Abstract
Type 2 innate lymphoid cells (ILC2s) are a subset of ILCs with critical roles in immunoregulation. However, the possible role of ILC2s as immunotherapy against allograft rejection remains unclear. Here, we show that IL‐33 significantly prolonged islet allograft survival. IL‐33‐treated mice had elevated numbers of ILC2s and regulatory T cells (Tregs). Depletion of Tregs partially abolished the protective effect of IL‐33 on allograft survival, and additional ILC2 depletion in Treg‐depleted DEREG mice completely abolished the protective effects of IL‐33, indicating that ILC2s play critical roles in IL‐33‐mediated islet graft protection. Two subsets of ILC2s were identified in islet allografts of IL‐33‐treated mice: IL‐10 producing ILC2s (ILC210) and non‐IL‐10 producing ILC2s (non‐ILC 10). Intravenous transfer of ILC210 cells, but not non‐ILC 10, prolonged islet allograft survival in an IL‐10‐dependent manner. Locally transferred ILC210 cells led to long‐term islet graft survival, suggesting that ILC210 cells are required within the allograft for maximal suppressive effect and graft protection. This study has uncovered a major protective role of ILC210 in islet transplantation which could be potentiated as a therapeutic strategy.
Keywords: IL‐10, IL‐33, innate lymphoid cells, islet transplantation, type 1 diabetes
Subject Categories: Immunology, Metabolism, Regenerative Medicine
This study reveals a major protective role of the IL‐33/ILC2 axis in islet transplantation that could be potentiated as a therapeutic strategy. Adoptive transfer of ex vivo expanded IL‐10‐producing ILC2s (ILC210) significantly prolonged allograft survival.

The paper explained.
Problem
Pancreatic islet transplantation is a promising treatment option for patients with type 1 diabetes. However, islet graft rejection remains one of the main obstacles to successful transplantation. Clinically applicable strategies for immunomodulation need to be developed to achieve long‐term graft tolerance. One attractive therapy to prevent allograft rejection relies on harnessing the potential of regulatory immune cells. Mounting evidence indicates that ILC2s play immune regulatory roles in acute and chronic inflammatory diseases. The current study explores whether ILC2s could suppress allograft rejection in an islet transplantation model.
Results
Here, we show that IL‐33 treatment significantly prevented islet allograft rejection and improved islet function. This remarkable therapeutic benefit was shown to be mediated by inducing regulatory immune cells including Tregs and ILC2s. A short course of IL‐33 treatment induced a sustained increase in ILC2 abundance in islet graft which was associated with IL‐33‐mediated islet graft protection. Importantly, we further demonstrated that IL‐10‐producing ILC2s (ILC210), a subset of ILC2, are important inhibitors of islet graft rejection. Co‐transplantation of ILC210 with islets led to long‐term allograft survival, suggesting that ILC210 cells are required within the allograft for maximal suppressive effect and graft protection.
Impact
Our study offers new insights into the role of IL‐33 and ILC210 in islet allograft survival. We propose administration of IL‐33 and ILC210 as an adjunctive therapy to prevent allograft rejection, bringing potential novel therapeutics to the field of transplantation.
Introduction
Innate lymphoid cells (ILCs) are a novel group of immune cells with critical roles in immunity, tissue homeostasis, and pathological inflammation (Eberl et al, 2015; Sonnenberg & Artis, 2015; Vivier et al, 2018). ILCs are subdivided into three groups: ILC1, ILC2, and ILC3, based on their cytokine profiles and expression of specific transcription factors, mirroring the classification of CD4+ T helper cell subsets into TH1, TH2, and TH17 cells. Type 2 ILCs (ILC2s) resemble TH2 cells as they require the transcription factor GATA‐3; produce type 2 cytokines IL‐4, IL‐5, IL‐9, and IL‐13; and play important roles in immunity against pathogens and type 2 inflammation (Krabbendam et al, 2018). ILC2s also promote tissue recovery following acute injury in multiple organs, such as lung, intestine, and kidney (Monticelli et al, 2011; Cao et al, 2018). For example, in influenza virus infection of mice, ILC2s were activated by lung epithelial cell‐derived IL‐25 and IL‐33 and promoted repair of the airway epithelium via producing amphiregulin (Areg) (Monticelli et al, 2011). More recently, IL‐10 producing ILC2s, namely ILC210, have been identified in lung where they play important roles in resolution of lung inflammation (Seehus et al, 2017), indicating that ILC2s include different functional subsets. However, the possible immunoregulatory role of ILC2 in transplant rejection has not been addressed so far. Modulation of ILC2 activity may provide a therapeutic approach to maintain allograft tolerance.
Type 1 diabetes mellitus (T1DM) is an autoimmune disease in which pancreatic β cells are destroyed by autoreactive T cells, resulting in lifelong insulin dependency (Burrack et al, 2017; Paschou et al, 2018). Pancreatic islet transplantation is a promising treatment option for patients with type 1 diabetes that restores both endogenous insulin production and glycemic stability (Anazawa et al, 2019). However, islet graft rejection caused by immune cells remains one of the main obstacles to successful transplantation. Although advances in immunosuppressive therapies have promoted excellent short‐term graft survival after islet transplantation, immunosuppressive drugs with severe side effects remain ineffective at preventing late‐stage allograft rejection (Gibly et al, 2011; Anazawa et al, 2019). Therefore, it is critical to develop applicable strategies that specifically target anti‐islet immune responses to achieve long‐term graft tolerance without use of immunosuppressive drugs. One attractive alternative therapy to prevent allograft rejection relies on harnessing the potential of regulatory T cells (Treg) (Gagliani et al, 2010; Lam et al, 2017). Recent studies have shown that ex vivo‐expanded human Treg can prevent the development of islet and skin allograft rejection in a humanized mouse model (Issa et al, 2010; Yi et al, 2012). IL‐33 significantly prolonged allograft survival in organ transplantation partially via increasing numbers of Tregs (Turnquist et al, 2011; Matta et al, 2016). We recently reported that IL‐33‐expanded kidney resident ILC2s prevented renal ischemia‐reperfusion injury (IRI) via production of Areg (Cao et al, 2018). In the present study, we sought to determine the role of the IL‐33‐ILC2 pathway in islet allograft survival. Here, we report that IL‐33 induced long‐term survival of islet allografts via increasing both Tregs and ILC2s in vivo. Importantly, we further demonstrated that ex vivo‐expanded ILC2s significantly prolonged allograft survival in an IL‐10‐dependent manner.
Results
IL‐33 prevented rejection of islet allograft
To determine whether IL‐33 could prolong islet graft survival, we treated diabetic C57BL/6 mice with mouse recombinant IL‐33 (0.3 μg/mouse/day, intraperitoneally) for five consecutive days prior to islet transplantation (Fig 1A). As expected, blood glucose measurement showed that PBS‐treated control mice all rejected their grafts rapidly, with a mean survival time of 12 days. In contrast, pretreatment with IL‐33 led to prominent long‐term graft survival (Fig 1B). In this case, a small proportion of the mice rejected their grafts around days 14–20, but the remaining 75% (n = 9 out of 12) of the mice retained their grafts indefinitely (> 80 days) (Fig 1C). Intraperitoneal glucose tolerance tests (IPGTTs) were performed to investigate islet graft function in vivo. The glucose tolerance in islet transplant mice treated with IL‐33 was significantly improved compared with mice receiving islets alone (Fig 1D). The values of the area under the glucose curve (AUC) for IPGTT in the group with IL‐33 treatment were significantly smaller than in those receiving islets alone (Fig 1E). Histology in PBS‐treated control mice showed that islet architecture was lost, with only a few remaining insulin‐positive cells and massive leukocyte infiltration. In contrast, the group with IL‐33 treatment showed well‐preserved islet morphology with reduced/minimal leukocyte infiltration (Fig 1F). These results showed that IL‐33 treatment prevented islet allograft rejection and improved islet function. We also found that IL‐33 treatment in STZ‐induced diabetic mice without islet transplantation improved fasting and non‐fasting glycemia at day 15 and 18 post‐STZ injection, but did not enhance survival of STZ‐induced diabetic mice (Appendix Fig S1). The improved glycemia status of diabetic mice with IL‐33 treatment could possibly contribute to prolonged islet allograft survival.
Figure 1. IL‐33 prolonged islet allograft survival.
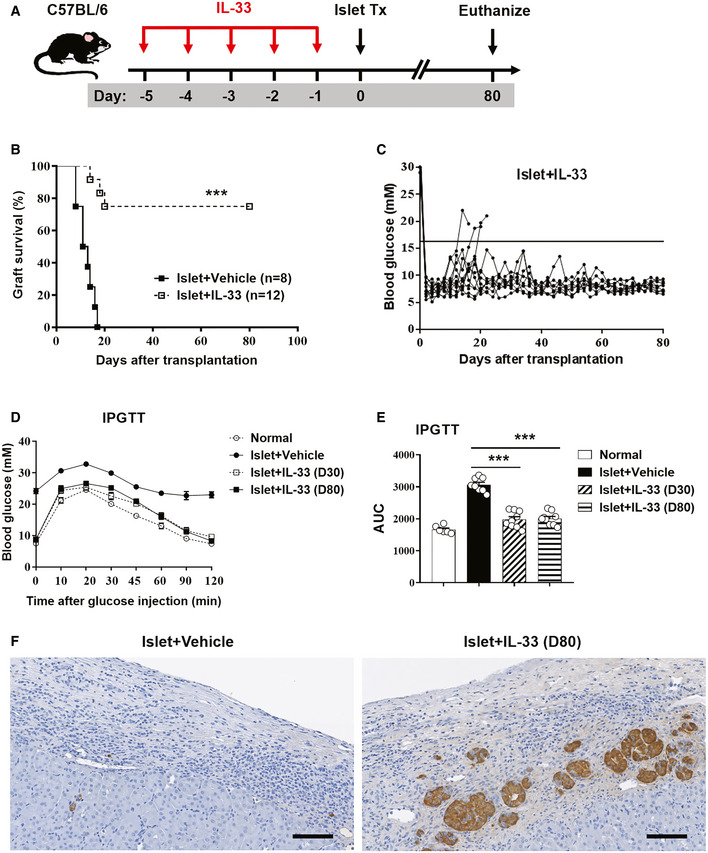
-
AStreptozotocin‐induced diabetic C57BL/6 (H2b) mice were treated with mouse recombinant IL‐33 daily for 5 consecutive days before islet transplantation. On day 0, mice were transplanted with BALB/c (H2d) islets. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected after two consecutive BGLs > 16 mmol/l (mM) after a period of normoglycemia.
-
BIslet graft survival of mice receiving vehicle (PBS) or IL‐33 was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001 vs. islet+vehicle.
-
CBlood glucose level of mice treated with IL‐33 (the horizontal black line indicates a BGL of 16 mmol/l, the threshold for rejection). Each line represents one mouse.
-
DIntraperitoneal glucose tolerance test (IPGTT) was assessed in normal mice, islet transplant mice receiving vehicle (on the day when grafts were considered rejected), and islet transplant mice treated with IL‐33 (at day 30 and day 80 post‐islet transplantation). Data shown are the mean ± SEM (n = 6–9 per group).
-
EArea under the curve (AUC) for IPGTT was assessed. Data shown are the mean ± SEM (n = 6–9 per group), and a one‐way ANOVA was performed, ***P < 0.001.
-
FRepresentative immunohistochemical staining for insulin in graft samples from mice receiving vehicle or IL‐33. Scale bar = 100 μm.
IL‐33 induced Th2 cytokine, ILC2s, and regulatory T cells in vivo
We then investigated the mechanism by which IL‐33 prevents rejection of islet allografts. In islet transplant mice treated with IL‐33, the serum levels of the Th1‐related cytokines IFN‐γ and IL‐6 were significantly reduced when compared with those of PBS‐treated control mice (Appendix Fig S2A). Meanwhile, IL‐33 treatment markedly reduced the expression of Th1‐related cytokines IFN‐γ and IL‐6 in allografts (Appendix Fig S2B). In contrast, IL‐33 treatment enhanced the serum levels of the Th2‐related cytokines IL‐4 and IL‐13 and the expression of IL‐4 and IL‐13 in allografts (Appendix Fig S2C and D). Regarding serum levels of IL‐10, we found no significant differences between the IL‐33‐treatment group and the controls. However, IL‐33 treatment markedly increased the expression of IL‐10 in allografts (Appendix Fig S2). These experiments suggest that IL‐33 treatment has large effects on the systemic and local Th1/Th2 response, promoting polarization of the immune response toward a Th2 phenotype, and simultaneously inhibiting Th1 reactivity. Moreover, we observed a significant increase in the ratio of CD4 T cells/CD8 T cells in islet grafts of mice treated with IL‐33 (Appendix Fig S2E and F), suggesting that IL‐33 treatment may prevent islet graft rejection through modulating CD4 and CD8 T‐cell responses.
We and others have previously shown that IL‐33 can induce expansion of regulatory T cells (Tregs) and ILC2s in multiple anatomical sites where they display an immunosuppressive role in various disease conditions (Turnquist et al, 2011; Schiering et al, 2014; Matta et al, 2016; Cao et al, 2018). Here, we aimed to examine whether short‐term IL‐33 treatment can induce long‐term accumulation of Tregs and ILC2s in multiple anatomical sites. Firstly, we analyzed the effect of IL‐33 on Tregs and ILC2s in spleen and kidney of normal C57BL/c mice at different time points after IL‐33 administration. Analysis of leukocytes isolated from the spleen and kidneys of C57BL/6 mice 3 days after short‐term IL‐33 treatment showed a moderate increase in CD4+Foxp3+Treg frequencies (Fig 2A and B) and a massive increase in Lin(−)GATA‐3+ ILC2 frequencies (Fig 2C and D) as compared with PBS‐treated control mice. IL‐33‐induced Treg accumulation in the spleen and kidney was only maintained for 1 week after a single course of five IL‐33 injections (Fig 2E), whereas IL‐33‐induced ILC2 accumulation in the kidney was maintained at a high level for up to 8 weeks and the ILC2 increase was more transient in the spleen (Fig 2F). Furthermore, we examined the Tregs and ILC2 accumulation in spleen, kidney, and islet graft of islet transplant mice at different time points after IL‐33 administration (Fig 3A). As expected, IL‐33 treatment induced the accumulation of Tregs in spleen, kidney, and islet graft at day 7 post‐islet transplantation, but not at day 30 and day 80 (Fig 3B–D), indicating IL‐33 induced only a short‐term increase in Tregs in different tissues, especially islet graft. In contrast, IL‐33 treatment induced a long‐term increase in ILC2s in kidney and islet graft, but not in the spleen (Fig 3E–I). A greater amount of ILC2s were found in islet graft than in kidney and liver, which indicates that ILC2s tend to migrate to islet graft undergoing immune response (Appendix Fig S3). Moreover, we observed a consistent increase in IL‐33, but not IL‐25 or thymic stromal lymphopoietin (TSLP), in islet graft tissue of mice treated with IL‐33 (Fig 3J). These data could partly explain why ILC2s are found within the graft for so long. Taken together, a short course of IL‐33 treatment induced a sustained increase in ILC2 abundance in kidney and islet graft which may be involved in IL‐33‐mediated islet graft protection.
Figure 2. IL‐33 induced Tregs and ILC2s in vivo .
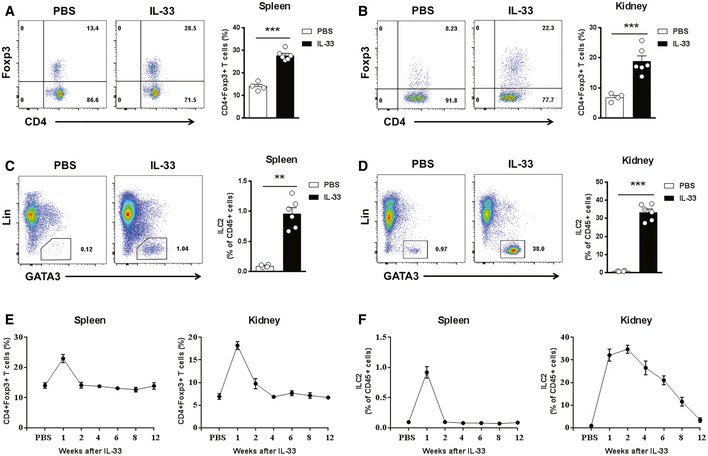
-
C57BL/6 mice were treated with mouse recombinant IL‐33 or PBS daily for 5 consecutive days.
-
A, BRepresentative FACS analysis showing the proportion of Tregs (CD4+Foxp3+) in the CD4+T‐cell compartment from the spleens (A) and kidneys (B) at day 3 after treatment in C57BL/6 mice receiving PBS (n = 4) or IL‐33 (n = 6).
-
C, DRepresentative FACS analysis showing the proportion of ILC2s (Lin‐GATA-3+) in the CD45+leukocyte compartment from the spleens (C) and kidneys (D) at day 3 after treatment in C57BL/6 mice receiving PBS (n = 4) or IL‐33 (n = 6).
-
E, Fproportion of Tregs (E) and ILC2s (F) in the spleen and kidney in PBS‐injected controls (n = 4) and at weeks 1–12 after IL‐33 treatment (n = 4–6 per IL‐33–treated group).
Figure 3. IL‐33 induced Tregs and ILC2s in mice with islet transplantation.
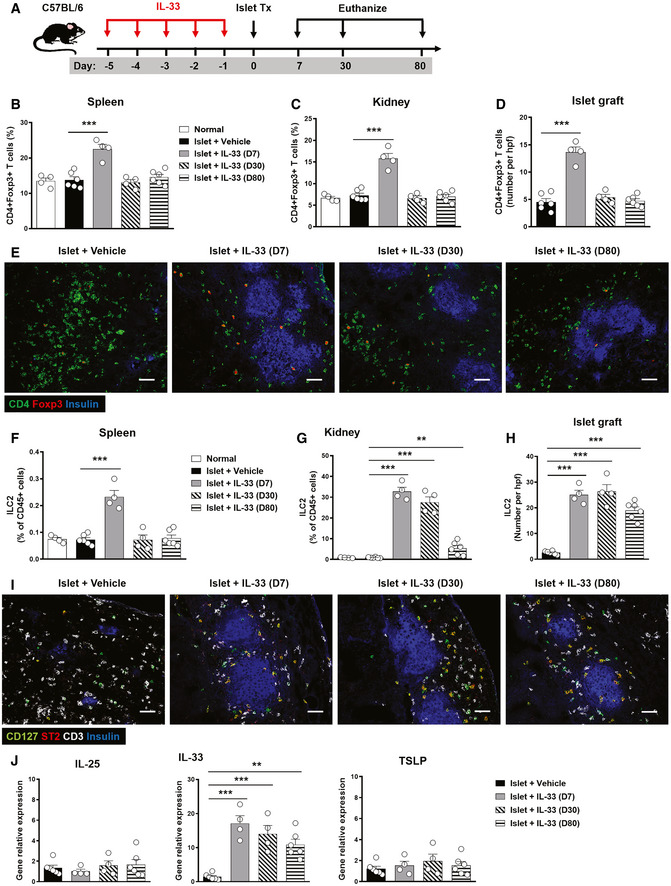
-
AStreptozotocin‐induced diabetic C57BL/6 (H2b) mice with IL‐33 treatment were transplanted with BALB/c (H2d) islets. Mice were sacrificed at day 7, 30, and 80 post‐islet transplantation.
-
B–DProportion or numbers of CD4+Foxp3+Tregs in the spleens, kidneys, and islet grafts of normal, islet transplant mice receiving vehicle and islet transplant mice with IL‐33 treatment (at day 7, 30, and 80 post‐islet transplantation). Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
-
ERepresentative confocal microscopy images of immunostaining for CD4, Foxp3, and insulin in islet grafts. Scale bar = 50 μm.
-
F–HProportion or numbers of ILC2s in the spleens, kidneys, and islet graft of mice with or without IL‐33 treatment. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.
-
IRepresentative confocal microscopy images of immunostaining for CD127, ST2, CD3, and insulin in islet grafts. Scale bar = 50 μm.
-
JThe mRNA expression of IL‐25, IL‐33, and TSLP in islet grafts of mice with or without IL‐33 treatment was examined by qPCR, and expressed relative to the control of each experiment. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.
Tregs and ILC2s played critical roles in IL‐33‐mediated islet graft protection
Previous studies have shown that ex vivo‐expanded Tregs protected against islet graft rejection (Shi et al, 2012; Yi et al, 2012). IL‐33‐mediated cardiac allograft survival and acute graft‐versus‐host disease (GVHD) protection was dependent on Tregs (Turnquist et al, 2011; Matta et al, 2016). To examine whether Treg accumulation in vivo contributes to IL‐33‐mediated islet graft protection, depletion of regulatory T cells (DEREG) mice was administered diphtheria toxin (DT) to selectively deplete Tregs in vivo (Fig 4A). Treg depletion in DEREG mice was confirmed in the spleen and kidney by flow cytometry at day 5 post‐islet transplantation (Fig 4B). Treg depletion significantly reduced the survival rate of islet graft in IL‐33‐treated mice, suggesting that Treg accumulation is an important mechanism in IL‐33‐mediated islet graft protection (Fig 4D and E). ILC2s play important roles in tissue repair and immunoregulation (Sonnenberg & Artis, 2015). We and other groups found that IL‐33‐activated ILC2s expressed significantly higher levels of CD25 in vivo (Appendix Fig S4; Roediger et al, 2015), suggesting that administration of anti‐CD25 antibodies could be utilized to deplete ILC2s in vivo. However, administration of anti‐CD25 antibody has been used to successfully deplete Tregs in vivo as CD25 is also highly expressed on Tregs (Lu et al, 2013). Here, the potential contribution of ILC2s to IL‐33‐mediated islet graft protection was assessed in Treg‐depleted mice (DEREG mice treated with DT), in which administration of anti‐CD25 antibodies (PC61) leads to depletion of ILC2s (Fig 4A). Flow cytometric analysis of Lin‐GATA3+ ILC2s in spleen and kidney confirmed significant depletion of ILC2s in mice with anti‐CD25 antibody treatment compared with Treg‐depleted DEREG mice (Fig 4C). Additional ILC2 depletion in Treg‐depleted DEREG mice completely abolished the protective effects of IL‐33 on islet transplantation (Fig 4D and E). Taken together, these results demonstrate that both Tregs and ILC2s play critical roles in IL‐33‐mediated islet graft protection.
Figure 4. ILC2s and Tregs contributed to IL‐33‐mediated islet protection in vivo .
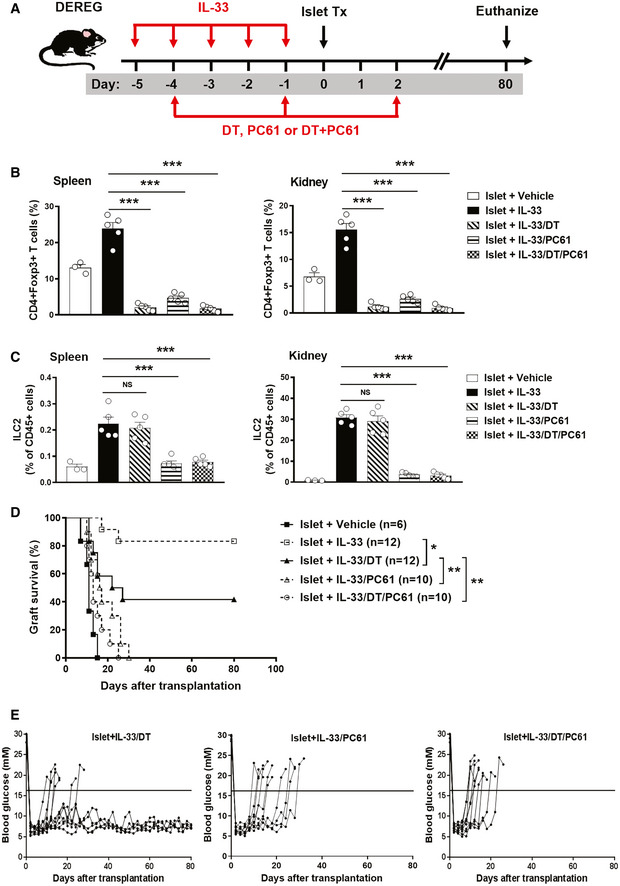
-
AStreptozotocin‐induced diabetic DEREG C57BL/6 mice were treated with mouse recombinant IL‐33 daily for 5 consecutive days, as well as diphtheria toxin (DT), PC61 or DT+PC61 on days −4 and sletallogr1 prior to and on day 2 post‐islet transplantation. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected.
-
B, CProportion of CD4+Foxp+Tregs (B) and Lin-GATA‐3+ILC2s (C) from the spleens and kidneys of islet transplant mice receiving vehicle, IL‐33, IL‐33/DT, IL‐33/PC61, or IL‐33/DT/PC61 at day 5 post‐islet transplantation. Data shown are the mean ± SEM (n = 4–5 per group), and a one‐way ANOVA was performed; NS: non‐significant, ***P < 0.001.
-
DIslet graft survival of five groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. *P < 0.05, **P < 0.01.
-
EData are shown as blood glucose measurement in islet transplant mice treated with IL-33/DT, IL‐33/PC61, or IL‐33/DT/PC61. The horizontal black line indicates a BGL of 16 mmol/l, the threshold for rejection. Each line represents one mouse.
IL‐33 and IL‐2/anti‐IL‐2 antibody complex induced ILC210
IL‐10 producing ILC2s (ILC210) have been described in lung and intestine (Seehus et al, 2017; Wang et al, 2017). IL‐33 and IL‐2 have been shown to induce ILC210 in vivo (Seehus et al, 2017). Here, we identified two subsets of ILC2s in islet graft and kidney of islet transplant mice treated with IL‐33, ILC210, and non‐IL‐10 producing ILC2s (Fig 5A and B). The IL‐2/anti‐IL‐2 antibody complex (IL‐2C) has been found to directly induce expansion of ILC2 that express the high‐affinity IL‐2 receptor CD25 (Seehus et al, 2017; Cao et al, 2020). Using IL‐10 reporter mice, we further demonstrated that combined IL‐33 and IL‐2C treatment markedly enhanced the ILC210 expansion in the kidney when compared with IL‐33 treatment alone (Fig 5C). In vitro, ILC2s isolated from kidneys were treated with IL‐33 and IL‐2C and analyzed for IL‐10 by flow cytometry and ELISA. IL‐33 and IL‐2C treatment induced more ILC210 expansion in cultured ILC2s (Fig 5D). ILC2s when cultured with IL‐33 and IL‐2C produced a high level of IL‐10 in the supernatant (Fig 5E). IL‐2 is known to activate STAT5 which has been shown to promote IL‐10 expression in T cells (Polhill et al, 2012). Therefore, we evaluated the degree of phosphorylation of STAT5 (p‐STAT5) in ILC2s. As expected, kidney ILC2s treated with IL‐33 and IL‐2C rapidly increased the expression of p‐STAT5 in vitro (Fig 5F). Moreover, inhibition of STAT5 significantly reduced IL‐10 production by ILC2s cultured with IL‐33 and IL‐2C (Fig 5G).
Figure 5. IL‐33 and IL‐2C induced ILC210 .
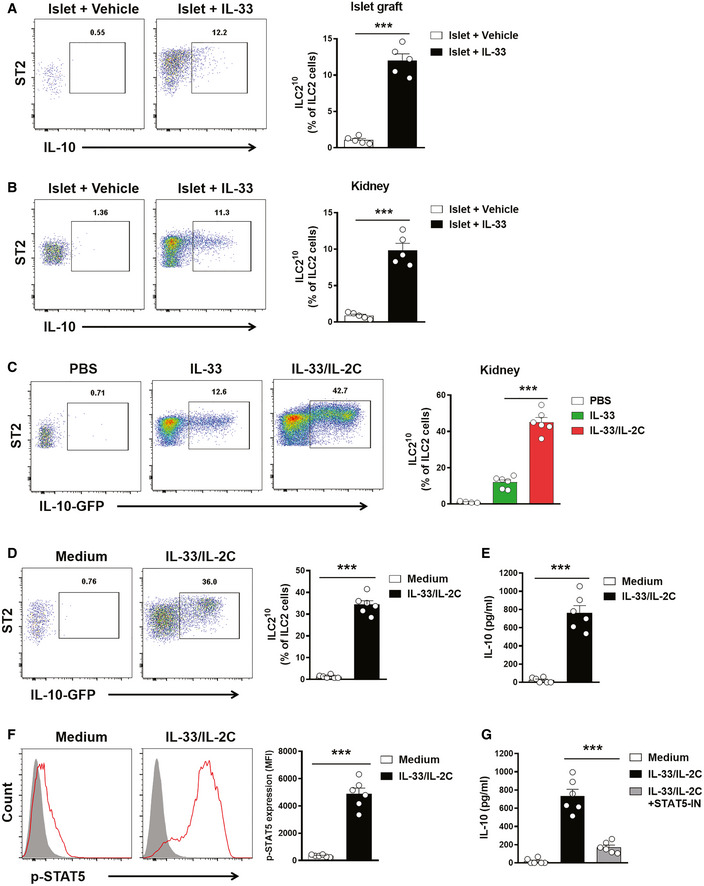
-
A, BILC210 and non‐IL-10 producing ILC2s were assessed in islet graft (A) and kidney (B) by intracellular IL‐10 staining at day 5 post‐islet transplantation. Data shown are the mean ± SEM (n = 5 per group), and an unpaired t‐test was performed; ***P < 0.001. ST2: suppression of tumorigenicity 2.
-
CIL‐10 reporter C57BL/6 mice were treated with PBS, IL‐33 alone or IL‐33 and IL‐2C daily for 5 consecutive days. Proportion of lin‐CD127+ST2+IL‐10-GFP ILC210 from the kidneys at day 3 after treatment in IL‐10 reporter C57BL/6 mice. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
-
DKidney ILC2s isolated from normal IL‐10 reporter C57BL/6 mice were cultured with medium only or medium with IL‐33 and IL‐2C for 6 days. Proportion of ILC210 was assessed by flow cytometry. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001. ST2: suppression of tumorigenicity 2.
-
EThe secreted cytokine IL‐10 was analyzed via ELISA. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001.
-
FKidney ILC2s isolated from C57BL/6 mice were cultured with medium only or medium with IL‐33 and IL‐2C for 30 min. The expression of phosphorylated STAT5 was examined in kidney ILC2s by flow cytometry. p‐STAT5 (red lines) and isotype controls (gray‐filled areas) are shown. Data represent the mean ± SEM of evaluations of MFI from each group (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001. MFI, mean fluorescence intensity.
-
GThe kidney ILC2s were cultured with medium, IL‐33/IL‐2C, or IL-33/IL-2C and STAT5 inhibitor (STAT5‐IN) for 3 days. The secreted cytokine IL‐10 was analyzed via ELISA. Data shown are the mean ± SEM (n = 6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
ILC210 prolonged islet graft survival in an IL‐10‐dependent manner
ILC210 produced large amounts of IL‐10, an anti‐inflammatory cytokine, which has been demonstrated to suppress graft rejection in different transplant models (Gagliani et al, 2010; Yi et al, 2012). To assess the importance of IL‐10 for ILC210 cell function in vivo, we deleted IL‐10 in ILC210 using CRISPR‐Cas9. ILC210 transfected with control empty vector produced a large amount of IL‐10 in the supernatant, whereas ILC210 transfected with IL‐10 CRISPR‐Cas9 did not (Fig 6A). We then adoptively transferred ILC210, IL‐10‐deleted ILC210, and non‐ILC210 into islet transplant C57BL/6 mice (Fig 6B). Fluorescently labeled ILC210, IL‐10‐deleted ILC210, and non‐ILC210 were observed in islet grafts of islet transplant mice, and there was no difference in the number of transfused ILC2s among the three groups (Fig 6C). The expression of IL‐10 in islet graft was significantly increased in mice transfused with ILC210, but not in mice transfused with IL‐10‐deleted ILC210 or non‐ILC210 (Fig 6D). There was no difference in IL‐10 levels in plasma among the four groups (data not shown). ILC210 treatment significantly prolonged islet allograft survival, whereas non‐ILC210 and IL‐10‐deleted ILC210 did not provide protection (Fig 6E). These results indicated that ILC210 prolong islet allograft survival via production of IL‐10. The immunosuppressive effect of ILC210 on CD4 T cells was assessed by co‐culture in vitro. ILC210 effectively suppressed allogeneic splenocyte‐induced CD4 T‐cell proliferation in a dose‐dependent manner, and neutralizing anti‐IL‐10 antibody or genetic ablation of IL‐10 diminished the suppressive role of ILC210 on CD4 T‐cell proliferation (Fig 6F and G). Therefore, these results demonstrated that IL‐10 is an important mediator in ILC210‐mediated islet allograft survival.
Figure 6. Transfused ILC210 suppressed islet rejection via IL‐10.
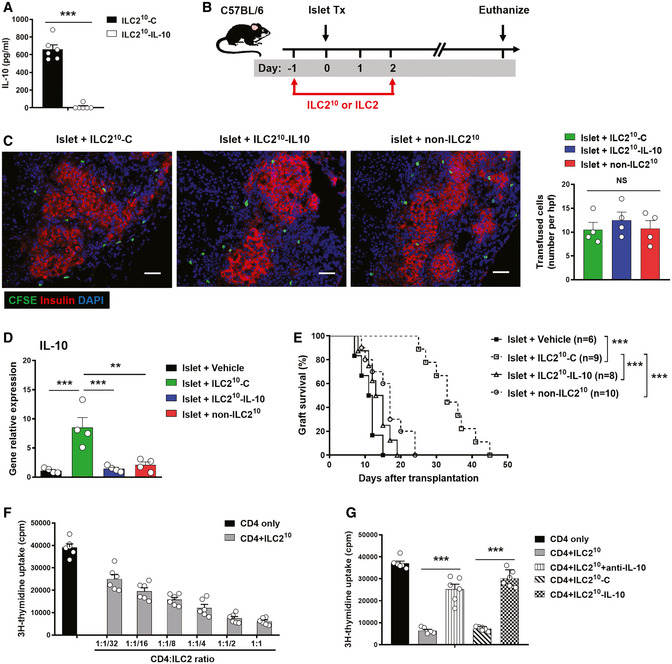
-
AILC210 and non‐ILC210 were isolated from ex vivo‐expanded kidney ILC2 by flow sorting. ILC210 were transfected with control (ILC210‐C) or IL‐10 CRISPR‐Cas9 (ILC210‐IL-10). IL‐10 was measured in culture supernatant of ILC210‐C and ILC210‐IL-10 via ELISA. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001.
-
BTransfected ILC210‐C and ILC210‐IL-10, and non‐ILC210 were adoptively transferred into diabetic C57BL/6 mice twice at 1 day prior to and 2 days post‐islet transplantation. Mice were sacrificed at the day when grafts were considered rejected.
-
CTransfused ILC2s (CFSE labeled, Green) were observed in islet graft at day 5 post‐islet transplantation. Scale bar = 50 μm. The numbers of CFSE‐labeled ILC2s in the islet graft were counted. Data shown are the mean ± SEM, and a one‐way ANOVA was performed (n = 4 per group). NS, non‐significant.
-
DThe mRNA expression of IL‐10 in islet grafts at day 5 post‐islet transplantation was examined by qPCR. Data shown are the mean ± SEM (n = 4 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.
-
EIslet graft survival of four groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001.
-
FCD4+ T cells isolated from C57BL/6 splenocytes were cultured with irradiated splenocytes derived from BALB/c in the presence of ILC210 at the indicated ratios for 4 days. CD4+ T‐cell proliferation was assessed using [3H]‐thymidine incorporation assays. Data shown are the mean ± SEM (n = 4–6 per group).
-
GNeutralizing anti‐IL-10 antibodies or genetic ablation of IL‐10 were used to block the effect of ILC210 on CD4+ T‐cell proliferation. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed. ***P < 0.001.
Co‐transplantation of ILC210 with islet prolonged allograft survival
We next determined whether migration to islet allografts was important for ILC210 cell effector function. To explore this, we compared the protective effects of intravenous and local transfer of ILC210 cells on islet rejection (Fig 7A). Intravenous transfer of 2 × 106 ILC210 cells modestly prolonged graft survival from 10.4 ± 2.5 days to 36.3 ± 4.5 days, whereas locally transferred 1 × 106 ILC210 cells led to long‐term islet graft survival—37.5% (n = 3 out of 8) of the mice retained their grafts for more than 80 days. Local transfer of 1 × 106 ILC210 cells and 2 × 105 ILC210 cells exhibited similar islet allograft protection, whereas local transfer IL‐10‐deleted ILC210 did not prolong islet graft survival (Fig 7B). Furthermore, we locally transferred CD45.2+ ILC210 cells into islet transplanted congenic C57BL/6 mice (CD45.1+) and examined their frequency in islet graft over time. The locally transferred CD45.2+ST2+ILC210 were detected in islet graft at day 5 post‐islet transplantation, and their proportion and numbers in islet graft were significantly reduced at the day when grafts were rejected or at day 80 post‐islet transplantation (Fig 7C–E). The proportion of CD45.2+ST2+ILC210 in accepted islet graft (surviving for 80 days post‐islet transplantation) was higher than that in rejected islet graft, but there was no difference in the number of CD45.2+ST2+ILC210 in rejected islet graft and in accepted islet graft (surviving for 80 days post‐islet transplantation) (Fig 7D and E), suggesting that the occurrence of islet rejection is not because the number of ILC2 reduces in the graft over time. Phenotypic alteration of ILC2 has been reported in various inflammatory disease conditions (Ohne et al, 2016; Golebski et al, 2019); here, we found that the expression of IL‐10, IL‐5, and IL‐13 by locally transferred ILC210 (Fig 7F and Appendix Fig S5) was markedly reduced in mice with islet graft rejection in comparison with that in mice that retained their islet graft for 80 days. Phenotypic change in ILC210 could possibly explain why locally transferred ILC210 did not lead to long‐term islet graft survival in 5 out of 9 islet transplanted mice. There results suggested that ILC210 cells were required within the allograft for maximal suppressive effect and graft protection.
Figure 7. Co‐transplantation of IL‐210 with islet prolonged allograft survival.
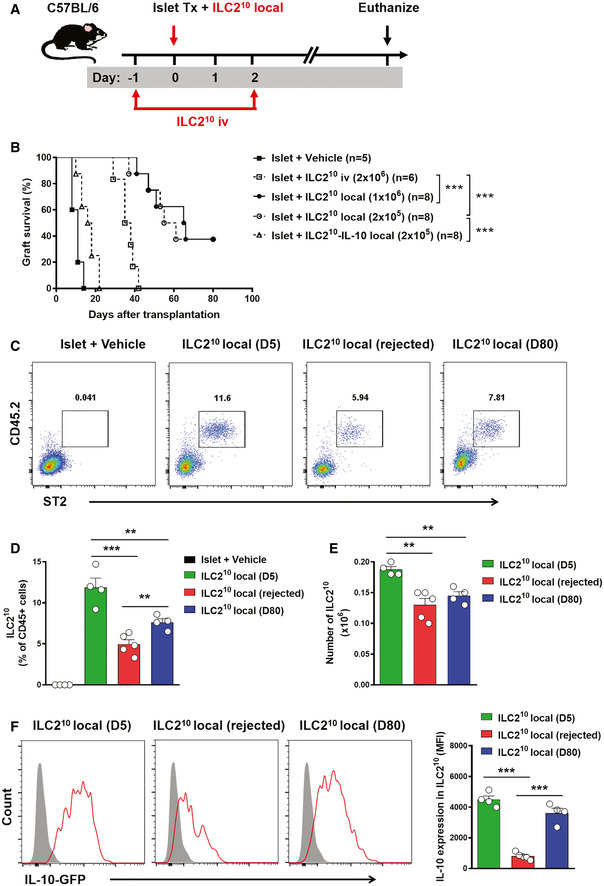
-
AILC210 were isolated from ex vivo‐expanded kidney ILC2s by flow sorting. ILC210 were co‐transplanted with islet locally or adoptively transferred into diabetic C57BL/6 mice twice at 1 day prior to and 2 days post‐islet transplantation. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected.
-
BIslet graft survival of five groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001.
-
CTo track ILC210 in vivo, CD45.2+ILC210 were co‐transplanted with islets in diabetic CD45.1+C57BL/6 mice. Representative FACS analysis showing the proportion of locally transplanted ILC210 (CD45.2+ST2+) in the total CD45+ cell compartment from islet graft at day 5 and 80 post‐islet transplantation or at the day when grafts were considered rejected.
-
D, EProportion and numbers of locally transplanted CD45.2+ST2+ILC210 in islet grafts of islet transplant mice over time. Data shown are the mean ± SEM (n = 4–5 per group), and a one‐way ANOVA was performed, **P < 0.01, ***P < 0.001.
-
FThe expression of IL‐10-GFP was examined in locally transplanted CD45.2+ST2+ILC210 by flow cytometry. IL‐10-GFP (red lines) and controls (gray‐filled areas) are shown. Data represent the mean ± SEM of evaluations of MFI from each group (n = 4–5 per group), and a one‐way ANOVA was performed; ***P < 0.001. MFI, mean fluorescence intensity.
Discussion
A growing body of evidence suggests ILC2s play immune regulatory roles in acute and chronic inflammatory diseases (Rak et al, 2016; Dalmas et al, 2017; Gadani et al, 2017; Cao et al, 2018; Krabbendam et al, 2018), whereas their possible importance in suppressing allograft rejection is unclear. Here, we found that short‐term IL‐33 treatment significantly prolonged islet allograft survival in STZ‐induced type 1 diabetic mice. The mechanisms underlying the protective effect of IL‐33 are associated with its initiation of suppressing alloimmune responses through expansion of ILC2s and Tregs. Furthermore, two functional subpopulations of ILC2s were identified in islet grafts of IL‐33‐treated mice. IL‐10 producing ILC2s (ILC210) were demonstrated to play a critical role in IL‐33‐mediated islet allograft protection.
IL‑33 is a novel member of the IL‐1 cytokine family and acts as a dual‐function molecule, namely as a nuclear gene regulator and extracellular cytokine (Molofsky et al, 2015; Martin & Martin, 2016). IL‐33 plays an anti‐inflammatory role in atherosclerosis, cutaneous wound healing, intestinal, and adipose tissue inflammation via expansion of Tregs (Miller et al, 2008; Schiering et al, 2014; Brestoff et al, 2015; Monticelli et al, 2015; Rak et al, 2016). Recent reports have highlighted that Tregs contribute to the protective effect of IL‐33 in models of cardiac allograft rejection and graft‐versus‐host disease (Turnquist et al, 2011; Matta et al, 2016). In this study, we demonstrated for the first time that IL‐33 promoted long‐term islet allograft survival in a fully MHC mismatched murine islet transplantation model. Tregs were significantly increased in spleen, kidney, and allograft of IL‐33‐treated mice. Tregs exhibit potent anti‐inflammatory properties and play an immunosuppressive role in transplant rejection (Issa et al, 2010; Yi et al, 2012). As expected, an increase in Tregs contributes to IL‐33‐mediated allograft survival, which is consistent with previous studies showing that ex vivo‐expanded Tregs can prolong islet allograft survival (Shi et al, 2012; Yi et al, 2012). Furthermore, ILC2s were shown to be crucial for the function of IL‐33 in promoting islet allograft survival, as additional ILC2 depletion in Treg‐depleted DEREG mice counteracted any therapeutic effect of the IL‐33 treatment. We also examined the spatial and temporal distribution of Tregs and ILC2s induced by IL‐33 treatment. A short course of IL‐33 treatment induced a sustained increase in ILC2s in kidney and islet graft, but only a short‐term increase in Treg in those tissues, suggesting ILC2s accumulated in islet allografts to play critical roles in IL‐33‐mediated islet graft protection. Interesting, the reason why ILC2s stay longer than Tregs may be related to preservation of a high concentration of IL‐33 in islet graft that extends the life of ILC2s. Another potential mechanism of IL‐33‐mediated islet graft protection is associated with induction of a Th1‐to‐Th2 switch in the periphery and allograft, which has been shown to contribute to IL‐33‐mediated cardiac allograft survival (Turnquist et al, 2011).
In further exploring diversity of ILC2 lineage, we found that ILC2s can be divided into two subsets: one producing IL‐10 (ILC210) and another not producing IL‐10 (non‐ILC210). An important finding in the current study is that ILC210, but not non‐ILC210, play a critical role in islet allograft survival. We have previously reported that IL‐33 induced significant expansion of ILC2s in kidney, which play a protective role in renal IRI (Huang et al, 2015; Cao et al, 2018). As ILC2s expressed high level of receptors for IL‐2 and IL‐33, we examined whether the combination of IL‐2/anti‐IL‐2 antibody complex (IL‐2C) and IL‐33 treatment would influence generation of ILC210 in vivo. We found that the administration of IL‐2C and IL‐33 significantly increased the ILC210 population in kidney, which is consistent with an earlier report in lung (Seehus et al, 2017). IL‐10 is a well‐characterized anti‐inflammatory cytokine and plays a central role in controlling inflammation and suppressing alloimmune responses after transplantation (Hara et al, 2001; Ouyang & O'Garra, 2019). The importance of IL‐10 in Treg or Tr1‐mediated suppression of alloimmune responses has been demonstrated in many transplantation models (Gagliani et al, 2010; Yi et al, 2012). ILC210 produced large amounts of the anti‐inflammatory cytokine IL‐10, which could suppress the alloimmune response to allogeneic islets. This is supported by our data showing that ILC210 effectively suppressed allogeneic splenocyte‐induced CD4 T‐cell proliferation via secretion of IL‐10 and transfused ILC210 prolonged islet graft survival in an IL‐10‐dependent manner. Moreover, another important finding is that local transfer of ILC210 together with islet exhibited a stronger protective effect on allogeneic islets than systemic transfer of ILC210 cells. Also, the phenotypic stability of locally transferred ILC210 is an important factor in determining the fate of islet grafts. Similar to our findings, Treg migration from blood to the islet allograft was necessary for the suppression of alloimmunity (Zhang et al, 2009). These results indicate that ILC210 cells are required within the allograft in order to exert maximal suppressive effect and graft protection. This also suggests that ILC210 can efficiently suppress alloimmune response at the site of the inflamed allograft. Furthermore, mounting evidence has shown that ILC2s directly promote would healing in models of respiratory infection, acute kidney injury, and intestinal inflammation via producing Areg (Monticelli et al, 2011, 2015; Cao et al, 2018), which allows a better re‐epithelialization of the tissue. These observations are very important in the field of transplantation, where correct repair of damaged tissue is essential for proper function of the allograft. This could be another important mechanism associated with ILC2‐mediated allograft protection, which needs to be further investigated.
In conclusion, this study has revealed a strong protective effect of IL‐33 on the survival of islet allografts. The allograft protective effect of IL‐33 depends on its ability to drive expansion of ILC2s and Tregs in vivo. Furthermore, this study demonstrates the importance of IL‐10 in ILC2‐mediated islet graft protection. Migration of ILC210 cells into the islet graft was necessary for maximal suppressive effect and graft protection. Local delivery of ILC210 could be a promising tool to promote long‐term islet graft survival. Therefore, this study suggests the strategy of using IL‐33 and ILC210 as adjunctive therapy to prevent allograft rejection, bringing new therapeutics to the transplantation field.
Materials and Methods
Mice
C57BL/6 (CD45.2+), congenic C57BL/6 (CD45.1+), and BALB/c mice were purchased from the Animal Resources Centre (Perth, Australia) and Shanghai Laboratory Animal Center of Chinese Academy of Science. DEREG mice (C57BL/6‐Tg23.2Spar/Mmjax) (Lahl et al, 2007) and IL‐10‐GFP mice (B6.129S6‐Il10tm1FlvJ) (Kamanaka et al, 2006) were obtained from Jackson Lab and bred at the Department of Animal Care at Xinxiang Medical University (XMU) and Westmead Hospital Animal House. For all studies, adult (8–10 weeks of age) male mice were used in accordance with the animal care and use protocols approved by Animal Ethics Committee of XMU or Western Sydney Local Health District (WSLHD).
Islets transplantation and IL‐33 treatment
C57BL/6 recipient mice were rendered diabetic using a single i.v. injection of streptozotocin (185 mg/kg). Mice with a blood glucose value > 16 mmol/l were selected as transplant recipients. Pancreatic islets were separated from the pancreata of donor (BALB/c) mice at a ratio of four pancreata per recipient. Briefly, islets were isolated from adult BALB/c mice by pancreas perfusion using Liberase TL Enzyme (Roche), digestion, and purification by Ficoll (Sigma) discontinuous gradient centrifugation. The interface between 1.081 and 1.037 was the separated islet cells. Islet transplantation was performed as previously described (Szot et al, 2007), kidney was exposed via a small incision in the peritoneum, a small nick was made in the kidney capsule at the inferior renal pole, and the islets (2,000 IEQ/recipient mouse) were deposited through the nick toward the superior pole of the kidney. Graft rejection was defined as a rise in blood glucose above > 16 mmol/l for two consecutive days after a period of euglycemia. Nephrectomy was performed at day 80 post‐islet transplantation to determine whether the euglycemia was graft dependent.
For IL‐33 treatment, C57BL/6 mice were given 0.3 μg mouse recombinant IL‐33 (BioLegend) intraperitoneally daily for five consecutive days before islet transplantation. The dose and duration were selected according to previous published studies (Monticelli et al, 2015; Cao et al, 2018). Control animals received phosphate‐buffered saline (PBS) only. To deplete Tregs and/or ILC2s, DEREG mice were injected i.p. with diphtheria toxin (DT; 30 mg/kg, Sigma‐Aldrich) and/or anti‐CD25 antibody (PC61, 500 μg/mouse, BioLegend). Tregs and ILC2s were examined in spleen and kidney by flow cytometry. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected after two consecutive BGLs > 16 mmol/l (mM). For STZ‐induced diabetic C57BL/6 mice without islet transplantation, hyperglycemic mice were injected with IL‐33 daily for 5 days (starting from day 6 after STZ injection). Fasting and non‐fasting blood glucose concentrations and survival rate of diabetic mice were monitored for 30 days.
Intraperitoneal glucose tolerance test
Intraperitoneal glucose tolerance test was performed in normal mice, islet transplant mice, and islet transplant mice treated with IL‐33. After 16 h overnight fasting, mice were injected with 1 g/kg body weight of glucose intraperitoneally. Blood glucose was measured at the indicated minutes after glucose injection.
In vitro suppression assay
CD4+ T cells isolated from C57BL/6 splenocytes (responders; 1 × 105 cells/well) were cultured in round‐bottom 96‐well plates with irradiated splenocytes (stimulators; 2 × 105/well) derived from BALB/c in the presence of ILC210 at various ratios for 4 days. Neutralizing anti‐IL‐10 antibodies (10 μg/ml, JES5‐16E3, BioLegend) were used to block the effect of ILC210 on CD4+ T cells proliferation. Cells were pulsed with 1 μCi [3H]thymidine per well for the last 16 h of a 4‐day culture period. Cells were harvested using a Packard Filtermate Harvester 96 and counted by Microbeta counter (PerkinElmer).
CRISPR‐Cas9 transfection
To knock out IL‐10 gene in ILC210, we co‐transfected IL‐10 CRISPR‐Cas9 KO plasmid with an IL‐10 HDR plasmid (Santa Cruz Biotechnology), following the manufacturer's instructions. In brief, ILC210 were transfected with IL‐10 CRISPR‐Cas9 plasmid or its control (sc‐421076) and incubated for 24 h. Media were replaced 24 h post‐transfection. Puromycin antibiotic (2 μg/ml) was added to allow for positive selection of transfected cells. IL‐10 was measured in culture supernatant of ILC210 via enzyme‐linked immunosorbent assay (ELISA). ELISA was performed according to the manufacturer's protocol (eBioscience). Briefly, pre‐coated microtiter plates were blocked at room temperature for 2 h with PBS containing 2% BSA. Each sample and its control were added to adjacent wells and incubated overnight at 4°C. After washing, avidin‐conjugated HRP and tetramethylbenzidine were used for color development. Optical densities were measured using an ELISA reader, and the concentration of cytokine was calculated.
ILC210 cell isolation and administration in islet transplant mice
ILC210 and non‐ILC210 were isolated from kidney ILC2s in IL‐10‐GFP C57BL/6 mice treated with IL‐33 and IL‐2C daily for five consecutive days. To examine in vivo functions of ILC210 in islet transplant mice, ILC210‐C (1 × 106), ILC210‐IL‐10 (1 × 106), and non‐ILC210 (1 × 106) were adoptively transferred into islet transplant C57BL/6 mice. In parallel, these three types of ILC2s labeled with CFSE were transfused into islet transplant C57BL/6 mice. All mice were euthanized at day 5 post‐islet transplantation. The number of CFSE‐labeled ILC2s was quantitated in 4–6 non‐overlapping high power fields of islet allograft. For co‐transplantation experiments, CD45.2+ ILC210 (1 × 106 or 2 × 105/mouse) were co‐transplanted with islets into kidneys of diabetic CD45.2+ or CD45.1+ congenic C57BL/6 mice. The frequency and phenotypes of locally transferred ILC210 were examined at days 5 and 80 post‐islet transplantation or at the day when grafts were considered rejected. Graft function was monitored using blood glucose measurements.
IL‐33 and IL‐2/IL‐2Ab complex administration
IL‐2/anti‐IL‐2 mAb (JES6‐1) complexes (IL‐2C) were prepared as previously reported (Polhill et al, 2012). IL‐33 (0.3 μg) alone or IL‐33 (0.3 μg) and IL‐2C (1 μg/5 μg) was administered intraperitoneally to IL‐10‐GFP C57BL/6 for five consecutive days. Frequency of ILC2 and ILC210 in kidneys was analyzed by flow cytometry at day 3 post‐administration.
ILC210 induction in vitro
Kidney ILC2s (2 × 104/well) isolated from normal IL‐10‐GFP reporter mice were cultured in round‐bottom 96‐well plates with RPMI 1640 medium containing IL‐7 (20 ng/ml), IL‐33 (50 ng/ml), and IL‐2C (20 ng/ml of IL‐2; 100 ng/ml of anti‐IL‐2) for 6 days. Proportion of ILC210 was assessed by flow cytometry. In parallel, kidney ILC2s isolated from C57BL/6 mice were cultured with IL‐33 and IL‐2C for 30 min. The expression of phosphorylated STAT5 was examined by flow cytometry. STAT5 inhibitor (50 μM, CAS 285986‐31‐4, Sigma) was used to suppress IL‐10 production in ILC2s. The secreted cytokine IL‐10 in culture supernatants was analyzed via ELISA.
Cell suspension preparation
Spleen was isolated and digested with collagenase D (1 mg/ml, Roche) and DNase I (100 μg/ml, Roche) for 30 min at 37°C. Kidney was perfused with saline before removal and digested with collagenase IV (1 mg/ml, Sigma) and DNase I (100 μg/ml, Roche) for 40 min at 37°C as previously described (Cao et al, 2015). The digested cell suspensions were then passed through a 40‐μm cell strainer. Tregs and ILC2s in single‐cell suspensions from spleen and kidney were analyzed by flow cytometry.
Flow cytometry and cell sorting
For FACS analysis of different organ samples, single‐cell suspensions were stained with Fc block/anti‐CD16/32 (1:200; 2.4G2) and antibodies to CD45.2 (1:100; 104), CD127 (1:100; A7R34), GATA3 (1:40; TWAJ), ST2 (1:50; RMST2‐2), as well as antibodies to immune cell lineages (referred as lin): CD3 (1:100; 145‐2C11), CD5 (1:200; 53‐7.3), TCRβ (1:100; H57‐597), TCRγδ (1:100; eBioGL3), CD19 (1:100; 1D3), B220 (1:100; RA3‐6B2), CD49b (1:100; DX5), CD11b (1:200; M1/70), CD11c (1:100; N418), FcεRIα (1:100; MAR‐1), Gr‐1 (1:200; RB6‐8C5), and Ter‐119 (1:100). Other antibodies used in this study included those against CD4 (1:100; GK1.5), Foxp3 (1:50; FJK‐16s), CD25 (1:100; PC61), IL‐10 (1:50; JES5‐16E3), phospho STAT5 (1:100; SRBCZX), and corresponding isotype controls, all purchased from eBioscience or BioLegend. Cells were analyzed on an LSR Fortessa flow cytometer. For FACS sorting, single‐cell suspensions were pregated on hematopoietic cells using anti‐CD45.2 antibody, then lineage markers were used to exclude immune cells, and DAPI was used to exclude dead cells. ILC2 [CD45+Lin(−)CD127+ST2+], ILC210 [CD45+Lin(−)CD127+ST2+IL‐10‐GFP], or non‐ILC210 [CD45+Lin(−)CD127+ST2+IL‐10(−)] were sorted using a FACSAria II (BD).
Quantitative PCR
Total RNA was isolated from allograft by RNeasy Mini Kit (Qiagen) and then reverse‐transcribed with First Strand cDNA Synthesis Kit (Fermentas). Real‐time PCR was performed on the CFX384 (Bio‐Rad) using the SYBR mastermix (Invitrogen). The analysis method was as described before, and the PCR primer sequences are presented in Appendix Table S1.
Immunohistochemistry and immunofluorescence
Immunohistochemistry of insulin staining was performed as described previously. Briefly, paraffin sections were stained for insulin using polyclonal guinea pig anti‐insulin (Dako). The secondary antibody, HRP‐conjugated rabbit anti‐guinea pig (Dako), was used to detect insulin. Sections were visualized with diaminobenzidine and were counterstained with hematoxylin.
For immunofluorescence staining of ILC2s, frozen sections were stained with polyclonal rabbit anti‐mouse CD127 (1:200; Thermofisher), rat anti‐mouse ST2 (1:20; RMST2‐2), hamster anti‐mouse CD3e (1:100; 145‐2C11), and polyclonal guinea pig anti‐insulin (1:100; Dako) antibodies, and then incubated with the secondary antibodies, AF488 goat anti‐rabbit IgG, AF546 goat anti‐rat IgG, and AF647 goat anti‐hamster IgG and AF405 goat anti‐guinea pig. For immunofluorescence staining of Tregs, rabbit monoclonal anti‐mouse CD4 (1:50; EPR19514), rat anti‐mouse Foxp3 (1:50; FJK‐16s) and polyclonal guinea pig anti‐insulin (Dako) was used as the primary antibody and AF488 goat anti‐rabbit IgG, AF546 goat anti‐rat IgG, and AF405 goat anti‐guinea pig as the secondary antibodies. Control rabbit, rat, hamster, and guinea pig IgG to primary antibodies were included in staining. The sections were viewed under an Olympus FV1000 (Olympus). The numbers of ILC2s [CD3(−)CD127+ST2+] and Tregs [CD4+Foxp3+] were quantitated in 4–6 non‐overlapping high power fields of islet allograft. The numbers of ILC2s [CD3(−)CD127+ST2+] were quantitated in 8–10 non‐overlapping high power fields of liver, lung, and kidney.
Statistics
Statistical tests included unpaired, two‐tailed Student's t‐test using Welch's correction for unequal variances and one‐way ANOVA with Tukey's multiple comparison test. Graft survival was analyzed using the Kaplan–Meier method, and survival curves were compared using the log‐rank test. The animals were randomized according to their body weight and blood glucose before starting therapy to avoid any bias and analysis was blinded. Statistical analyses were performed using Prism (Version 7, GraphPad). The exact P‐values are listed in Appendix Table S2. Results are expressed as the mean ± SEM. A P < 0.05 was considered statistically significant.
Author contributions
QC, WW, and DCHH designed and supervised the study. QH, XM, YW, ZN, RW, GL, and MW performed animal experiments and in vitro experiments, and analyzed data; QH, XM, RW, YW, ZN, and FY contributed to analysis of islet transplantation model. PR, HW, and WW provided reagents and expertise; QC, WW, HW, YW, and DCHH participated in discussions, provided intellectual input, and wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
For more information
Dr. Qi Cao's website: https://www.westmeadinstitute.org.au/research/research-divisions/infection-and-immunity/renal-inflammation-and-immunology-group/overview
Supporting information
Appendix
Review Process File
Acknowledgements
We thank the WSLHD and XMU animal facilities for their conscientious care of mice. This work was supported by the National Natural Science Foundation of China grants U1804167 (to Q.H., and Z.N.), 81570624 (to Q.C., Y.W., and Z.N.), 81770721 (to Q.C., and Q.H.), and 81671752 (to W.W., and X.M.); and the National Health & Medical Research Council of Australia grants 1141330 (to Y.W., Q.C., and D.H.) and 1146156 (to Q.C.).
EMBO Mol Med (2020) 12: e12305
Contributor Information
Wei Wang, Email: cjr.wangwei@vip.163.com.
Qi Cao, Email: qi.cao@sydney.edu.au.
Data availability
Additional data are provided in the Appendix and are available online.
References
- Anazawa T, Okajima H, Masui T, Uemoto S (2019) Current state and future evolution of pancreatic islet transplantation. Ann Gastroenterol Surg 3: 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P et al (2015) Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519: 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack AL, Martinov T, Fife BT (2017) T cell‐mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne) 8: 343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang Y, Wang XM, Lu J, Lee VW, Ye Q, Nguyen H, Zheng G, Zhao Y, Alexander SI et al (2015) Renal F4/80+CD11c+ mononuclear phagocytes display phenotypic and functional characteristics of macrophages in health and in adriamycin nephropathy. J Am Soc Nephrol 26: 349–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang Y, Niu Z, Wang C, Wang R, Zhang Z, Chen T, Wang XM, Li Q, Lee VWS et al (2018) Potentiating tissue‐resident type 2 innate lymphoid cells by IL‐33 to prevent renal ischemia‐reperfusion injury. J Am Soc Nephrol 29: 961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang R, Wang Y, Niu Z, Chen T, Wang C, Jin L, Huang Q, Li Q, Wang XM et al (2020) Regulatory innate lymphoid cells suppress innate immunity and reduce renal ischemia/reperfusion injury. Kidney Int 97: 130–142 [DOI] [PubMed] [Google Scholar]
- Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, Stawiski M, Traunecker E, Lucchini FC, Dapito DH et al (2017) Interleukin‐33‐activated islet‐resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity 47: 928–942 [DOI] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, McKenzie AN (2015) Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348: aaa6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J (2017) Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med 214: 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, Battaglia M (2010) Antigen‐specific dependence of Tr1‐cell therapy in preclinical models of islet transplant. Diabetes 59: 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibly RF, Graham JG, Luo X, Lowe WL Jr, Hering BJ, Shea LD (2011) Advancing islet transplantation: from engraftment to the immune response. Diabetologia 54: 2494–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, Kradolfer CMA, Shikhagaie MM, Seys S, Hellings PW et al (2019) IL‐1beta, IL‐23, and TGF‐beta drive plasticity of human ILC2s towards IL‐17‐producing ILCs in nasal inflammation. Nat Commun 10: 2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ (2001) IL‐10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo . J Immunol 166: 3789–3796 [DOI] [PubMed] [Google Scholar]
- Huang Q, Niu Z, Tan J, Yang J, Liu Y, Ma H, Lee VW, Sun S, Song X, Guo M et al (2015) IL‐25 elicits innate lymphoid cells and multipotent progenitor type 2 cells that reduce renal ischemic/reperfusion injury. J Am Soc Nephrol 26: 2199–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K (2010) Ex vivo‐expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation 90: 1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara‐Tejero M, Galan JE, Harhaj E, Flavell RA (2006) Expression of interleukin‐10 in intestinal lymphocytes detected by an interleukin‐10 reporter knockin tiger mouse. Immunity 25: 941–952 [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Bal SM, Spits H, Golebski K (2018) New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol Rev 286: 74–85 [DOI] [PubMed] [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T (2007) Selective depletion of Foxp3 + regulatory T cells induces a scurfy‐like disease. J Exp Med 204: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AJ, Hoeppli RE, Levings MK (2017) Harnessing advances in T regulatory cell biology for cellular therapy in transplantation. Transplantation 101: 2277–2287 [DOI] [PubMed] [Google Scholar]
- Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, Wang Y, Lee VW, Zheng G, Tan TK et al (2013) Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int 84: 745–755 [DOI] [PubMed] [Google Scholar]
- Martin NT, Martin MU (2016) Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 17: 122–131 [DOI] [PubMed] [Google Scholar]
- Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ et al (2016) Peri‐alloHCT IL‐33 administration expands recipient T‐regulatory cells that protect mice against acute GVHD. Blood 128: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY (2008) IL‐33 reduces the development of atherosclerosis. J Exp Med 205: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Savage AK, Locksley RM (2015) Interleukin‐33 in tissue homeostasis, injury, and inflammation. Immunity 42: 1005–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T et al (2011) Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D (2015) IL‐33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin‐EGFR interactions. Proc Natl Acad Sci USA 112: 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohne Y, Silver JS, Thompson‐Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu YJ (2016) IL‐1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol 17: 646–655 [DOI] [PubMed] [Google Scholar]
- Ouyang W, O'Garra A (2019) IL‐10 family cytokines IL‐10 and IL‐22: from basic science to clinical translation. Immunity 50: 871–891 [DOI] [PubMed] [Google Scholar]
- Paschou SA, Papadopoulou‐Marketou N, Chrousos GP, Kanaka‐Gantenbein C (2018) On type 1 diabetes mellitus pathogenesis. Endocr Connect 7: R38–R46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhill T, Zhang GY, Hu M, Sawyer A, Zhou JJ, Saito M, Webster KE, Wang Y, Wang Y, Grey ST et al (2012) IL‐2/IL‐2Ab complexes induce regulatory T cell expansion and protect against proteinuric CKD. J Am Soc Nephrol 23: 1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, Artis D, Volk SW (2016) IL‐33‐dependent Group 2 innate lymphoid cells promote cutaneous wound healing. J Invest Dermatol 136: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Tay SS, Mitchell AJ, Bolton HA, Guy TV, Tan SY, Forbes‐Blom E, Tong PL, Koller Y et al (2015) IL‐2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol 136: 1653–1663 [DOI] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN et al (2014) The alarmin IL‐33 promotes regulatory T‐cell function in the intestine. Nature 513: 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, Kaye J (2017) Alternative activation generates IL‐10 producing type 2 innate lymphoid cells. Nat Commun 8: 1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Lees JR, Scott DW, Farber DL, Bartlett ST (2012) Endogenous expansion of regulatory T cells leads to long‐term islet graft survival in diabetic NOD mice. Am J Transplant 12: 1124–1132 [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Artis D (2015) Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med 21: 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot GL, Koudria P, Bluestone JA (2007) Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp 9: 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX et al (2011) IL‐33 expands suppressive CD11b+ Gr‐1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell‐dependent promotion of cardiac allograft survival. J Immunol 187: 4598–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE et al (2018) Innate lymphoid cells: 10 years on. Cell 174: 1054–1066 [DOI] [PubMed] [Google Scholar]
- Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, Du Y, Tian Y, Yin Z, Xu Z et al (2017) Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 171: 201–216 [DOI] [PubMed] [Google Scholar]
- Yi S, Ji M, Wu J, Ma X, Phillips P, Hawthorne WJ, O'Connell PJ (2012) Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin‐10 in humanized mice. Diabetes 61: 1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y et al (2009) Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 30: 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Review Process File
Data Availability Statement
Additional data are provided in the Appendix and are available online.


