of CHB increased from 1973 to 2015, with one paper published in 1973 and 683 papers published in 2015, a 683-fold increase. In 2016 and 2017, slightly fewer papers were published than in 2015 (Figure 1). These results suggested that the interest of researchers in CHB treatment generally rose from 1973 to 2015 and that there was a slight drop from 2016 to 2018.
Figure 1.
Annual publication on the treatment of chronic hepatitis B.
Top topics addressed in CHB treatment-related literature
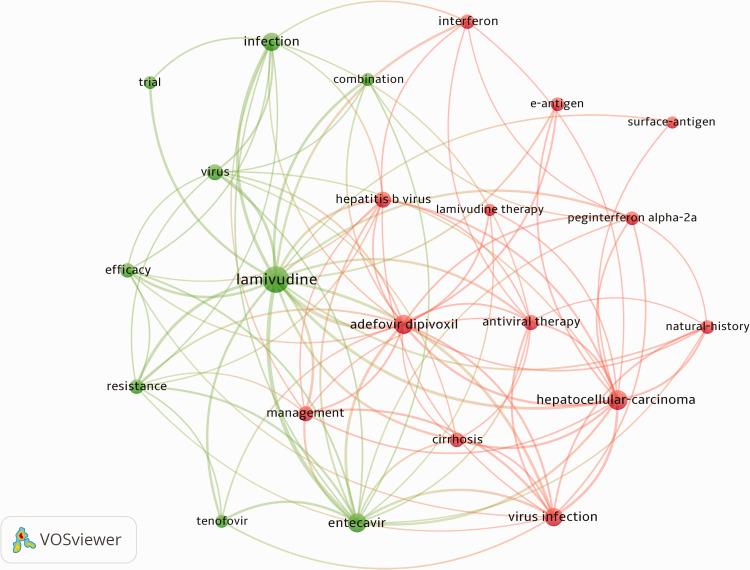
The results (data collected from Web of Science; Figure 2) demonstrated that the co-occurrence of all keywords (except for chronic hepatitis B, hepatitis B, or therapy) focused on lamivudine (788, 19.70%), hepatocellular carcinoma (403, 10.07%), entecavir (390, 9.75%), adefovir dipivoxil (376, 9.40%), virus infection (340, 8.50%), infection (333, 8.32%), hepatitis B virus (273, 6.82%), virus (254, 6.35%), management (248, 6.20%), antiviral therapy (246, 6.15%), cirrhosis (223, 5.57%), efficacy (213, 5.32%), interferon (209, 5.22%), resistance (207, 5.17%), e-antigen (190, 4.75%), natural-history (184, 4.60%), peginterferon alpha-2a (180, 4.50%), trial (174, 4.35%), combination (164, 4.10%), tenofovir (162, 4.05%), surface-antigen (152, 3.80%) and lamivudine therapy (151, 3.77%). Each of these topics was addressed by at least 150 studies, and together were broadly interpreted as “antiviral therapy for CHB and its progressive diseases”, and “antiviral drug-related resistance” using cluster analysis. This suggested that research on the treatment of CHB concentrated on antiviral drugs, especially lamivudine, entecavir and adefovir dipivoxil. Consistent with the results from the Web of Science database, antiviral drugs were also frequent topics related to CHB treatment in studies from PubMed (Table 1). In addition, development of this disease, including cirrhosis and hepatocellular carcinoma, as well as drug resistance were also frequent topics in research on CHB treatment over the past decades.
Figure 2.
Network visualization map showing co-occurrence of all keywords analyzed by VOSviewer. The map was created using a threshold of n=150 for analyzing papers from Web of Science. Twenty-two keywords appear on the map. More occurrences of each keyword result in larger circles. A color represents a cluster. The red cluster can be broadly interpreted as “antiviral therapy for CHB and its progressive diseases”, while the green cluster can be interpreted as “antiviral drug-related resistance”. CHB, chronic hepatitis B.
Table 1.
Top 15 terms in publications concerning treatment of CHB from GoPubMed.
| Top terms | Frequency | %/total |
|---|---|---|
| Humans | 8073 | 85.10% |
| Patients | 7131 | 75.17% |
| Hepatitis B virus | 6191 | 65.26% |
| Male | 4961 | 52.30% |
| Female | 4613 | 48.63% |
| Adult | 4342 | 45.77% |
| Antiviral agents | 3950 | 41.64% |
| Middle aged | 3487 | 36.76% |
| Hepatitis B e antigens | 3255 | 34.31% |
| DNA | 2739 | 28.87% |
| Immunization | 2672 | 28.17% |
| Immunity | 2654 | 27.98% |
| Antigens | 2591 | 27.31% |
| Serum | 2463 | 25.96% |
| Fibrosis | 2395 | 25.25% |
CHB, chronic hepatitis B.
Country/regions, city distribution and collaborations on CHB treatment-related research
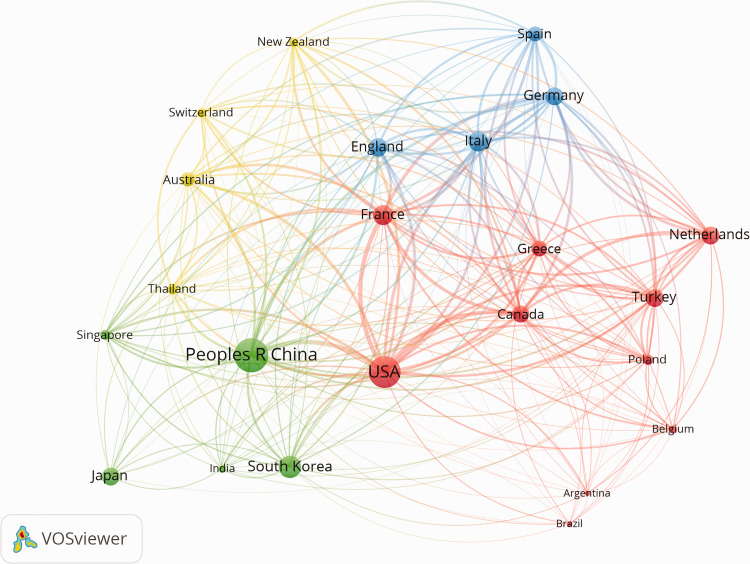
According to the world map from GoPubMed related to treatment of CHB, the literature was concentrated in Europe, East Asia, and North America (data not shown). The 10 leading countries in publications related to CHB treatment were China (n = 1978, 20.85%), the United States (n = 1258, 13.26%), Japan (n = 496, 5.23%), Germany (n = 457, 4.82%), Italy (n = 443, 4.67%), France (n = 433, 4.56%), South Korea (n = 413, 4.35%), Turkey (n = 284, 2.99%), the United Kingdom (n = 266, 2.80%), and Spain (n = 229, 2.41%). Figure 3 shows the collaborations between the top-publishing countries/regions. Among 75 countries, 23 countries were responsible for more than 30 publications. The map was divided into four collaboration clusters: 10 countries surrounding the United States (red), five countries surrounding China (green), four countries surrounding England (blue), and four countries surrounding Australia (yellow) (Figure 3). The results suggested that the most prolific countries collaborated strongly with one another on research related to CHB treatment.
Figure 3.
Network visualization map for top publication countries/regions. The map was created using a threshold of n = 30 for analyzing the papers searching from Web of Science. Twenty-three countries/regions appear on the map. The more publications, the larger the circle. The thickness of the line represents link strength of collaboration between the countries, and the collaboration cluster between the countries is shown as four colors.
The top nine cities producing CHB publications were Beijing (n = 423, 4.46%), Taipei (n = 320, 3.37%), Shanghai (n = 299, 3.15%), Hong Kong (n = 284, 2.99%), Seoul (n = 278, 2.93%), Guangzhou (n = 223, 2.35%), London (n = 181, 1.91%), Paris (n = 156, 1.64%), and Rotterdam (n = 135, 1.42%). This demonstrates that the most prolific cities producing CHB treatment-related research were concentrated in China.
Top authors and organizations for research on the treatment of CHB
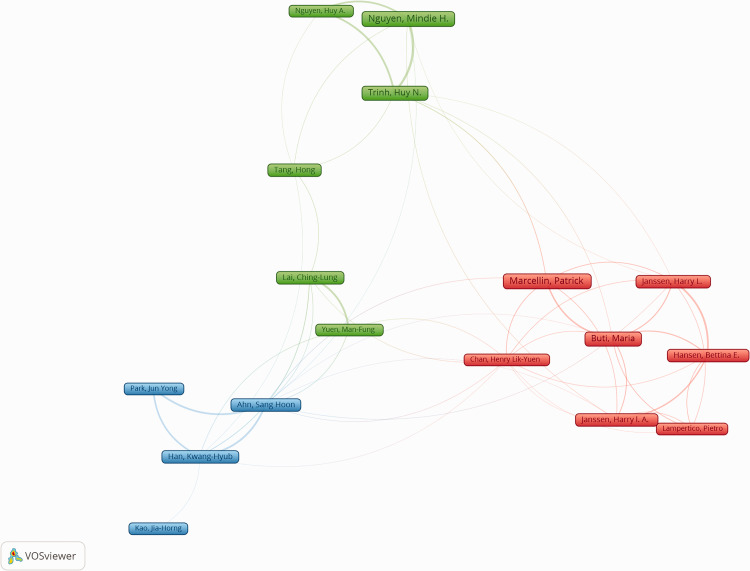
The co-authorship network map from VOSviewer (Figure 4) showed the publications and collaborations between authors. Seventeen authors had at least 40 publications, and Patrick Marcellin was the most active author (102 papers, 1.7%).
Figure 4.
Network of top authors on the treatment of CHB analyzed from VOSviewer. The map was created using a threshold of 40 for analyzing papers identified from Web of Science. The thickness of the line represents collaborations between authors. CHB, chronic hepatitis B.
Table 2 shows the most productive organizations, with the most prolific organization being Assistance Publique Hopitaux Paris (264 papers, Table 2).
Table 2.
Top 10 organizations producing CHB-related publications.
| Organization | Country | Publications | %/total papers |
|---|---|---|---|
| Assistance Publique Hopitaux Paris | France | 264 | 4.49% |
| Erasmus University Rotterdam | Netherlands | 243 | 4.13% |
| Erasmus University Medical Center | Netherlands | 226 | 3.84% |
| University of Toronto | Canada | 197 | 3.35% |
| Gilead Sciences | USA | 191 | 3.25% |
| Hopital Universitaire Beaujon Aphp | France | 190 | 3.23% |
| Institut National De La Sante Et De La Recherche Medicale Inserm | France | 189 | 3.21% |
| University of Hong Kong | China | 183 | 3.11% |
| University of London | UK | 163 | 2.77% |
| Capital Medical University | China | 140 | 2.38% |
CHB, chronic hepatitis B.
The 10 most productive journals and the 20 most influential papers for research on treatment of CHB
Table 3 shows the 10 most prolific journals publishing research on CHB treatment. The three most prolific journals were Journal of Hepatology (314 papers, 3.31%), Hepatology (313 papers, 3.30%), and Chinese Journal of Hepatology (278 papers, 2.93%).
Table 3.
Top journals publishing papers on the treatment of CHB identified from GoPubMed.
| Journal | Publications | %/total |
|---|---|---|
| Journal of Hepatology | 314 | 3.31% |
| Hepatology | 313 | 3.30% |
| Chinese Journal of Hepatology | 278 | 2.93% |
| World Journal of Gastroenterology | 258 | 2.72% |
| Journal of Viral Hepatitis | 229 | 2.41% |
| Journal of Medical Virology | 194 | 2.05% |
| Journal of Gastroenterology and Hepatology | 181 | 1.91% |
| Liver International | 149 | 1.57% |
| Gastroenterology | 134 | 1.41% |
| Antiviral Therapy | 132 | 1.39% |
CHB, chronic hepatitis B.
As shown in Table 4, the most highly cited papers were primarily concerned with the effects of antiviral drugs, especially lamivudine, adefovir dipivoxil, interferon, and entecavir, on CHB and associated diseases including fibrosis and cirrhosis, consistent with the results of the top topics for CHB treatment. These results further suggested the major theme of antiviral therapy as a central topic for CHB and CHB-associated research.
Table 4.
Top 20 cited articles on CHB treatment.
| Title | Author | Journal | Year | Total Cited |
|---|---|---|---|---|
| Lamivudine as initial treatment for chronic hepatitis B in the United States21 | Dienstag JL, Schiff ER, Wright TL, et al. | New England Journal of Medicine | 1999 | 1105 |
| Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B22 | Marcellin P, Chang T, Lim SG, et al. | New England Journal of Medicine | 2003 | 1057 |
| Effect of alpha-interferon treatment in patients with hepatitis-B e-antigen-positive chronic hepatitis-B – a meta analysis23 | Wong DKH, Cheung AM, Orourke K, et al. | Annals of Internal Medicine | 1993 | 865 |
| Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B24 | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. | New England Journal of Medicine | 2003 | 763 |
| Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years25 | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. | Gastroenterology | 2006 | 685 |
| A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B26 | Perrillo RP, Schiff ER, Davis GL, et al. | New England journal of Medicine | 1990 | 684 |
| Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study27 | Marcellin P, Gane E, Buti M, et al. | Lancet | 2013 | 682 |
| Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B28 | Niederau C, Heintges T, Lange S, et al. | New England Journal of Medicine | 1996 | 670 |
| Long-term safety of lamivudine treatment in patients with chronic hepatitis B29 | Lok ASF, Lai CL, Leung N, et al. | Gastroenterology | 2003 | 621 |
| Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B30 | Liaw YF, Leung NWY, Chang TT, et al. | Gastroenterology | 2000 | 620 |
| Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy31 | Werle-Lapostolle B, Bowden S, Locarnini S, et al. | Gastroenterology | 2004 | 602 |
| AASLD guidelines for treatment of chronic hepatitis B32 | Terrault NA, Bzowej N H, Chang K-M, et al. | Hepatology | 2016 | 597 |
| Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: Results after 3 years of therapy33 | Leung NWY, Lai CL, Chang TT, et al. | Hepatology | 2001 | 542 |
| Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B34 | Chang TT, Liaw YF, Wu SS, et al. | Hepatology | 2010 | 541 |
| Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B35 | Lai CL, Dienstag J, Schiff E, et al. | Clinical Infectious Diseases | 2003 | 518 |
| Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B36 | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. | New England Journal of Medicine | 2005 | 453 |
| Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial37 | Schalm SW, Heathcote J, Cianciara J, et al. | Gut | 2000 | 427 |
| Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B38 | Boni C, Bertoletti A, Penna A, et al. | Journal of Clinical Investigation | 1998 | 380 |
| A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update39 | Keeffe EB, Dieterich DT, Han S-H, et al. | Clinical Gastroenterology and Hepatology | 2008 | 376 |
| Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B40 | Villeneuve JP, Condreay LD, Willems B, et al. | Hepatology | 2000 | 374 |
CHB, chronic hepatitis B.
Discussion
We analyzed literature on CHB treatment, involving 9486 papers retrieved from PubMed and 5883 papers from Web of Science database, by applying quantitative and qualitative bibliometric methods. Through bibliometric analysis, our results provided an overview of the development of CHB treatment and issues of special interest based on literature over the past 45 years.
According to the increasing amount of literature in PubMed and Web of Science over the past 45 years, great progress has been made in the therapy of CHB. The results highlighted frequent topics in CHB treatment including a focus on antiviral drugs. There was a large body of literature that described antiviral therapies for CHB, especially lamivudine, entecavir, adefovir dipivoxil, interferon, peginterferon alpha-2a (PegIFNα-2a) and tenofovir. These highlights were likely related to the discovery and development of antiviral agents. Between 1990 and 2013, several antiviral agents were successively approved for CHB treatment.41 Six antiviral agents (peginterferon-2a, lamivudine, telbivudine, entecavir, adefovir, and tenofovir) were approved for the treatment of CHB in adults, and five antiviral agents (IFN-Α-2B, lamivudine, entecavir, adefovir, and tenofovir) were used for treatment of CHB in children.32 These approved antiviral agents were classified into interferons and nucleotide/nucleoside analogues, including lamivudine, adefovir, entecavir, telbivudine and tenofovir.32 Our results for both types of antiviral agents were correlated, with studies on interferon and nucleoside analogues both being areas of high interest for CHB treatment over the past decades. In addition, drug resistance was also one of the top topics in CHB treatment, which may suggest that new therapeutic strategies for CHB need to be explored.
Progressive conditions associated with CHB, including cirrhosis and hepatocellular carcinoma, were also frequently described in the literature on CHB treatment, suggesting that CHB treatment also focused on cirrhosis and hepatocellular carcinoma, consistent with previous studies.41 The risk of progression to cirrhosis and liver cancer is elevated in patients with CHB. CHB is the most frequent of hepatocellular carcinoma. A recent study reported that patients with hepatitis B virus-associated hepatocellular carcinoma receiving antiviral drugs display attenuated hepatocarcinogenesis.42 Moreover, three of the 20 most cited papers on CHB treatment investigated antiviral therapy, including tenofovir disoproxil, entecavir and lamivudine, for CHB-associated cirrhosis, further indicating that antiviral therapy for cirrhosis has been of high interest to researchers. A meta-analysis demonstrated that antiviral therapy reduced the risk of cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B viral infection.43
There was also a great deal of work indexed in PubMed showing the possible therapeutic effects of immunization treatment on CHB. This result is consistent with previous reports.1,44–46 Activation of innate immunity is a novel strategy for treatment of CHB by directly targeting hepatitis B virus.46 For immune-active CHB in adults, peg-IFN, entecavir, or tenofovir were the preferred antiviral agents recommended by the American Association for the Study of Liver Diseases.32 Taken together with the results from Web of Science database, these findings showed that treatments for CHB focused not only on antiviral therapy, but also on immunization. Interestingly, few terms related to traditional Chinese medicine were discovered in the terms or keywords from GoPubMed and the network from VOSviewer, even though China is the country with the most publications on CHB treatment. This finding is likely related to the databases we retrieved studies from. More work needs to be done to develop novel treatments for CHB.
Our results also showed the contributions of countries, organizations, and authors to CHB treatment research. We presented a network visualization map in productive countries by applying VOSviewer. Research related to the treatment of CHB varied greatly globally but was mostly produced in Europe, Asia, and America. China contributed the most publications related to CHB treatment, consistent with the results of a literature review reporting high prevalence of this disease in China.47 These advances may benefit from a vast network of collaborations between countries, and between researchers. All contributions from countries, institutions and researchers promoted the development of CHB treatment.
Approximately 75% of papers with high influence were related to antiviral agents, which was consistent with the analysis of top terms or keywords. Interestingly, from a productivity and citation perspective, only three journals published the top twenty most highly cited articles (Table 3 and 4) between 1973 to 2018. These three journals were Gastroenterology, Hepatology and Gut. Gastroenterology accounted for 20.1% of the top-20 manuscript citations. Two papers were related to adefovir dipivoxil, and the other two were related to lamivudine in Gastroenterology. Hepatology accounted for 16.4% of the top-20 manuscript citations. Two papers were related to lamivudine, and the others were related to entecavir and guidelines, respectively. Gut published one paper among the top twenty most highly cited manuscripts, accounting for 3.4% of all citations. That paper was related to lamivudine and alpha interferon, which further confirmed that antiviral agents, especially lamivudine, were very interesting to researchers.
Our study had some limitations. we qualitatively and quantitatively analyzed the literature related to CHB treatment over the past 40 years, but the literature databases used were limited to MEDLINE and Web of Science. Other databases, such as Scopus and Embase, also can index relevant papers. Although MEDLINE is one of the most authoritative biomedical databases in the world, and Web of Science is the core database of Science Citation Index Expanded, the papers indexed both two literature databases in this study are still not comprehensive. In addition, we may not have searched comprehensively based on the search terms used, and use of other search terms may also affect the results. Therefore, all the results and conclusions of this study should be interpreted in light of these limitations.
Conclusions
This study illustrated the features of publications on CHB treatment from 1973 to 2018 by applying bibliometric analysis. Great progress has been made in the treatment of CHB over the past 45 years. Overall, our study found that a large amount of research on treatment of CHB concentrated on antiviral therapy, CHB progressive conditions including cirrhosis and hepatocellular carcinoma, immunization, and antiviral drug resistance. Although work on CHB treatment has made considerable progress in the past 45 years, many unresolved issues such as drug resistance and inability to eliminate all HBsAg, remain. New therapeutic strategies and approaches for CHB remain to be explored.
Acknowledgements
We would like to thank for National Natural Science Foundation of China (No. 81803976) and the Science and Technology Bureau of Chengdu (Sichuan, China. No. 2016-XT00-00033-GX) for grant support.
Authors’ contributions
Quansheng Feng and Li Wen designed the study. All authors analyzed the data. Guiyu Li and Jiyong Lin drafted the manuscript. Guiyu Li and Jiyong Lin contributed equally to this work. Quansheng Feng and Li Wen revised and reviewed the manuscript. All authors approved the final version of the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval
Not required.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81803976) and the Science and Technology Bureau of Chengdu (No. 2016-XT00-00033-GX).
ORCID iD
Quansheng Feng https://orcid.org/0000-0003-3437-8987
References
- 1.Boni C, Barili V, Acerbi Get al. HBV immune-therapy: from molecular mechanisms to clinical applications. Int J Mol Sci 2019; 20: pii: E2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le MH, Yeo YH, Cheung Ret al. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999–2016. Hepatology. Epub ahead of print 22 June 2019. DOI: 10.1002/hep.30831. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer A, Horn J, Mikolajczyk RTet al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386: 1546–1555. [DOI] [PubMed] [Google Scholar]
- 4.Shih C, Yang CC, Choijilsuren Get al. Hepatitis B virus. Trends Microbiol 2018; 26: 386–387. [DOI] [PubMed] [Google Scholar]
- 5.Akinyemiju T, Abera S, Ahmed Met al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol 2017; 3: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannini P, Sokal EM. Hepatitis B: changing epidemiology and interventions. Arch Dis Child 2017; 102: 676–680. [DOI] [PubMed] [Google Scholar]
- 7.Caes L, Boerner KE, Chambers CTet al. A comprehensive categorical and bibliometric analysis of published research articles on pediatric pain from 1975 to 2010. Pain 2016; 157: 302–313. [DOI] [PubMed] [Google Scholar]
- 8.Mao Z, Liu C, Chen Set al. A bibliometric analysis of exertional heat stroke research in Web of Science. Mil Med Res 2016; 3: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed MF, Marais O, Qureshi AIet al. The top 100 most-cited articles in stroke imaging: a bibliometric analysis. Curr Probl Diagn Radiol 2018; 47: 161–167. [DOI] [PubMed] [Google Scholar]
- 10.Zeleznik D, Blazun Vosner H, Kokol P. A bibliometric analysis of the Journal of Advanced Nursing, 1976-2015. J Adv Nurs 2017; 73: 2407–2419. [DOI] [PubMed] [Google Scholar]
- 11.Zyoud SH, Smale S, Waring WSet al. Global research trends in microbiome-gut-brain axis during 2009-2018: a bibliometric and visualized study. BMC Gastroenterol 2019; 19: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si J, Zhao C, Liu Net al. Statistical analysis of acupuncture-moxibustion literature based on SCIE and GoPubMed. Zhongguo Zhen Jiu 2015; 35: 1309–1314. [PubMed] [Google Scholar]
- 13.Ang HM, Kwan YH. Bibliometric analysis of journals in the field of geriatrics and gerontology. Geriatr Gerontol Int 2017; 17: 357–360. [DOI] [PubMed] [Google Scholar]
- 14.Muller AM, Ansari P, Ebrahim NAet al. Physical activity and aging research: a bibliometric analysis. J Aging Phys Act 2016; 24: 476–483. [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Wilson CS, Lipton RBet al. Growth of headache research: a 1983–2014 bibliometric study. Cephalalgia 2017; 37: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Li J, Seehus Cet al. Bibliometric analysis of recent sodium channel research. Channels (Austin) 2018; 12: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders TFC, Rymer BC, McNamara KJ. A global bibliometric analysis of otolaryngology: head and neck surgery literature. Clin Otolaryngol 2017; 42: 1338–1342. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Wu L. Trend in H(2)S biology and medicine research-A bibliometric analysis. Molecules 2017; 22: pii: E2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezaee Zavareh MS, Alavian SM. Ten-year analysis of hepatitis-related papers in the Middle East: a web of science-based scientometric study. Turk J Gastroenterol 2017; 28: 20–25. [DOI] [PubMed] [Google Scholar]
- 20.Doms A, Schroeder M. GoPubMed: exploring PubMed with the gene ontology. Nucleic Acids Res 2005; 33: W783–W786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dienstag JL, Schiff ER, Wright TLet al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999; 341: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Chang TT, Lim SGet al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003; 348: 808–816. [DOI] [PubMed] [Google Scholar]
- 23.Wong DK, Cheung AM, O'Rourke Ket al. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med 1993; 119: 312–323. [DOI] [PubMed] [Google Scholar]
- 24.Hadziyannis SJ, Tassopoulos NC, Heathcote EJet al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003; 348: 800–807. [DOI] [PubMed] [Google Scholar]
- 25.Hadziyannis SJ, Tassopoulos NC, Heathcote EJet al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006; 131: 1743–1751. [DOI] [PubMed] [Google Scholar]
- 26.Perrillo RP, Schiff ER, Davis GLet al. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med 1990; 323: 295–301. [DOI] [PubMed] [Google Scholar]
- 27.Marcellin P, Gane E, Buti Met al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381: 468–475. [DOI] [PubMed] [Google Scholar]
- 28.Niederau C, Heintges T, Lange Set al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996; 334: 1422–1427. [DOI] [PubMed] [Google Scholar]
- 29.Lok AS, Lai CL, Leung Net al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 30.Liaw YF, Leung NW, Chang TTet al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology 2000; 119: 172–180. [DOI] [PubMed] [Google Scholar]
- 31.Werle-Lapostolle B, Bowden S, Locarnini Set al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004; 126: 1750–1758. [DOI] [PubMed] [Google Scholar]
- 32.Terrault NA, Bzowej NH, Chang KMet al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63: 261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung NW, Lai CL, Chang TTet al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33: 1527–1532. [DOI] [PubMed] [Google Scholar]
- 34.Chang TT, Liaw YF, Wu SSet al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010; 52: 886–893. [DOI] [PubMed] [Google Scholar]
- 35.Lai CL, Dienstag J, Schiff Eet al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis 2003; 36: 687–696. [DOI] [PubMed] [Google Scholar]
- 36.Hadziyannis SJ, Tassopoulos NC, Heathcote EJet al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352: 2673–2681. [DOI] [PubMed] [Google Scholar]
- 37.Schalm SW, Heathcote J, Cianciara Jet al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut 2000; 46: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boni C, Bertoletti A, Penna Aet al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest 1998; 102: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keeffe EB, Dieterich DT, Han SHet al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6: 1315–1341; quiz 1286. [DOI] [PubMed] [Google Scholar]
- 40.Villeneuve JP, Condreay LD, Willems Bet al. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B. Hepatology 2000; 31: 207–210. [DOI] [PubMed] [Google Scholar]
- 41.Halegoua-De Marzio D, Hann HW. Then and now: the progress in hepatitis B treatment over the past 20 years. World J Gastroenterol 2014; 20: 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin XL, Hong SK, Kim Het al. Antiviral therapy may decrease HBx, affecting cccDNA and MSL2 in hepatocarcinogenesis. Oncol Lett 2019; 18: 4984–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lok AS, McMahon BJ, Brown RS, Jret al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 2016; 63: 284–306. [DOI] [PubMed] [Google Scholar]
- 44.Lang J, Neumann-Haefelin C, Thimme R. Immunological cure of HBV infection. Hepatol Int 2019; 13: 113–124. [DOI] [PubMed] [Google Scholar]
- 45.Ji LS, Gao QT, Guo RWet al. Immunomodulatory effects of combination therapy with Bushen formula plus entecavir for chronic hepatitis B patients. J Immunol Res 2019; 2019: 8983903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suslov A, Wieland S, Menne S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr Opin Virol 2018; 30: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collaborators PO. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3: 383–403. [DOI] [PubMed] [Google Scholar]