Abstract
Objective
No data are available to develop uniform recommendations for reperfusion therapies in ST-segment elevation myocardial infarction (STEMI) during the coronavirus disease 2019 (COVID-19) pandemic. We aimed to fill the evidence gap regarding STEMI reperfusion strategy during the COVID-19 era.
Methods
Clinical characteristics and outcomes for 17 patients with STEMI who received fibrinolysis during the COVID-19 pandemic were compared with 20 patients who received primary percutaneous coronary intervention (PPCI), and were further compared with another 41 patients who received PPCI in the pre-COVID-19 period.
Results
In patients with STEMI, fibrinolysis achieved a comparable in-hospital and 30-day primary composite end point, as compared with those who received PPCI during the COVID-19 pandemic. No major bleeding was detected in either group. Compared patients with STEMI who received PPCI in the pre-COVID-19 period, we found a remarkable extension of chest pain onset-to-first medical contact (FMC) and FMC-to-wire crossing times, significantly increased number and length of stents, and much worse thrombolysis in myocardial infarction flow in patients with STEMI who received PPCI during the COVID-19 pandemic.
Conclusion
Owing to its considerable efficacy and safety and advantages in conserving medical resources, we recommend fibrinolysis as a reasonable alternative for STEMI care during the COVID-19 pandemic.
Keywords: Coronavirus disease 2019, ST-segment elevation myocardial infarction, fibrinolysis, primary percutaneous coronary intervention, safety, efficacy
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which emerged in December 2019.1,2 This novel coronavirus spreads extremely fast and has led to the global COVID-19 pandemic. According to World Health Organization data, up to 18 September 2020, this virus has infected up to 30,000,000 individuals and caused more than 950,000 deaths worldwide. The current mortality rate ranges from 2% to 5% of all patients with COVID-19.3,4 During the initial COVID-19 outbreak in China, Wuhan was the worst-affected city. The local government and hospitals responded quickly to the outbreak and implemented a series of strategies to contain the spread of SARS-CoV-2. These stringent infection control measures effectively limited local contagion. However, the health care system in Wuhan has been seriously affected in unexpected ways.
ST-segment elevation myocardial infarction (STEMI) is one of the most life-threatening cardiovascular emergencies, with high mortality and morbidity worldwide. STEMI requires an expeditious diagnosis and treatment, thus making adequate STEMI care one of the greatest unmet needs in the field of cardiology during the COVID-19 pandemic. With the goal of restoring patency of the infarct-related artery (IRA) as well as reducing the morbidity and mortality of STEMI, both fibrinolytic therapy and primary percutaneous coronary intervention (PPCI) are well-established reperfusion strategies for patients with symptoms of ischemia of ≤12 hours duration and persistent ST-segment elevation.5 As the superiority of PPCI over fibrinolytic therapy has been shown in many studies, PPCI is the preferred strategy recommended in the main guidelines when revascularization is feasible soon after the first medical contact (FMC).5,6 Notably, the strategy of reperfusion is determined according to the ischemia duration and medical resources, especially during the COVID-19 pandemic.
Mechanical reperfusion with PPCI should be performed by an experienced team, including not only interventional cardiologists but also skilled support staff. However, as the pandemic of COVID-19 seriously overwhelmed the clinical workforce and medical supplies, especially in Wuhan, the delivery of PPCI for patients with STEMI presented numerous challenges. The optimal therapeutic approach for STEMI care during the COVID-19 pandemic is currently under debate. Cardiovascular societies in different countries have come out with guidelines in this regard. In China, fibrinolytic therapy is recommended as the primary therapeutic option for treating patients with STEMI,7,8 although globally, the prevailing preference remains continuing a PPCI approach.9–11
To date, there is no available evidence regarding the management of reperfusion strategies in the face of an ongoing infectious disease pandemic. In this retrospective analysis, we aimed to compare the outcomes of two separate groups, patients who underwent fibrinolysis and a PPCI strategy, in Wuhan during the COVID-19 pandemic, to investigate the optimized reperfusion strategy in STEMI care. In this study, we enrolled 41 consecutive patients who underwent PPCI prior to the COVID-19 outbreak and compared their pre-procedural, procedural, and post-procedural characteristics.
Methods
Study design
The study population comprised contemporary patients diagnosed with STEMI at two hospitals in Wuhan, China, during the COVID-19 period (from 23 January to 20 March 2020), and another consecutive group of patients with STEMI who received PPCI during the pre-COVID-19 period (from 1 September to 20 December 2019) at one of the centers. This study was approved by the Ethics Committee of Union Jiangbei Hospital and People's Hospital of Dongxihu District. Data were collected after obtaining informed consent from the patients. Both centers were PCI-capable sites in Wuhan. People's Hospital of Dongxihu District continued to execute PPCI for STEMI care even during the COVID-19 outbreak, with the help of the local government. Therefore, all patients receiving PPCI in this study were recruited from this hospital. During the COVID-19 outbreak, all patients with STEMI were treated as suspected COVID-19 cases until they were confirmed to be free of SARS-CoV-2 infection. This study consisted of three phases: baseline assessment, in-hospital assessment, and 30-day follow-up after reperfusion therapy. Furthermore, we recorded and analyzed the key time points as well as pre-procedural, procedural, and post-procedural characteristics of patients receiving PPCI treatment.
Patients and medical care
Because Wuhan was the epicenter of the COVID-19 outbreak, all patients requiring hospitalization in Wuhan, even those without fever and/or respiratory symptoms (such as chills, sore throat, or cough) initially underwent COVID-19 screening, which included complete blood count, chest computed tomography (CT) scan, SARS-CoV-2 nucleic acid detection, and/or serum IgM/IgG testing in the emergency department (ED).
STEMI diagnosis is mainly based on chest pain owing to ischemia lasting for at least 30 minutes and electrocardiogram (ECG) presenting ST-segment elevation ≥0.1 mV in at least two frontal plane leads or ≥0.2 mV in at least two precordial leads. The maximum time from the FMC to ECG and STEMI diagnosis should be ≤10 minutes.5 Patients with a suspected initial STEMI diagnosis that was later ruled out were excluded from this analysis. Unless contraindicated, all patients with STEMI received aspirin (300 mg) and clopidogrel (300 mg). Patients were then managed by cardiologists for further reperfusion therapy. Decisions about the use of other medications were made at the discretion of the cardiologists in charge, who were encouraged to practice evidence-based medicine and follow appropriate guidelines in treating STEMI. Data on key time points in STEMI care, defined according to the guideline,5 were recorded. Symptom onset to FMC time is defined as the time from onset of patient-reported chest discomfort to the FMC. FMC to needle/wire crossing time is defined as the time from the FMC to prourokinase injection/wire crossing.
The two study hospitals carried out different reperfusion strategies in the included patients. In Union Jiangbei Hospital, cardiologists followed a protocol recommended by the Chinese Society of Cardiology for patients presenting at regional hospitals ≤12 hours after symptom onset; during the COVID-19 outbreak, these patients received full-dose intravenous fibrinolysis. In People's Hospital of Dongxihu District during both the pre-COVID-19 period and during the outbreak period, patients received PPCI according to the existing STEMI management guidelines.5,6 During the COVID-19 period, PPCI proceeded via the radial artery approach, in compliance with the standard algorithms, and was performed by a skilled medical team under strict protective measures against SARS-CoV-2 infection. PPCI was only performed for the affected vessel, in principle. Because COVID-19 screening would lead to a significant delay in STEMI treatment, despite the final results of screening, all patients with STEMI were initially treated as suspected COVID-19 cases when receiving PPCI or systemic thrombolytic therapy. If COVID-19 was diagnosed after reperfusion therapy, patients were transferred to the designated infectious disease ward for further treatment. If COVID-19 and other infectious diseases were ruled out after reperfusion therapy, patients were transferred to the cardiac intensive care unit.
Fibrinolytic therapy
Patients with STEMI received an intravenous injection of 20 mg prourokinase (Tasly Pharmaceuticals, Shanghai, China), followed by another 30-mg prourokinase infusion within 30 minutes.12 Unfractionated heparin (loading dose 60 U/kg with maximum 4000 U, and continuous infusion at 12 U/kg/hour with maximum 1,000 U/hour for 48 hours) and clopidogrel 75 mg (instead of a 300-mg load) were used to minimize bleeding risk; these were given only to patients age >75 years in the fibrinolytic therapy group.11 The activated partial thromboplastin time (aPTT) was maintained between 50 and 70 s by adjusting the dose of heparin. If fibrinolysis failed, defined as persistent chest discomfort or <50% resolution of ST elevation 60 to 90 minutes after fibrinolytic drug administration, the decision to perform emergency rescue PCI was made by the physician responsible for each individual patient. If fibrinolysis was contraindicated, emergency PCI would be performed in a separate catheterization laboratory in this hospital, under strict infection control measures. Otherwise, drug therapy was performed with intensive follow-up via telemedicine or occasional visits to the ED when necessary. Coronary angiography and elective catheterization, if necessary, were performed within 3 months once the COVID-19 outbreak was relatively under control.
The contraindications of fibrinolysis are as follows: known intracranial tumors or cerebrovascular malformations; suspected aortic dissection; various blood diseases or bleeding tendency; severe liver or kidney dysfunction; previous history of cerebral hemorrhage, hemorrhagic stroke or other cerebrovascular events within 6 months; history of bleeding disorders, trauma, or visceral surgery in the past 4 weeks.12
Staff protection against SARS-CoV-2 infection and catheterization laboratory disinfection
All health care providers were required to wear effective personal protective equipment (PPE), especially when caring for patients. Maximum protection was implemented, to prevent staff exposure to SARS-CoV-2. Effective PPE includes a disposable surgical cap, N95 mask, disposable waterproof isolation gown, protection suit, protective eyewear, full face shield, double medical gloves, disposable shoe covers, and rapid hand disinfection solution. In performing PPCI, the number of health care professionals was reduced to two doctors, one nurse, and one technician; the staff underwent a disinfection protocol between each PPCI procedure, to minimize exposure and potential transmission of SARS-CoV-2.
Meticulous deep post-intervention cleaning and disinfection of all equipment in the catheterization laboratory that could potentially be contaminated with SARS-CoV-2 were essential components of infection control. Thorough environmental and ultraviolet light-based disinfection was performed after each PCI. The time required for cleaning and disinfection was ≥ 1 hours. Because the virus can survive on contaminated surfaces for a long time, staff members responsible for cleaning wore full PPE.
Evaluation of IRA reperfusion
All participants were closely monitored within 24 hours of reperfusion therapy. During that period, a 12-lead ECG (18-lead ECG for posterior wall and right ventricular MI) examination was repeated at 30, 60, 90, and 120 minutes after fibrinolysis. When appropriate, an ECG examination could be done at the discretion of the responsible physician. Clinical symptoms and signs were evaluated, especially regarding the duration and relief of chest pain. Creatine kinase-MB (CK-MB) and high-sensitivity troponin I (hsTnI; if available) were measured at 10, 12, 14, 16, 18, and 24 hours after symptom onset. In addition, IRA patency was evaluated according to the non-invasive clinical indexes mentioned below, within 24 hours of fibrinolytic therapy.
In this study, we used clinical patency to confirm successful clinical reperfusion with fibrinolytic therapy. Successful coronary artery recanalization was diagnosed indirectly using the following non-invasive clinical indexes: (1) chest pain substantially (≥ 70%) or completely relieved within 120 minutes after thrombolytic therapy; (2) ST-segment resolution ≥ 50% of the most elevated lead in the original ECG within 60 to 90 minutes after fibrinolysis; (3) electrocardiographic arrhythmias abruptly manifested, improved, or resolved within 2 to 3 hours after thrombolytic therapy; and (4) premature serum CK-MB enzyme peak within 14 hours of symptom onset. IRA patency was determined if any two of the above four items (excluding 3 and 4) were achieved.
Diagnosis and treatment of acute coronary reocclusion
Acute coronary reocclusion was defined as: (1) recurrence of chest pain within 48 hours after thrombolysis, lasting ≥ 30 minutes and that is not resolved with administration of nitroglycerin tablets; (2) re-elevated ST segment in the original ECG leads; (3) and re-elevated serum CK-MB enzyme levels. Emergency treatment for acute reocclusion involved immediate re-evaluation of aPTT and use of additional heparin or dual antiplatelet therapy (aspirin and clopidogrel) if this failed to meet the required standard. Other treatments were determined by the physician responsible for each individual patient.13
Study end points
The primary efficacy end point was the occurrence of major adverse cardiac events (MACEs) in-hospital and up to 30 days after reperfusion therapy, comprising all-cause death, cardiac death, stroke, re-infarction/reocclusion, and target vessel revascularization.13
The key safety end point comprised the frequency of severe bleeding events, assessed according to Thrombolysis In Myocardial Infarction (TIMI) criteria.14 Major bleeding was defined as any intracranial bleeding, or significant clinically relevant hemorrhage, with a ≥ 5 g/dL drop in hemoglobin (Hb) or ≥ 15-point decrease in hematocrit. Minor bleeding was defined as any significant, clinically relevant hemorrhage with a 3 to 5 g/dL drop in Hb and decrease of 9 to 14 points in hematocrit.13 Other bleeding events that did not meet the above two bleeding criteria were classified as minimal bleeding.
Secondary end points included: (1) IRA patency within 24 hours of fibrinolysis;12 (2) individual components of MACE; (3) re-hospitalization; and (4) characteristics of patients with STEMI who received PPCI treatment, including pre-procedural, procedural, and post-procedural characteristics.
Statistical analysis
Continuous variables are reported as mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Categorical variables are summarized as frequency (percentage). Differences between continuous variables were assessed using the unpaired Student t-test when normally distributed and the Mann–Whitney U test for those with a non-normal distribution. Categorical variables were summarized as percentages and compared using the χ2 test or Fisher’s exact test, as appropriate. For all tests, a two-sided P value of <0.05 was considered statistically significant. The analysis was performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
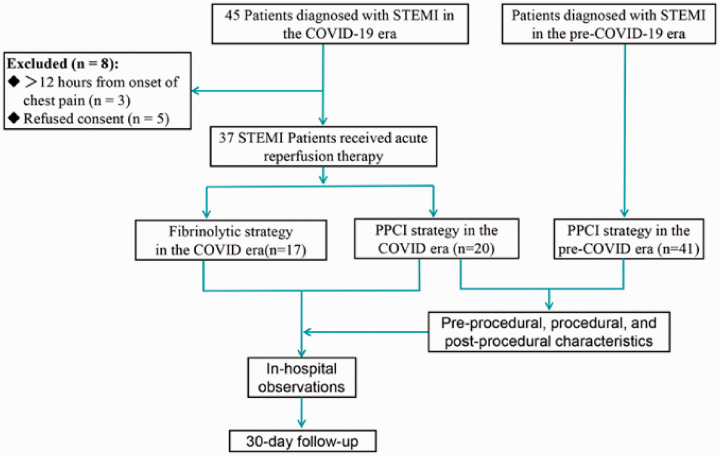
After excluding eight patients, a total of 78 patients were included in this analysis. Of these, 41 (53%) underwent PPCI in late 2019 (from 1 September to 20 December 2019), the pre-COVID-19 period, and 37 (47%) received reperfusion therapy during the COVID-19 outbreak; among patients treated in the COVID-19 period, 17 (22%) underwent fibrinolysis and 20 (26%) underwent PPCI (Figure 1).
Figure 1.
Flow of patients in this study.
COVID-19, coronavirus disease 2019; STEMI, ST-segment elevation myocardial infarction; PPCI, primary percutaneous coronary intervention.
The baseline demographic and clinical characteristics of patients in the pre-COVID-19 and COVID-19 periods were basically similar (Table 1). The mean patient age (SD) was 56.12 (11.02) years, and 68 patients (87.17%) were men. The ECG-derived STEMI location was similar between the two groups, with anterior and inferior locations being the most common. In this observational study, patients who presented to the hospital with STEMI and who underwent PPCI had both higher Killip class and peak of troponin I; however, in these patients, heart structure and function, reflected in left ventricular end-diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF), were not significantly different at admission compared with patients who received fibrinolysis. Of 37 patients, 4 were confirmed to have COVID-19 infection.
Table 1.
Baseline characteristics of patients included in this study.
|
COVID-19 period |
Pre-COVID-19 period |
*P value | †P value | ||
|---|---|---|---|---|---|
| Fibrinolysis (n = 17) | PPCI group (n = 20) | PPCI group (n = 41) | |||
| Demographics‡ | |||||
| Age (years) | 59.29 ± 11.46 | 59.05 ± 14.07 | 55.49 ± 11.89 | 0.95 | 0.31 |
| Male sex, No./total No. (%) | 15/17 (88.23) | 18/20 (90.00) | 35/41 (85.37) | 1.00 | 0.61 |
| Median body weight (kg) | 65.91 ± 9.06 | 71.75 ± 9.51 | 68.80 ± 8.27 | 0.07 | 0.22 |
| BMI (kg/m2) | 22.95 ± 2.65 | 24.61 ± 2.59 | 23.78 ± 2.12 | 0.06 | 0.19 |
| Smoker, No./total No. (%) | 12/17 (70.59) | 15/20 (75.00) | 31/41 (75.61) | 0.76 | 0.96 |
| Clinical features‡ | |||||
| Heart rate (bpm) | 81.41 ± 20.56 | 87.85 ± 19.52 | 81.20 ± 14.62 | 0.17 | 0.14 |
| Systolic BP (mmHg) | 134.40 ± 26.53 | 121.60 ± 24.32 | 125.2 ± 20.40 | 0.13 | 0.54 |
| Diastolic BP (mmHg) | 81.76 ± 12.54 | 74.45 ± 15.86 | 75.98 ± 11.01 | 0.13 | 0.66 |
| Cardiac arrest | 2/17 (11.76) | 2/20 (10) | 3/41 (7.32) | 1.00 | 1.00 |
| COVID-19 | 2/17 (11.76) | 2/20 (10) | 0/41 (0) | 1.00 | 0.10 |
| Medical history, No./total No. (%) | |||||
| Diabetes mellitus | 2/17 (11.76) | 4/20 (20) | 5/41 (12.20) | 0.67 | 0.46 |
| Hypertension | 10/17 (58.82) | 10/20 (50) | 18/41 (43.90) | 0.74 | 0.79 |
| Hypercholesterolemia | 6/17 (35.29) | 7/20 (35) | 15/41 (36.59) | 1.00 | 1.00 |
| Peripheral artery disease | 0/17 (29.41) | 1/20 (5) | 1/41 (2.44) | 1.00 | 1.00 |
| TIA/stroke | 1/17 (5.88) | 3/20 (15) | 3/41 (7.32) | 0.61 | 0.38 |
| Angina | 5/17 (29.41) | 4/20 (20) | 13/41 (31.71) | 0.70 | 0.38 |
| History of heart failure | 2/17 (11.76) | 2/20 (10) | 3/41 (7.32) | 1.00 | 1.00 |
| History of myocardial infarction | 3/17 (17.65) | 3/20 (15) | 4/41 (9.76) | 1.00 | 0.67 |
| Prior PCI/CABG | 1/17 (5.88) | 1/20 (5) | 4/41 (9.76) | 1.00 | 1.00 |
| History of bleeding | 1/17 (5.88) | 2/20 (10) | 1/41 (2.44) | 1.00 | 0.25 |
| ECG findings at study enrollment, No./total No. (%) | |||||
| ST elevation (anterior alone) | 8/17 (47.06) | 9/20 (45) | 16/41 (39.02) | 1.00 | 0.66 |
| ST elevation (anterior and inferior) | 1/17 (5.88) | 1/20 (5) | 0/41 (0) | 1.00 | 0.33 |
| ST elevation (inferior alone) | 5/17 (29.41) | 10/20 (50) | 25/41 (60.98) | 0.32 | 0.42 |
| ST elevation (other) | 3/17 (17.65) | 0/20 (0) | 0/41 (0) | 0.09 | − |
| Infarct size | |||||
| Peak CK-MB (median, IQR) | 23.00 (15.50–44.50) | 45.50 (20.25–73.76) | 46.00 (34.00–56.50) | 0.09 | 0.98 |
| Peak hsTNI (median, IQR) | 1844 (368.50–3783) | 10691 (774.80–29385) | 12477 (3508–22697) | 0.04* | 0.84 |
| BNP (median, IQR) | 150 (80.50–554.50) | 200 (87.25–1014) | 217 (99–856) | 0.56 | 0.82 |
| Heart structure and function | |||||
| LVEDD | 47.53 ± 2.85 | 48.20 ± 4.49 | 46.73 ± 5.84 | 0.97 | 0.75 |
| LVEF | 59.12 ± 6.73 | 56.60 ± 8.86 | 53.37 ± 8.41 | 0.34 | 0.16 |
| Killip, n (%) | 0.04* | 0.04† | |||
| I | 15/17 (88.23) | 9/20 (45) | 32/41(78.05) | ||
| II | 1/17 (5.88) | 7/20 (35) | 5/41 (12.20) | ||
| III/IV | 1/17 (5.88) | 4/20 (20) | 4/41 (9.76) | ||
*P < 0.05, fibrinolysis group versus PPCI, in the COVID-19 period.
†P < 0.05, PPCI in the COVID-19 period versus PPCI, in the pre-COVID-19 period.
‡ Unless otherwise noted, data in the table are frequency (%) or mean (standard deviation).
BMI, body mass index; bpm, beats per minute; COVID-19, coronavirus disease 2019; BP, blood pressure; TIA, transient ischemic attack; PPCI, primary percutaneous coronary intervention; CABG, coronary artery bypass grafting; ECG, electrocardiogram; CK-MB, creatine kinase-MB; hsTNI, high-sensitivity troponin I; BNP, brain natriuretic peptide; IQR, interquartile range; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
In-hospital and 30-day clinical outcomes
Fifteen of 17 patients (88.2%) achieved successful clinical reperfusion after fibrinolysis with prourokinase, according to the indirect indicators of clinical patency. In the fibrinolysis group, the mean onset of chest pain to FMC time was 338.8 minutes, and the mean FMC to needle time was 77.53 minutes (Table 2). The in-hospital and 30-day primary composite end point rates as well as the individual components are shown in Table 2. No significant difference was observed between the fibrinolysis group and PPCI group as far as the frequency of MACEs during the index hospitalization and at 30-day follow-up during the COVID-19 period. During the in-hospital period, the incidence of all-cause mortality was 11.76% in patients who underwent fibrinolysis and 10% in patients who underwent PPCI in the COVID-19 period. Among 33 early post-STEMI survivors, all were followed for more than 30 days. Rates of cardiac death, stroke re-infarction/reocclusion, and target vessel revascularization were similarly low in both groups. No major bleeding (TIMI criteria) was detected during the follow-up period in either group. Rates of re-hospitalization owing to heart failure (HF) were similar between groups. The fibrinolysis group had lower levels of brain natriuretic peptide (BNP) at 30-day follow-up; however, these did not reach statistical significance. There was no difference in LVEF and LVEDD.
Table 2.
In-hospital and 30-day efficacy and safety outcomes.
|
COVID-19 period |
Pre-COVID-19 era |
*P value | †P value | ||
|---|---|---|---|---|---|
| Fibrinolysis (n = 17) | PPCI group (n = 20) | PPCI group (n = 41) | |||
| In-hospital, No./total No. (%) | |||||
| All-cause mortality | 2/17 (11.76) | 2/20 (10) | 0/41 | 1.00 | 0.10 |
| Cardiac death | 2/17 (11.76) | 2/20 (10) | 0/41 | 1.00 | 0.10 |
| Stroke | 0/17 | 0/20 | 0/41 | − | − |
| Re-infarction/reocclusion | 1/17 (5.88) | 1/20 (5) | 0/41 | 1.00 | 0.33 |
| Target vessel revascularization | 1/17 (5.88) | 1/20 (5) | 0/41 | 1.00 | 0.33 |
| MACE | 3/17 (17.65) | 3/20 (15) | 0/41 | 0.61 | 0.03† |
| Major bleeding | 0/17 | 0/20 | 0/41 | − | − |
| IRA patency | 15/17 (88.2) | NA | NA | − | − |
| Onset of chest pain-to-FMC time, min (mean ± SD) | 338.8 ± 145.40 | See Table 3 | See Table 3 | 0.80 | − |
| FMC-to-needle time, min (mean ± SD) | 77.53 ± 32.88 | NA | NA | − | − |
| 30-day follow-up, No./total No. (%) | |||||
| All-cause mortality | 2/17 (11.76) | 2/20 (10) | 2/41 (4.89) | 1.00 | 1.00 |
| Cardiac death | 2/17 (11.76) | 2/20 (10) | 1/41 (2.44) | 1.00 | 1.00 |
| Stroke | 0/17 | 0/20 | 1/41 (2.44) | − | 1.00 |
| Re-infarction/reocclusion | 1/17 (5.88) | 1/20 (5) | 0/41 | 1.00 | 1.00 |
| Target vessel revascularization | 1/17 (5.88) | 1/20 (5) | 0/41 | 1.00 | 1.00 |
| MACE | 3/17 (17.65) | 3/20 (15) | 2/41 (4.89) | 1.00 | 0.32 |
| Major bleeding | 0/17 | 0/20 | 0/41 | − | − |
| Re-hospitalization | 2/17 (11.76) | 3/20 (15) | 1/41 (2.44) | 1.00 | 0.10 |
| Re-hospitalization owing to HF | 1/17 (5.88) | 2/20 (10) | 1/41 (2.44) | 1.00 | 0.25 |
| BNP (median, IQR) | 200 (110–550) | 400 (110–927) | 340 (180–602) | 0.53 | 0.89 |
| LVEDD | 49.67 ± 3.89 | 50.44 ± 4.42 | 48.39 ± 5.26 | 0.60 | 0.30 |
| LVEF | 48.40 ± 7.79 | 47.17 ± 8.89 | 48.51 ± 6.37 | 0.68 | 0.51 |
*P < 0.05, fibrinolysis group versus PPCI in the COVID-19 period.
†P < 0.05, PPCI in the COVID-19 period versus PPCI in the pre-COVID-19 period.
COVID-19, coronavirus disease 2019; PPCI, primary percutaneous coronary intervention; MACE, major adverse cardiac event; IRA, infarct-related artery; FMC, first medical contact; SD, standard deviation; HF, heart failure; BNP, brain natriuretic peptide; HF, heart failure; IQR, interquartile range; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
To evaluate the impact of the COVID-19 pandemic on PPCI procedures and outcomes, we collected data for another consecutive 41 patients with STEMI who underwent PPCI in the People’s Hospital of Dongxihu District during the pre-COVID-19 period in late 2019. As shown in Table 1, the baseline characteristics of these patients, who received PPCI in the same hospital, were basically similar to those of patients who received PPCI during the COVID-19 period. However, no in-hospital clinical events occurred in patients who underwent PPCI during the pre-COVID-19 period. Compared with patients who received PPCI in the pre-COVID-19 period, significantly higher rates of in-hospital MACEs and a trend of higher rates of MACEs and re-hospitalization were observed in patients who received PPCI in the COVID-19 period (Table 2).
Preprocedural, procedural, and post-procedural characteristics of patients with STEMI who underwent PPCI during and prior to the COVID-19 pandemic
Details of the procedural characteristics of patients are summarized in Table 3. We detected a remarkable delay in reperfusion of patients with STEMI. The average time from symptom onset to FMC was 166.8 minutes in the pre-COVID-19 period, but this was doubled in the COVID-19 period. Similarly, the time from FMC to reperfusion with PPCI (wire crossing) was 118.71 ± 27.10 minutes and 165.5 ± 30.11 minutes in these groups, respectively. The right coronary artery was the IRA in most cases in both groups (50%), and the proximal segment was the occlusion site. Drug-eluting stents were used in over 95% of PPCI cases. Notably, the number and length of stents used during the procedure were markedly increased in patients who underwent PPCI during the COVID-19 outbreak. Furthermore, PPCI during the COVID-19 period was associated with reduced post-procedural TIMI flow (2.20 ± 1.01 vs. 2.78 ± 0.53; P = 0.01), compared with PPCI performed before the outbreak of COVID-19. Rates of reinfarction/reocclusion and revascularization after PPCI performance were similar in both groups.
Table 3.
Preprocedural, procedural, and post-procedural characteristics of patients with STEMI who received PPCI.
| COVID-19 period |
Pre-COVID-19 period |
†P value | |
|---|---|---|---|
| PPCI group (n = 20) | PPCI group (n = 41) | ||
| Pre-procedural characteristics | |||
| Onset of chest pain-to-FMC time, min (mean ± SD) | 350.6 ± 134.90 | 166.8 ± 76.64 | <0.01† |
| FMC-to-wire crossing time, min (mean ± SD) | 165.5 ± 30.11 | 118.71 ± 27.10 | <0.01† |
| Procedural characteristics, No./total No. (%) | |||
| Culprit vessel | |||
| LAD | 9/20 (45) | 14/41 (34.15) | 0.41 |
| LCX | 1/20 (5) | 5/41 (12.20 | 0.65 |
| RCA | 10/20 (50) | 22/41 (53.66) | 0.79 |
| Other | 0/20 (0) | 0/41 (0) | − |
| Site of occlusion | |||
| Ostial | 0/20 (0) | 0/41 (0) | − |
| Proximal segment | 9/20 (45) | 22/41 (53.66) | 0.53 |
| Mid segment | 8/20 (40) | 12/41 (29.27) | 0.40 |
| Distal segment | 3/20 (15) | 7/41 (17.07) | 1.00 |
| Drug-eluting stent use | 20/20 (100) | 39/41 (95.12) | 1.00 |
| Drug-eluting stent use (mean ± SD) | 2.00 ± 0.73 | 1.32 ± 0.61 | <0.01† |
| Mean stent length, mm (mean ± SD) | 57.35 ± 23.75 | 39.93 ± 18.46 | <0.01† |
| TIMI flow grade (mean ± SD) | 2.20 ± 1.01 | 2.78 ± 0.53 | 0.01† |
| Post-procedural characteristics, No./total No. (%) | |||
| Re-infarction/reocclusion (48 h) | 1/20 (5) | 0/41 (0) | 0.33 |
| Target vessel revascularization (48 h) | 1/20 (5) | 0/41 (0) | 0.33 |
†P < 0.05, PPCI in the COVID-19 period versus PPCI in the pre-COVID-19 period.
STEMI, ST-segment elevation myocardial infarction; PPCI, primary percutaneous coronary intervention; COVID-19, coronavirus disease 2019; SD, standard deviation; FMC, first medical contact; LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
Management of patients with STEMI during the COVID-19 pandemic
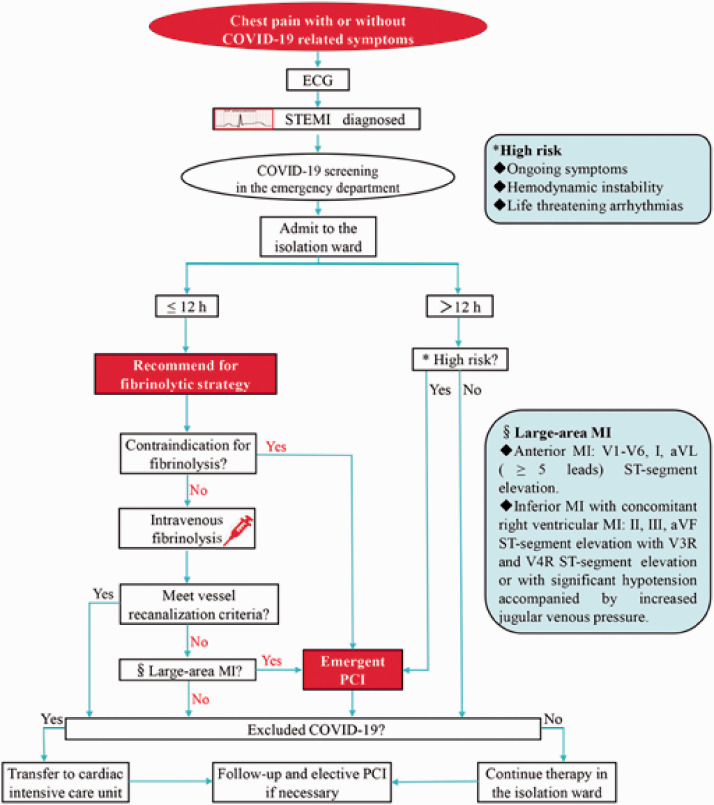
A proposed management algorithm for STEMI during the COVID-19 pandemic is displayed in Figure 2. In summary, patients with acute chest pain who are highly suspected of STEMI should be transferred to an isolation ward for acute reperfusion therapy. In patients presenting ≤12 hours after onset of symptoms, emergency intravenous fibrinolysis is recommended, unless contraindicated. Emergency PCI should be conducted for patients who do not meet the criteria for recanalization after fibrinolysis, those who are contraindicated to thrombolysis, or patients in a life-threatening state even >12 hours after onset of chest pain.
Figure 2.
Proposed management algorithm for patients with STEMI during the COVID-19 pandemic.
COVID-19, coronavirus disease 2019; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
The evolving and expanding outbreak of SARS-CoV-2 infection has caused important challenges in myocardial reperfusion efforts. The pandemic situation requires potential modification of established processes and practices in care for STEMI, which is a major public health problem with high mortality worldwide.6 Taking into consideration the lack of medical resources, together with the risk of spreading infection with highly contagious SARS-CoV-2, the Committee of the Chinese Society of Cardiology recommends fibrinolytic therapy as the first-choice of treatment for STEMI,7,8 although the prevailing guidance globally still favors continuing a PPCI approach.2,10 However, there is no available evidence regarding modified treatment for STEMI during the era of COVID-19.
To address this gap, we analyzed data of 37 patients from two PCI-capable centers in Wuhan, who received different primary reperfusion treatments for STEMI from 23 January to 20 March 2020. Of these, 20 patients received PPCI at Dongxihu District People's Hospital, and 17 patients underwent fibrinolytic treatment at Jiangbe Union Hospital, both groups during the COVID-19 outbreak. Given the epidemiology, all patients in Wuhan were treated as suspected COVID-19 cases until they were confirmed to be free of SARS-CoV-2 infection. In our study, 4 out of 37 patients (2 patients in each group) had COVID-19 infection. Most of our patients (89.2%) who required acute reperfusion therapy for the management of STEMI were not infected with COVID-19, although an association has been found between cardiovascular diseases and COVID-19 infection.15–17 It is well accepted that obtaining timely reperfusion of the IRA via PPCI as early as possible is associated with better outcomes, in comparison with the use of fibrinolytic drugs. Unexpectedly, there was no difference between the fibrinolysis group and the PPCI group in all-cause death, cardiac death, stroke, re-infarction/reocclusion, and revascularization among patients with STEMI presenting ≤12 hours after symptom onset during the COVID-19 outbreak. No major bleeding was detected in either group. In addition, the LVEF, LVEDD, and BNP level at 30-day follow-up were comparable between these two groups. In summary, we demonstrated that, compared with fibrinolysis, PPCI had comparable in-hospital and 30-day follow-up clinical efficiency and safety end points; however, PPCI requires more PPE and a larger health care workforce under conditions of the COVID-19 pandemic.
To explore the mechanisms underlying our findings, we enrolled another 41 consecutive patients with STEMI who received PPCI from 1 September to 20 December 2019, prior to the COVID-19 outbreak. Compared with these patients, we observed a significant delay in the time of chest pain onset to FMC in the 37 patients who underwent acute reperfusion therapy during the COVID-19 outbreak. All patients admitted during the outbreak did not achieve guideline-recommended times to reperfusion (FMC to wire crossing time < 90 minutes for regional patients, and FMC to needle time < 30 minutes).11 It is understandable that people are reluctant to go to a hospital during the COVID-19 pandemic; however, this reluctance can lead to delays in seeking care. Moreover, when comparing the data of emergency PCI in the pre-COVID-19 period and during the COVID outbreak in People's Hospital of Dongxihu District, we found that during the epidemic, the FMC to wire crossing time of emergency PCI was significantly prolonged, the total operation time was dramatically lengthened, and the average number and length of implanted stents were markedly increased. These might be related to the high intensity level of protective measures during the procedure not only increasing in difficulty in the fine manipulation of guide wires but also by disturbing the accurate decision-making by the operator under the conditions of a pandemic. Moreover, the shortage of medical staff is an important factor that cannot be ignored.
We observed that 15 of 17 patients (88.2%) achieved successful clinical reperfusion after fibrinolysis with prourokinase. This was consistent with phase IV clinical trials of prourokinase, in which successful clinical reperfusion was observed in 85.4% of patients, and the incidence of intracranial hemorrhage was as low as 0.32%.12 As the efficiency and safety of fibrinolysis has improved during the past few years,11,18 fibrinolysis can be considered in patients with STEMI when it is not possible to execute PPCI in a timely manner.
With reference to exceeding hospital capacity, conserving limited medical resources and minimizing provider as well as patient exposure to SARS-CoV-2 are particularly important in the setting of the COVID-19 pandemic. Compared with PPCI, fibrinolytic therapy not only saves PPE and the necessary workforce but it also minimizes exposure of health care providers and patients to the virus. However, in the current study, we observed two cases of cardiac death in the fibrinolysis group. Both of these patients were found to have large-area MI. Patients with large-area MI require special attention post fibrinolytic therapy. Emergency PCI under intensive infection control measures should be promptly carried out if fibrinolytic therapy fails in these patients.
Finally, according to the findings of this evidence-based study, we propose the following management algorithm for patients with STEMI during the COVID-19 pandemic (Figure 2). In patients with acute chest pain, an ECG should be recorded promptly on arrival to the ED, and COVID-19 screening should be initiated simultaneously. Once STEMI is diagnosed, the patient should be admitted to an isolation ward for acute reperfusion therapy. Intravenous fibrinolysis is recommended for patients presenting ≤12 hours after onset of symptoms, unless contraindicated to fibrinolysis. Of note, emergency PCI is indicated in patients who do not meet vessel recanalization criteria after fibrinolysis, those who are contraindicated to thrombolysis, or those presenting with life-threatening status even >12 hours after the onset of chest pain. Once COVID-19 infection has been ruled out in patients with STEMI, they should be transferred to the cardiac intensive care unit or otherwise continue therapy in the isolation ward. If necessary, elective PCI should be performed 1 to 3 months after primary reperfusion or once the COVID-19 epidemic is relatively controlled.
Study limitations
First, the data were collected from different hospitals during the COVID-19 pandemic period, which may lead to potential confounding. Both study centers were PCI-capable sites; however, only People's Hospital of Dongxihu District was able to continue to execute PPCI for STEMI care during the COVID-19 outbreak owing to an insufficient medical workforce and PPE during that period in Union Jiangbei Hospital. Nevertheless, we believe that this situation represents the real-world landscape during the COVID-19 epidemic. Furthermore, the data of patients with STEMI who underwent PPCI were all collected from the same hospital, including 41 patients in late 2019, prior to the COVID-19 outbreak, and 20 patients who received PPCI during the outbreak in 2020; this reduced potential bias to some extent. Finally, the number of patients in this retrospective study was small. However, many studies have reported a dramatic decrease in hospital admissions for patients with STEMI during the same months of the pandemic in both North America and Europe. In addition, Wuhan was the epicenter in China; many patients were reluctant to go the hospital in a timely manner owing to fear of contacting COVID-19 and a lack of public transportation. We believe that our data are reliable for these reasons.
Conclusion
Managing patients with STEMI is particularly challenging under conditions during the COVID-19 pandemic, considering the risk of infection with highly contagious SARS-CoV2. The pandemic has caused remarkable delays among patients with STEMI in seeking medical care and receiving reperfusion treatment, especially in performing PPCI. In view of the considerable efficacy and safety of fibrinolysis, as we observed in this study, and its inherent advantages in conserving medical resources and avoiding the spread of SARS-CoV-2 in the hospital, we recommend that emergency intravenous fibrinolysis as a reasonable alternative for STEMI care in the setting of the COVID-19 pandemic. Our findings might be helpful for health care providers treating patients with STEMI during current and subsequent outbreaks of COVID-19, which are predicted by many experts.
Acknowledgements
The authors thank all the coordinators and patients who participated in the study. We dedicate this work to the memory of health care workers who have given their lives in the care of patients with COVID-19.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China 81600187 (to M.Z.) and 81800891 (to N.W.).
ORCID iD: Min Zhang https://orcid.org/0000-0002-2199-1283
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. DOI: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J Am Coll Cardiol 2020; 75: 2352–2371. DOI: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. DOI: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19. JAMA 2020; Published online March 26, 2020. DOI: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. DOI: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: 529–555. DOI: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Fan Y, Lu Z. Experiences and lesson strategies for cardiology from the COVID-19 outbreak in Wuhan, China, by ‘on the scene’ cardiologists. Eur Heart J 2020; 41: 1788–1790. DOI: 10.1093/eurheartj/ehaa266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing ZC, Zhu HD, Yan XW, et al. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J 2020; 41: 1791–1794. Advance online publication. DOI: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People's Hospital. Intensive Care Med 2020; 46: 1111–1113. Advance online publication. DOI: 10.1007/s00134-020-05993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szerlip M, Anwaruddin S, Aronow HD, et al. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates. Catheter Cardiovasc Interv 2020; 96: 586–597. DOI: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 11.Siontis KC, Barsness GW, Lennon RJ, et al. Pharmacoinvasive and Primary Percutaneous Coronary Intervention Strategies in ST-Elevation Myocardial Infarction (from the Mayo Clinic STEMI Network). Am J Cardiol 2016; 117: 1904–1910. DOI: 10.1016/j.amjcard.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Zhao Z, Chen X, et al. Safety and efficacy of prourokinase injection in patients with ST-elevation myocardial infarction: phase IV clinical trials of the prourokinase phase study. Heart Vessels 2018; 33: 507–512. DOI: 10.1007/s00380-017-1097-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang HB, Ji P, Zhao XS, et al. Recombinant human TNK tissue-type plasminogen activator (rhTNK-tPA) versus alteplase (rt-PA) as fibrinolytic therapy for acute ST-segment elevation myocardial infarction (China TNK STEMI): protocol for a randomised, controlled, non-inferiority trial. BMJ Open 2017; 7: e016838. DOI: 10.1136/bmjopen-2017-016838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berwanger O, Nicolau JC, Carvalho AC, et al. Ticagrelor vs Clopidogrel After Fibrinolytic Therapy in Patients With ST-Elevation Myocardial Infarction. JAMA Cardiol 2018; 3: 391. DOI: 10.1001/jamacardio.2018.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260. DOI: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong TY, Redwood S, Prendergast B, et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 2020; 41: 1798–1800. Advance online publication. 10.1093/cvr/cvaa106. DOI: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 811–818. DOI: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vora AN, Holmes DN, Rokos I, et al. Fibrinolysis use among patients requiring interhospital transfer for ST-segment elevation myocardial infarction care: a report from the US National Cardiovascular Data Registry. JAMA Intern Med 2015; 175: 207–215. DOI: 10.1001/jamainternmed.2014.6573. [DOI] [PubMed] [Google Scholar]