Abstract
Bone disease is an important complication of hyperparathyroidism. We herein report a rare case of severe bone disease caused by primary hyperparathyroidism. A 33-year-old man presented with pain and restricted mobility in his right upper limb and right hip due to a fall 3 days previously. X-ray examination showed a fracture of the proximal and distal humerus. Computed tomography examination showed a supracondylar fracture of the right humerus, a fracture of the right femoral neck, a fracture of the right sciatic branch, and multiple brown tumors. Ultrasonography showed a 3.5- × 1.6-cm hypoechoic mass below the left lobe of the thyroid. The patient was diagnosed with primary hyperparathyroidism based on increased serum calcium and parathormone concentrations, pathological fractures, and multiple brown tumors. He therefore underwent bilateral lower parathyroidectomy. Pathological examination revealed a parathyroid adenoma. The patient recovered well after surgery and was followed up for 6 months with no symptoms of hyperparathyroidism. This case report suggests that clinicians should be aware of the possibility of severe bone disease secondary to primary hyperparathyroidism. Active and early diagnosis and surgical treatment are important in such cases.
Keywords: Primary hyperparathyroidism, bone disease, pathological fracture, parathyroid adenoma, parathyroidectomy, case report
Introduction
Primary hyperparathyroidism (PHPT) is a systemic disease caused by lesions of the parathyroid gland (such as parathyroid adenoma, parathyroid hyperplasia, and parathyroid carcinoma).1 PHPT leads to the synthesis and secretion of parathyroid hormone (PTH) and causes calcium, phosphorous, and bone metabolism disorders. The main clinical manifestations are bone diseases with increased bone resorption, hypercalciuria-induced urinary calculi, hypercalcemia, and hypophosphatemia.2 In China, the most common clinical manifestation of PHPT is bone disease. The PTH concentration is significantly increased in these patients. Osteoclasts are enhanced through the actions of multiple systems such as the skeletal system, urinary system, and digestive system; this can eventually cause a variety of bone diseases and pathological fractures.3 We herein report a case of severe bone disease caused by PHPT. Relevant studies were also reviewed to provide insight with respect to clinical practice.
Case presentation
A 33-year-old man with pain and limited activity of his right upper limb and right hip caused by a fall 3 days previously was admitted to our hospital. The patient had undergone extracorporeal lithotripsy for urinary calculi 3 years previously and surgical treatment for a left tibial fracture caused by a fall 1 year previously. In addition, the patient had developed multiple slight fractures caused by falls in recent years and improved after conservative treatment. The patient had also been admitted to several hospitals, but the cause was not clear. Physical examination on admission revealed stable vital signs; obvious swelling, deformity, and tumefaction of the right elbow; activity limitation of the right elbow; obvious swelling and tenderness of the right hip; activity limitation of the right hip; and positive Patrick’s test.
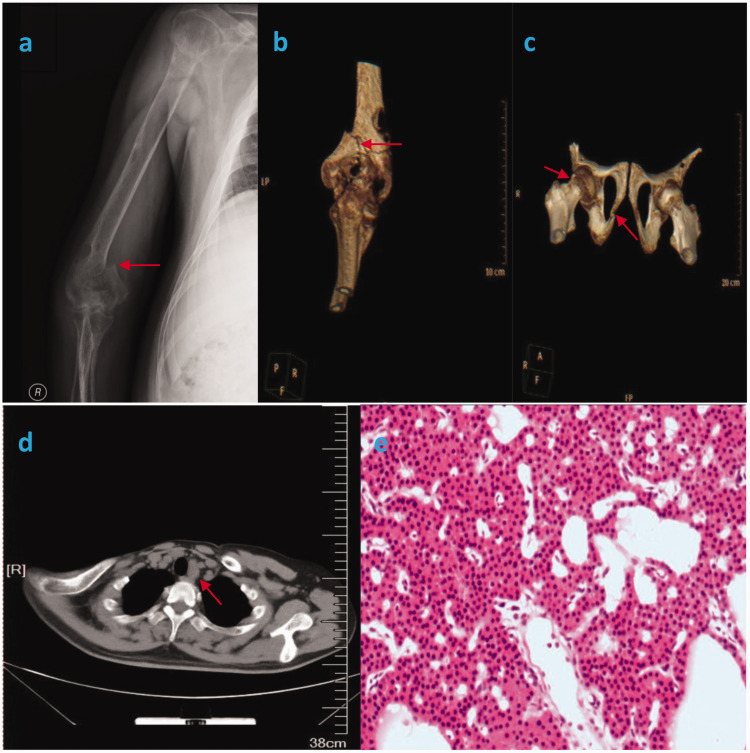
After admission, X-ray examination of the right humerus revealed a right humeral fracture (Figure 1(a)). Computed tomography of the right elbow joint, right hip joint, and chest demonstrated a right supracondylar humeral fracture (Figure 1(b)), right femoral neck and right ischial fractures (Figure 1(c)), multiple brown tumors, and a solid lesion behind the thyroid gland (Figure 1(d)). Routine blood examination showed a serum calcium concentration of 2.91 mmol/L (reference range, 2.08–2.60 mmol/L) and serum phosphorus concentration of 0.77 mol/L (reference range, 0.84–1.45 mol/L). The serum PTH concentration was 167.1 pmol/L (reference range, 1.6–6.9 pmol/L). Cervical color Doppler ultrasound demonstrated a 3.5- × 1.6-cm hypoechoic tumor below the left lobe of the thyroid gland. The patient was finally diagnosed with PHPT; pathologic fractures of the right supracondylar humerus, right femoral neck, and right ischium; and multiple brown tumors.
Figure 1.
(a) X-ray of the right humerus showing a right humeral fracture (red arrow). (b) Computed tomography scan of the right elbow showing a fracture of the right supracondylar humerus (red arrow). (c) Computed tomography scan of the right hip showing a fracture of the right femoral neck and right ischium (red arrows). (d) Computed tomography scan of the chest showing a solid lesion behind the thyroid gland (red arrow). (e) Pathological examination showing parathyroid chief cells arranged in nests and cords (hematoxylin–eosin, ×100).
Parathyroidectomy was performed. The abnormal parathyroid glands were excised; they exhibited obvious tumefaction, and parathyroid adenoma was diagnosed by intraoperative frozen section pathological examination. We also explored the remaining parathyroid glands. The shape, size, and color of the glands were normal, and they were retained. Postoperative pathology confirmed the diagnosis of parathyroid adenoma (Figure 1(e)). The serum PTH concentration was 3.7 pmol/L, and the serum calcium concentration was 2.30 mmol/L; both were within the reference range on the second day after surgery. The patient recovered well and was discharged after 1 week. Six months later, a follow-up by telephone revealed that the patient was able to perform easy manual work and had made a remarkable recovery overall.
Discussion
The incidence rate of PHPT varies from 34 to 120 cases per 100,000 individuals, and it increases with age.4–9 Some studies have reported that the prevalence of PHPT in postmenopausal women is >2%.10,11 In any case, PTHT is relatively rare in the clinical setting. As a result, some clinicians have an insufficient understanding of this disease, fail to make a timely and correct diagnosis, and are prone to misdiagnosis.12,13 Because this disease mainly manifests as pathological fractures, most patients are first diagnosed in the department of orthopedics or bone oncology, resulting in a high rate of delayed treatment or misdiagnosis.14,15 Misdiagnosis can lead to further aggravation of skeletal system diseases, which gradually develop into severe manifestations of pathological bone damage such as osteitis fibrosa cystica, repeated fractures, and delayed recovery after fracture treatment.16,17 Once confirmed, timely excision of the diseased parathyroid glands is considered to be the preferred method for the treatment of PHPT with severe bone disease, rather than treatment of the skeletal system disease only.18,19 After excision of the parathyroid gland lesions, PTH and other relevant indicators gradually return to normal levels, and new bone tissue gradually grows within and resolves the skeletal lesions.20
PHPT is caused by abnormal elevation of PTH due to parathyroid gland lesions such as parathyroid adenoma, parathyroid hyperplasia, and parathyroid carcinoma, all of which are mainly managed by surgical treatment.21 The incidence of PHPT is higher in Europe and the United States than in China, but most patients are asymptomatic.22 Most patients with PHPT have no obvious symptoms or have nonspecific symptoms such as osteoporosis; very few patients have specific manifestations such as bone pain, pathological fracture, or other bone diseases.23 In contrast, most patients with PHPT in China are symptomatic and exhibit different symptoms and signs involving one or more systems such as the skeletal system, urinary system, digestive system, nervous system, and muscular system.24
PHPT causes a series of symptoms and signs by acting on the skeletal system and urinary system; it also has certain effects on the digestive system, nervous system, muscular system, and other systems.22,23,25 Some patients mainly present with skeletal system diseases, which is the most common presentation in the clinical setting. These patients often have obvious symptoms, such as bone pain, osteoporosis, and pathological fracture; they may also develop brown tumors,26 osteitis fibrosa cystica,27 and other specific clinical manifestations. Some patients mainly present with symptoms involving the urinary system (e.g., kidney calculi, ureteral calculi, azotemia, or renal insufficiency).23 These symptoms are caused by PTH-induced enhancement of the effect of osteoclasts, which causes calcium phosphate in bones to be dissolved into the blood, resulting in hypercalcemia and hyperphosphatemia. When the concentration of calcium ions and phosphorus ions exceeds the renal threshold and these ions enter the urine, hypercalciuria and hyperphosphaturia develop, causing urinary calculi and secondary symptoms. Our patient developed both skeletal and urinary system disease but no clinical manifestations of the digestive, nervous, muscle, or other systems.
Approximately 5% of PHPT is reportedly the syndromic form, and multiple endocrine neoplasia type 1 (MEN1)-PHPT is the most common type.28 MEN1-PTHT penetrance occurs earlier (tending to occur in young people aged 20–30 years) than sporadic PHPT. One study showed that 50 years of age was the crucial threshold, and almost all patients with MEN1-PHPT had developed PHPT by 50 years of age.29 Moreover, an age of <50 years was found to be an independent risk factor for PHPT complications.30 MEN1-PTHT is characterized by asynchronous and asymmetrical parathyroid gland growth, and it can be confused with a single adenoma when only one abnormal gland is found at the time of the initial surgery.31 Therefore, periodic monitoring of parathyroid function and phospho-calcium metabolism should be performed after surgery. MEN1-PHPT also exhibits greater clinical aggressiveness than non-syndromic PHPT.32,33 The incidence of renal lithiasis is higher in patients aged <30 years, and 68% of patients with MEN1-PHPT present with urolithiasis complications as the first clinical manifestation (50% have urolithiasis as the only clinical manifestation of the syndrome at the time of diagnosis).34 Lourenço et al.35 reported a case of early reduction of bone mass and urolithiasis in a patient with MEN1-PHPT in 2010. Consequently, it is extremely important to gather useful data during clinical assessment of patients with the syndromic forms of PHPT.36 When a patient with PHPT is highly suspected to have or has already been determined to have syndromic PHPT, especially patients aged <30 years, appropriate DNA genetic tests are strongly recommended.37 Multiglandular asymmetric growth of the parathyroid tissue generally occurs in patients with syndromic PHPT; therefore, the choice of surgical approach is extremely important and challenging.38 In the present case, the patient most likely had multiple endocrine neoplasia-PHPT considering his age, multiple brown tumors, fractures, parathyroid adenoma, and urolithiasis.
Hypocalcemia is a common complication after parathyroidectomy, and a recent study suggested dividing it into different types;39 postoperative hypoparathyroidism (serum calcium concentration of <2 mmol/L 24 hours after surgery), protracted hypoparathyroidism (subnormal serum PTH concentration and/or need for calcium replacement at 4–6 weeks after surgery), and permanent hypoparathyroidism (the need for calcium replacement therapy 1 year after surgery). Hungry bone syndrome is another rare but serious complication characterized by severe and prolonged hypocalcemia despite normal or even elevated levels of serum PTH after surgery for PHTP,40 and postoperative calcium and vitamin D supplementation may benefit such patients.41
Most parathyroid glands are located on the back of the left and right lobes of the thyroid gland. The parathyroid gland originates in the dorsal part of the third and fourth pharyngeal pouches. The dorsal part of the third pharyngeal pouch develops to form the lower parathyroid gland, while the ventral part of the third pharyngeal pouch develops to form the thymus gland. In the process of development, the parathyroid gland and thymus descend together, but the parathyroid gland may stop descending at any time, becoming located at sites, such as the tracheoesophageal groove, thymus, mediastinum, thyroid gland, or inframandibular region.42 The location of the superior parathyroid gland is relatively fixed with few variations; it is mostly located near the intersection of the recurrent laryngeal nerve and the inferior thyroid artery. Less common sites include the tracheoesophageal groove, posterior esophagus, superior mediastinum, thyroid gland, and carotid sheath.43 An ectopic parathyroid gland may lead to the possibility of reoperation.44 Therefore, accurate preoperative positioning of the parathyroid gland is also important. Color Doppler ultrasound, nuclide imaging, computed tomography, and other comprehensive examinations of the parathyroid gland can improve the accuracy and sensitivity of localization,45 providing a reference and guidance for surgical methods.
It is currently believed that at least unilateral exploration of parathyroid adenomas should be performed; that is, another parathyroid gland on the same side of the lesion should be explored after resection of the diseased parathyroid gland, which is confirmed by frozen section pathological examination. If the second parathyroid gland is confirmed to be normal, the operation can be concluded. Some scholars also advocate bilateral exploration (i.e., intraoperative exposure of all four parathyroid glands) if necessary. The benefits of this treatment are that lesions of the parathyroid glands are not missed and that the possibility of a second operation due to incomplete surgery is reduced.
In conclusion, we have herein reported a rare case of severe bone disease secondary to PHPT. Through reflection of the whole process of diagnosis and treatment of this case and a literature review, we conclude that cervical ultrasound and measurement of the PTH, calcium, and phosphorus concentrations should be performed in patients with a history of recurrent fractures and multiple bone diseases to exclude multiple bone destruction caused by PHPT. Our findings showed that a clear diagnosis and surgical resection of the parathyroid gland as early as possible can yield good therapeutic results for patients with PHPT.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics: This study was approved by the local Clinical Research Ethics Committee, and the requirement for written informed consent for publication was waived because of the retrospective nature of the study. The patient provided informed consent for all procedures performed.
ORCID iD: Yu Wang https://orcid.org/0000-0001-8335-6050
References
- 1.Townsend CM, Jr, Beauchamp RD, Evers BM, et al. Sabiston textbook of surgery. 19th ed Philadelphia: Elsevier, 2012, p.933. [Google Scholar]
- 2.Costanzo L S. BRS physiology. 4th ed Philadelphia: Lippincott Williams Wilkins, 2011, pp.247–248. [Google Scholar]
- 3.Purrunsing Y, Zhang J, Cui Y, et al. Sixty-two-year-old male suffering from uremic leontiasis ossea caused by severe secondary hyperparathyroidism. JBMR Plus 2018; 2: 240–245. Published 2018 Mar 30. doi: 10.1002/jbm4.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser WD. Hyperparathyroidism. Lancet 2009; 374: 145–158. [DOI] [PubMed] [Google Scholar]
- 5.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med 2011; 365: 2389–2397. [DOI] [PubMed] [Google Scholar]
- 6.Clarke BL. Epidemiology of primary hyperparathyroidism. J Clin Densitom 2013; 16: 8–13. doi: 10.1016/j.jocd.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 2013; 98: 1122–1129. doi: 10.1210/jc.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg SJ, Clarke BL, Peacock M, et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab 2014; 99: 3580–3594. doi: 10.1210/jc.2014-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griebeler ML, Kearns AE, Ryu E, et al. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965–2010). Bone 2015; 73: 1–7. doi: 10.1016/j.bone.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundgren E, Rastad J, Thurfjell E, et al. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery 1997; 121: 287–294. [DOI] [PubMed] [Google Scholar]
- 11.Wermers RA, Khosla S, Atkinson EJ, et al. The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965–1992. Ann Intern Med 1997; 126: 433–440. [DOI] [PubMed] [Google Scholar]
- 12.Cusano NE, Cipriani C, Bilezikian JP. Management of normocalcemic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab 2018; 32: 837–845. doi: 10.1016/j.beem.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Pezzillo F, Di Matteo R, Liuzza F, et al. Isolated bone lesion secondary to hyperparathyroidism: diagnostic considerations. Clin Ter 2008; 159: 265–268. [PubMed] [Google Scholar]
- 14.Misiorowski W, Czajka-Oraniec I, Kochman M, et al. Osteitis fibrosa cystica-a forgotten radiological feature of primary hyperparathyroidism. Endocrine 2017; 58: 380–385. doi: 10.1007/s12020-017-1414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera L, Dolcino M, Mora M, et al. Primary hyperparathyroidism diagnosed after surgical ablation of a costal mass mistaken for giant-cell bone tumor: a case report. J Med Case Rep 2011; 5: 596. Published 2011 Dec 28. doi: 10.1186/1752-1947-5-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocher MS, Gebhardt MC, Jaramillo D, et al. Multiple lytic skeletal lesions and hypercalcemia in a 13-year-old girl. Clin Orthop Relat Res 2000; 374: 298–319. DOI: 10. 1007/s11999-007-0052-z. [DOI] [PubMed] [Google Scholar]
- 17.Scully RE, Mark EJ, McNeely WF, et al. Case records of the Massachusetts General Hospital. N Engl J Med 1993; 328: 1031–1035. DOI: 10.1056/NEJMoa054288.8461068 [Google Scholar]
- 18.Neves MC, Ohe MN, Rosano M, et al. A 10-year experience in intraoperative parathyroid hormone measurements for primary hyperparathyroidism: a prospective study of 91 previous unexplored patients. J Osteoporos 2012; 2012: 914214. doi: 10.1155/2012/914214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udelsman R, Åkerström G, Biagini C, et al. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab 2014; 99: 3595–3606. doi: 10.1210/jc.2014-2000. [DOI] [PubMed] [Google Scholar]
- 20.Edwards ME, Rotramel A, Beyer T, et al. Improvement in the health-related quality-of-life symptoms of hyperparathyroidism is durable on long-term follow-up. Surgery 2006; 140: 655–654. doi: 10.1016/j.surg.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Flint RS, Harman CR, Carter J, et al. Primary hyperparathyroidism: referral patterns and outcomes of surgery. ANZ J Surg 2002; 72: 200–203. [DOI] [PubMed] [Google Scholar]
- 22.Ventz M, Quinkler M. Primary hyperparathyroidism. Dtsch Med Wochenschr 2010; 135: 2024–2030. [DOI] [PubMed] [Google Scholar]
- 23.Goljan E. Rapid Review Pathology. 3rd ed Philadelphia: Elsevier, 2011, p.494. [Google Scholar]
- 24.Duan K, Mete O. Parathyroid carcinoma: diagnosis and clinical implications. Turk Patoloji Derg 2015; 31: 80–97. [DOI] [PubMed] [Google Scholar]
- 25.Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet 2018; 391: 168–178. doi: 10.1016/S0140-6736(17)31430-7. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Qu Y, Shi W, et al. Multiple bone brown tumor secondary to primary hyperparathyroidism: a case report and literature review. Gland Surg 2019; 8: 810–816. doi: 10.21037/gs.2019.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araújo SM, Bruin VM, Nunes AS, et al. Multiple brown tumors causing spinal cord compression in association with secondary hyperparathyroidism. Int Urol Nephrol 2013; 45: 913–916. doi: 10.1007/s11255-012-0123-2. [DOI] [PubMed] [Google Scholar]
- 28.Thakker RV. Genetics of parathyroid tumours. J Intern Med 2016; 280: 574–583. doi: 10.1111/joim.12523. [DOI] [PubMed] [Google Scholar]
- 29.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001; 86: 5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 30.Bilezikian JP, Potts JT, Jr, Fuleihan GH, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Miner Res 2002; 17: N2–N11. [PubMed] [Google Scholar]
- 31.Arnold A, Levine MA. Chapter 18 – Molecular Basis of Primary Hyperparathyroidism//The Parathyroids. Philadelphia: Elsevier Inc, 2015. pp. 279–296. [Google Scholar]
- 32.Eller-Vainicher C, Chiodini I, Battista C, et al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. J Bone Miner Res 2009; 24: S275. [DOI] [PubMed] [Google Scholar]
- 33.Duan K, Gomez Hernandez K, Mete O. Clinicopathological correlates of hyperparathyroidism. J Clin Pathol 2015; 68: 771–787. doi: 10.1136/jclinpath-2015-203186. [DOI] [PubMed] [Google Scholar]
- 34.Christopoulos C, Antoniou N, Thempeyioti A, et al. Familial multiple endocrine neoplasia type I: the urologist is first on the scene. BJU Int 2010; 96: 884–887. [DOI] [PubMed] [Google Scholar]
- 35.Lourenço DM, Jr, Coutinho FL, Toledo RA, et al. Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. J Bone Miner Res 2010; 25: 2382–2391. doi: 10.1002/jbmr.125. [DOI] [PubMed] [Google Scholar]
- 36.Alberto F. Genetics of parathyroids disorders: overview. Best Pract Res Clin Endocrinol Metab 2018; 32: 781–790. doi: 10.1016/j.beem.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Falchetti A, Marini F, Giusti F, et al. DNA-based test: when and why to apply it to primary hyperparathyroidism clinical phenotypes. J Intern Med 2009; 266: 69–83. doi: 10.1111/j.1365-2796.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 38.Cristina EV, Alberto F. Management of familial hyperparathyroidism syndromes: MEN1, MEN2, MEN4, HPT-jaw tumour, familial isolated hyperparathyroidism, FHH, and neonatal severe hyperparathyroidism. Best Pract Res Clin Endocrinol Metab 2018; 32: 861–875. doi: 10.1016/j.beem.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, et al. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg 2015; 4: 82–90. doi: 10.3978/j.issn.2227-684X.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witteveen JE, Van Thiel S, Romijn JA, et al. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol 2013; 168: R45–R53. Published 2013 Feb 20. doi: 10.1530/EJE-12-0528. [DOI] [PubMed] [Google Scholar]
- 41.Kaderli RM, Riss P, Dunkler D, et al. The impact of vitamin D status on hungry bone syndrome after surgery for primary hyperparathyroidism. Eur J Endocrinol 2018; 178: 1–9. doi: 10.1530/EJE-17-0416. [DOI] [PubMed] [Google Scholar]
- 42.Dudek RW. High-yield embryology . 4th ed Philadelphia: Lippincott Williams Wilkins, 2010, p.91. [Google Scholar]
- 43.Scharpf J, Kyriazidis N, Kamani D, et al. Anatomy and embryology of the parathyroid gland. Oper Techn Otolaryngol-Head Neck Surg 2016; 27: 117–121. [Google Scholar]
- 44.Liu X, Sun L, Shao M, et al. Primary hyperparathyroidism due to ectopic parathyroid adenoma in an adolescent: a case report and review of the literature. Endocrine 2019; 64: 38–42. doi: 10.1007/s12020-019-01875-3. [DOI] [PubMed] [Google Scholar]
- 45.Feng L, Zhang X, Liu ST. Surgical treatment of primary hyperparathyroidism due to parathyroid tumor: a 15-year experience. Oncol Lett 2016; 12: 1989–1993. doi: 10.3892/ol.2016.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]