Abstract
Objective
The clinical efficacy of platelet-rich plasma (PRP) in the treatment of osteoarthritis remains controversial. In this paper, we evaluated the clinical efficacy of PRP in the treatment of osteoarthritis using meta-analysis, providing evidence for the selection of clinical treatment options.
Methods
We performed a computer-based search of PubMed, Embase, and the Cochrane Library databases to retrieve articles using the search terms “platelet-rich plasma”, “osteoarthrosis”, and “knee joint”. Quality evaluation and data extraction were performed. The combined effect was assessed using RevMan 5.3 software.
Results
Five randomized controlled trials, involving 320 patients, were included in this study. No significant differences were observed in the International Knee Documentation Committee score, visual analog scale (VAS) score, or the absolute value of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score between the experimental and control groups. The absolute value of the VAS score and change in the WOMAC score were significantly decreased and patient satisfaction was increased in the experimental group, as compared with the control group.
Conclusion
The findings of this meta-analysis suggest that intra-articular injection of PRP is an effective treatment for osteoarthritis that can reduce post-operative pain, improve locomotor function, and increase patient satisfaction.
Keywords: Osteoarthritis, platelet-rich plasma, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, International Knee Documentation Committee (IKDC) score, randomized controlled trial, patient satisfaction
Introduction
Osteoarthritis of the knee is a progressive disease involving the intra-articular, tibiofemoral, and patellofemoral cartilage and the surrounding joints and structures.1 It is one of the most common causes of pain, loss of movement function, and walking-related disabilities in older adults (age > 65 years old) in the United States.2–4 The quality of life of patients with knee osteoarthritis can be severely reduced without intervention. Non-steroidal anti-inflammatory drugs, chondroitin sulfate, glucosamine drugs, hyaluronic acid, and glucocorticoids are the most commonly used conservative methods for treating the disease. However, use of these methods can result in different degrees of adverse reactions. Cartilage tissue has poor healing ability; therefore, only short-term analgesics and anti-inflammatory effects can be achieved, and the long-term clinical efficacy of these treatments is poor.5,6
Platelet-rich plasma (PRP) is an autologous blood product that contains a high concentration of platelets, specifically, 3 to 5 times that of normal blood.7 PRP contains a high concentration of autogenous growth factors, including vascular endothelial growth factor, platelet-derived growth factor, and transforming growth factor-β, which promote the proliferation of chondrocytes and the synthesis of the extracellular matrix.8 PRP is increasingly being used in the field of sports injury because of its simple preparation method, low cost, and high degree of safety.9
The use of PRP injections for the treatment of osteoarthritis has previously been reported.10–12 However, in most studies, PRP treatment has been compared with hyaluronic acid (HA) treatment; only two studies have compared PRP treatment with normal saline treatment. The conclusions of most studies have been that PRP is better than normal saline but that there is no significant difference compared with HA. Hence, there is little evidence to support the clinical efficacy of PRP injections for the treatment of osteoarthritis. In this paper, we analyzed the clinical efficacy of PRP (using normal saline as a control) in the treatment of osteoarthritis in terms of knee pain, knee joint function, and patient satisfaction, based on the latest published randomized controlled trials (RCTs) providing reliable evidence for the clinical treatment of osteoarthritis.
Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Owing to the nature of this study, ethical approval and patient consent were not needed.
Search methods for identification of studies
The search strategy was formulated according to the standards of the Cochrane Collaboration. Subject terms and free terms used were “platelet-rich plasma”, “thrombocyte rich plasma”, “osteoarthritis”, and “knee joint”. Boolean operators were used to search for relevant articles in PubMed, Embase, and the Cochrane Library databases. In addition, references in related papers were retrieved manually. Article retrieval was performed in PubMed applying the following string: Search (((((((Plasma, Platelet-Rich[Title/Abstract]) OR Platelet Rich Plasma[Title/Abstract]) OR thrombocyte rich plasma[Title/Abstract])) OR “Platelet-Rich Plasma”[Mesh])) AND ((“Osteoarthritis”[Mesh]) OR ((((((((((((Osteoarthritides[Title/Abstract]) OR Osteoarthrosis[Title/Abstract]) OR Osteoarthroses[Title/Abstract]) OR Arthritis, Degenerative[Title/Abstract]) OR Arthritides, Degenerative[Title/Abstract]) OR Degenerative Arthritides[Title/Abstract]) OR Degenerative Arthritis[Title/Abstract]) OR Arthrosis[Title/Abstract]) OR Arthroses[Title/Abstract]) OR Osteoarthrosis Deformans[Title/Abstract]) OR Arthritis[Title/Abstract]) OR Arthritides[Title/Abstract]))) AND ((“Knee Joint”[Mesh]) OR (((((((((Joint, Knee[Title/Abstract]) OR Joints, Knee[Title/Abstract]) OR Knee Joints[Title/Abstract]) OR Superior Tibiofibular Joint[Title/Abstract]) OR Joint, Superior Tibiofibular[Title/Abstract]) OR Joints, Superior Tibiofibular[Title/Abstract]) OR Superior Tibiofibular Joints[Title/Abstract]) OR Tibiofibular Joint, Superior[Title/Abstract]) OR Tibiofibular Joints, Superior[Title/Abstract]))
Eligibility criteria
Inclusion criteria
Studies were considered for inclusion if:
patients with osteoarthritis were included;
the study was an RCT and the follow-up time was not less than 6 months;
the study included patients who received PRP injection in the experimental group and normal saline injection in the control group;
the original article was complete and included at least one of following indicators: visual analog scale (VAS) score, International Knee Documentation Committee (IKDC) score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or patient satisfaction score;
the data were true and credible. Indicators that could be transformed into binary or continuous variables were used.
Exclusion criteria
Studies were excluded from this meta-analysis if one or more of the following conditions was met:
the study was a retrospective case report or non-controlled trial;
patients were included with meniscus injury of the knee joint, peripheral fracture, ligament injury, or other diseases;
the study was a case report or conference papers with non-available full-text, or the study included animal experiments or basic research on corpses;
valid data could not be extracted from the study for this meta-analysis.
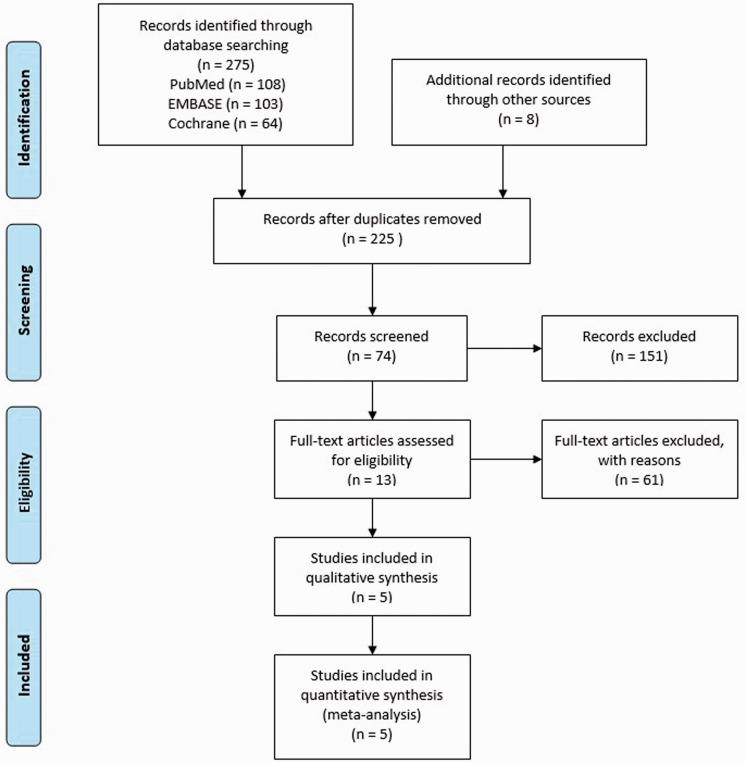
A flowchart of the study selection for this meta-analysis is shown in Figure 1.
Figure 1.
Flowchart of study selection in this meta-analysis.
Outcome measures
The following outcome measures were used to evaluate curative effects:
Absolute value of and change in VAS score: The lower the absolute value of the VAS score, the better the curative effect. The change in VAS score refers to the change in VAS score at 6 months post-surgery relative to the baseline value. A lower change in VAS score indicates better curative effect.13
Absolute value of and change in IKDC score: The IKDC score can be used to evaluate various diseases of the knee joint. It can be used to comprehensively evaluate subjective symptoms and objective signs of the knee joint, with a higher absolute value indicating better curative efficacy.14
Absolute value of and change in WOMAC score: The WOMAC score uses 24 parameters to evaluate osteoarthritis of the hip and knee, with a lower absolute value indicating better therapeutic effects. The change in WOMAC score refers to the WOMAC score at 6 months post-surgery relative to the baseline value. A lower change in WOMAC scores indicates better curative effect.15
Patient satisfaction: The number of patients who were satisfied with the surgery at 6 months post-surgery.16
Assessment of methodological quality
Two investigators independently used the Jadad scale to evaluate the quality of the included studies, with scores lower than 4 indicating low quality.17 When the two investigators did not agree with each other, a third investigator (G.W.L.) resolved the disagreement in discussion with the two investigators.
Data collection
Two investigators independently extracted data from all eligible studies according to a standard form of data extraction. Any disagreements were resolved as described above.
If the data reported in the article were incomplete, the corresponding author was contacted by e-mail to obtain the original data; however, in these cases, no responses were received. In some cases, if the standard deviation (SD) was not reported and no response was received from the authors, the article published by Hou et al.18 was referred to. The range or median was estimated, or the method described in the Cochrane Handbook for Systematic Reviews of Interventions was used to convert the data, and the SD was estimated based on the confidence interval (CI).
Statistical analysis
Heterogeneity among the included studies was tested and analyzed using the chi-squared test. When I2 > 50%, the random-effects model was used; otherwise, the fixed-effects model was used. The relative risk (RR) was calculated for binary variables and the standard median deviation for continuous variables.19 The 95% CI estimates and hypothesis test results for each variable are shown in a forest plot. Outcome indicators with significant heterogeneity were successively excluded from the literature, and sensitivity analysis was performed to assess the source of heterogeneity. Statistical analyses were performed using RevMan version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Search results and characteristics of selected studies
Of the 225 potential published studies, five met our inclusion criteria20–24 (Figure 1), of which five were RCTs. A total of 320 patients were included in this meta-analysis. There were 165 cases in the experimental group and 155 cases in the control group. The quality of each RCT was scored according to the Jadad scale. The basic characteristics and score results of the included studies are shown in Table 1. The Jadad scale scores ranged from 5 to 7, and all included studies were of high quality.
Table 1.
Main characteristics of all eligible studies included in this meta-analysis.
| Author, year | Study type |
Number |
Mean age |
Follow-up (months) |
Jadad | |||
|---|---|---|---|---|---|---|---|---|
| Surg | NS | Surg | NS | Surg | NS | |||
| Patel et al., 2013 [20] | RCT | 50 | 46 | 51.64 | 53.65 | 6 | 6 | 5 |
| Görmel et al., 2015 [21] | RCT | 39 | 40 | 53.7 | 52. | 6 | 6 | 5 |
| Smith et al., 2016 [22] | RCT | 15 | 15 | 53.53 | 46.6 | 12 | 12 | 6 |
| Lin et al., 2019 [23] | RCT | 31 | 27 | 61.17 | 62.23 | 12 | 12 | 7 |
| Elik et al., 2019 [24] | RCT | 30 | 27 | 61.3 | 60.19 | 6 | 6 | 7 |
RCT: randomized controlled trial; Surg: surgical intervention; NS: non-surgical intervention.
Absolute value and change value of VAS scores
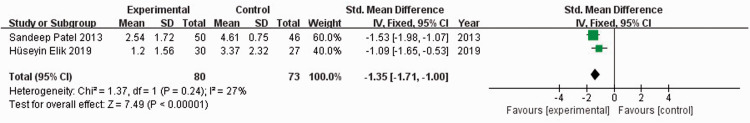
Two previous studies20,24 reported VAS scores for 153 patients. When I2 = 27%, the fixed-effects model was used to compare the absolute value of VAS scores. The absolute value of the VAS score in the experimental group was significantly lower than that of the control group (standardized mean difference [SMD] = −1.35, 95% CI: −1.71 to −1.09, P < 0.00001) (Figure 2). When I2 = 90%, the random-effects model was used to compare the change value of VAS scores. There was no significant difference in the change value of VAS scores between the experimental and control groups (SMD = −1.11, 95% CI: −2.24 to −0.03) (Figure 3). Sensitivity analysis could not be performed because only two studies were included.
Figure 2.
Forest plot showing absolute values of visual analog scale scores.
Figure 3.
Forest plot showing change values of visual analog scale scores.
Absolute value and change value of IKDC scores
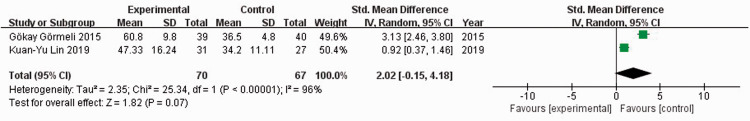
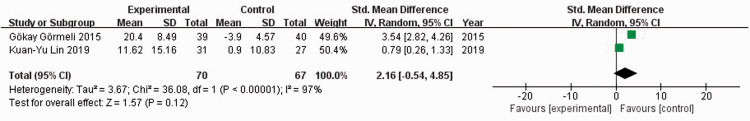
Two studies21,23 reported IKDC scores among 137 patients. When I2 = 96%, the random-effects model was used to compare the absolute value of IKDC scores. There was no significant difference in the absolute value of IKDC scores between the experimental and control groups (SMD = 2.02, 95% CI: −0.15 to 4.18) (Figure 4). When I2 = 97%, the random-effects model was used to compare the change value of IKDC scores. There was no significant difference in change values of IKDC scores between the experimental and control groups (SMD = 2.16, 95% CI: −0.54 to 4.85) (Figure 5). Sensitivity analysis could not be performed because only two studies were included.
Figure 4.
Forest plot showing absolute values of the International Knee Documentation Committee scores.
Figure 5.
Forest plot showing change values of the International Knee Documentation Committee scores.
Absolute value and change value of WOMAC score
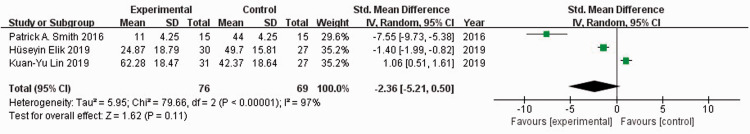
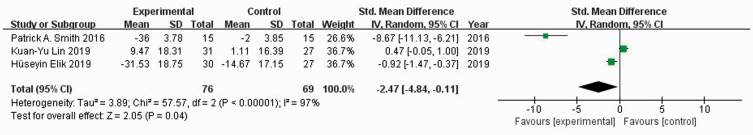
Three studies22–24 reported WOMAC scores in 278 patients. When I2 = 97%, the random-effects model was used to compare the absolute value of the WOMAC score. There was no significant difference in the absolute value of WOMAC scores between the experimental and control groups (SMD = −2.36, 95% CI: −5.21 to 0.50) (Figure 6). When I2 = 97%, the random-effects model was used to compare the change value of WOMAC scores. The WOMAC score of the experimental group was significantly lower than that of the control group (SMD = −2.47, 95% CI: −4.84 to −0.11, P = 0.04) (Figure 7). Sensitivity analysis did not reveal the source of heterogeneity.
Figure 6.
Forest plot showing absolute values of the Western Ontario and McMaster Universities Osteoarthritis Index scores.
Figure 7.
Forest plot showing the change in Western Ontario and McMaster Universities Osteoarthritis Index scores.
Patient satisfaction
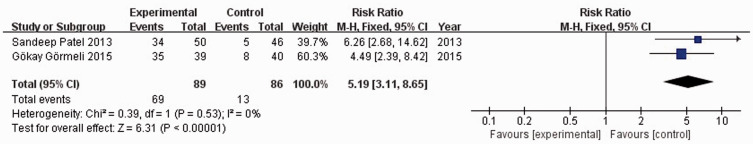
Two studies20,21 reported patient satisfaction among 175 patients. When I2 = 0%, the fixed-effects model was used to compare patient satisfaction. Patient satisfaction in the experimental group was significantly higher than that in the control group (RR = 5.19, 95% CI: 3.11 to 8.65, P < 0.00001) (Figure 8).
Figure 8.
Forest plot showing patient satisfaction.
Discussion
The findings of this meta-analysis indicated that PRP shows obvious clinical advantages in the treatment of osteoarthritis. Although there was no significant difference in IKDC scores between the experimental and control groups, the VAS and WOMAC scores were significantly decreased and patient satisfaction was increased in the experimental group compared with the control group.
VAS score is used to evaluate pain. The results of this meta-analysis showed that the change in VAS score in the experimental group was significantly lower than that in the control group; that is, pain intensity in the experimental group was lower than that in the control group. However, there was no significant difference in the change in VAS score. This could be because of the small sample size and differences between the experimental and control groups in baseline data. Strong evidence25–28 exists that PRP can relieve pain; intra-articular injection of PRP can reduce the expression of pain mediators, such as prostaglandin E2, substance P, dopamine, and 5-hydroxytryptamine. Moreover, PRP contains large amounts of platelet-derived growth factor, insulin-like growth factor, transforming growth factor-β, and vascular endothelial growth factor, which can promote the synthesis of cartilage matrix, stimulate the proliferation of chondrocytes, inhibit the local inflammatory response, and regulate the microenvironment. In addition, inflammatory regulatory factors in PRP can inhibit the nuclear factor kappa B pathway, thereby inhibiting the leukotriene-mediated degradation of the cartilage matrix and the expression of inflammatory factors. These factors can also promote the proliferation of stem cells and the secretion of proteoglycan and collagen, acting synergistically with multiple growth factors to repair articular cartilage injury and relieve pain. Therefore, PRP treatment can effectively relieve pain and achieve good clinical outcomes in combination with other treatments.
The WOMAC score is used to evaluate the severity of osteoarthritis, according to patient symptoms and physical signs. This scale consists of pain, stiffness, and function sub-scales. The results of this meta-analysis revealed that there were no significant differences in the absolute value of the WOMAC score, but the change in WOMAC scores in the experimental group was significantly smaller than that of the control group, that is, the clinical effect in the experimental group was superior to that in the control group. The high heterogeneity seen in the study by Lin et al.23 can be attributed to the use of leukocyte-poor PRP. In that study, although the absolute value of the WOMAC score in the experimental group was not significantly lower than that in the control group, the change in WOMAC score was significantly different between the experimental and control groups. Therefore, PRP is suggested to improve joint function. PRP can improve stiffness and joint function because it contains a large amount of growth factors, which greatly promote tissue injury repair.29 Moreover, Krüger et al.30 found that PRP can induce the migration and differentiation of subchondral mesenchymal progenitor cells to form cartilage. Cartilage damage occurs during the development of knee osteoarthritis, as seen by arthroscopic examination of over 60% patients with osteoarthritis of the knee joint.31 The chondrocytes of the joint have a poor ability to divide and self-heal after injury,32 and PRP can promote the repair of cartilage injury. In addition to lubricating the joints, PRP can prevent aseptic inflammation, which can reduce symptoms and improve joint function.
The results of this meta-analysis showed that there was no significant difference in IKDC scores between the experimental and control groups, in contrast to the difference in WOMAC scores. This may be owing to different emphases for each scoring standard, subjective assessment by patients, and the small sample size. The IKDC score can be used to evaluate various diseases of the knee joint. It is highly reliable and sensitive for the assessment of anterior cruciate ligament injury, but it has poor reliability in the assessment of the basic living condition of patients.
Patient satisfaction in the experimental group was higher than that in the control group. This possibly occurred because PRP can reduce pain and improve joint function, thereby increasing patient satisfaction.
Strengths and limitations
In this meta-analysis, we comprehensively evaluated several indicators related to the treatment of osteoarthritis. The change value, which removes the impact of different baseline conditions and makes the results more objective, was considered when scoring indicators. In addition, only RCTs were included, which led to high-quality results. However, this meta-analysis has the following limitations. (1) Regression analysis or other methods were not used to identify the source of heterogeneity, and publication bias was not evaluated because fewer than 10 studies were included. (2) There were no uniform standards for PRP preparation and application, which may have led to heterogeneity among studies.
Conclusion
The findings of this meta-analysis suggest that PRP exhibits obvious advantages in the treatment of osteoarthritis. PRP injection can reduce post-operative pain, improve knee function, and lead to high patient satisfaction. Greater efforts should be made to optimize and investigate the analgesic effect of PRP in terms of age, body mass index, and other indicators based on a larger number of studies.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Wenlai Guo https://orcid.org/0000-0002-3494-9076
References
- 1.Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthr Cartil 2011; 19: 478–472. [DOI] [PubMed] [Google Scholar]
- 2.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R 2012; 4: S10–S19. [DOI] [PubMed] [Google Scholar]
- 3.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006; 20: 3–25. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000; 133: 635–646. [DOI] [PubMed] [Google Scholar]
- 5.McCrum C. Therapeutic Review of Methylprednisolone Acetate Intra-Articular Injection in the Management of Osteoarthritis of the Knee - Part 1: Clinical Effectiveness. Musculoskeletal Care 2017; 15: 79–88. [DOI] [PubMed] [Google Scholar]
- 6.McCrum C. Therapeutic Review of Methylprednisolone Acetate Intra-Articular Injection in the Management of Osteoarthritis of the Knee -Part 2: Clinical and Procedural Considerations. Musculoskeletal Care 2016; 14: 252–266. [DOI] [PubMed] [Google Scholar]
- 7.Paterson KL, Hunter DJ, Metcalf BR, et al. Efficacy of intraarticular injections of platelet-rich plasma as a symptom- and disease-modifying treatment for knee osteoarthritis - the RESTORE trial protocol. BMC Musculoskelet Disord 2018; 19: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil 2013; 21: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R 2011; 3: 226–250. [DOI] [PubMed] [Google Scholar]
- 10.Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 2010; 18: 472–479. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez M, Anitua E, Azofra J, et al. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 2008; 26: 910–913. [PubMed] [Google Scholar]
- 12.Xu Z, Luo J, Huang X, et al. Efficacy of Platelet-Rich Plasma in Pain and Self-report Function in Knee Osteoarthritis. Am J Phys Med Rehabil 2017; 96: 793–800. [DOI] [PubMed] [Google Scholar]
- 13.Paul-Dauphin A, Guillemin F, Virion JM, et al. Bias and precision in visual analogue scales: a randomized controlled trial. Am J Epidemiol 1999; 150: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 14.Haverkamp D, Sierevelt I N, Breugem SJM, et al. Translation and validation of the Dutch version of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med 2006; 34: 1680–1684. [DOI] [PubMed] [Google Scholar]
- 15.Xie F, Li SC, Goeree R, et al. Validation of Chinese Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in patients scheduled for total knee replacement. Qual Life Res 2008; 17: 595. [DOI] [PubMed] [Google Scholar]
- 16.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of International Knee Documentation Committee Subjective Knee Form. Am J Sports Med 2001; 29: 600. [DOI] [PubMed] [Google Scholar]
- 17.Eppley BL, Pietrzak WS, Blanton M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast Reconstr Surg 2006; 118: 147e–159e. [DOI] [PubMed] [Google Scholar]
- 18.Xiaowen H, Pu WK, Xin C. How to Estimate the Mean and Standard Deviation Based on the Median, Range and Sample Size when Conducting Meta-Analysis. Chin J Evid Based Med 2015; 4: 484–487. [Google Scholar]
- 19.Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001; 323: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S, Dhillon MS, Aggarwal S, et al. Treatment With Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Trial. Am J Sports Med 2013; 41: 356–364. [DOI] [PubMed] [Google Scholar]
- 21.Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017; 25: 958–965. [DOI] [PubMed] [Google Scholar]
- 22.Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med 2016; 44: 884–891. [DOI] [PubMed] [Google Scholar]
- 23.Lin KY, Yang CC, Hsu CJ, et al. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy 2019; 35: 106–117. [DOI] [PubMed] [Google Scholar]
- 24.Elik H, Doğu B, Yılmaz F, et al. The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J Back Musculoskelet Rehabil 2020: 33: 127–138. [DOI] [PubMed] [Google Scholar]
- 25.Weiner BK, Walker M. Efficacy of Autologous Growth Factors in Lumbar Intertransverse Fusions. Spine 2003; 28: 1968–1970. [DOI] [PubMed] [Google Scholar]
- 26.Spaková T, Rosocha J, Lacko M, et al. Treatment of Knee Joint Osteoarthritis with Autologous Platelet-Rich Plasma in Comparison with Hyaluronic Acid. Am J Phys Med Rehabil 2012; 91: 411–417. [DOI] [PubMed] [Google Scholar]
- 27.Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive Effect of an Autologous Platelet Concentrate in Lateral Epicondylitis in a Double-Blind Randomized Controlled Trial: Platelet-Rich Plasma Versus Corticosteroid Injection With a 1-Year Follow-up. Am J Sports Med 2010; 38: 255–262. [DOI] [PubMed] [Google Scholar]
- 28.Oudsten BLD. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: A double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med 2011; 39: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 29.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85: 638–646. [DOI] [PubMed] [Google Scholar]
- 30.Krüger JP, Hondke S, Endres M, et al. Human Platelet-Rich Plasma Stimulates Migration and Chondrogenic Differentiation of Human Subchondral Progenitor Cells. J Orthop Res 2012; 30: 845–852. [DOI] [PubMed] [Google Scholar]
- 31.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007; 14: 177–182. [DOI] [PubMed] [Google Scholar]
- 32.Benthien J P, Schwaninger M, Behrens P. We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 2011; 19: 543–552. [DOI] [PubMed] [Google Scholar]