To the editor,
Profiling of the antibody responses to SARS-CoV-2 may be crucial to understand the immunological reaction and to design successful treatment strategies. Studies have confirmed the development of a typical antibody response to an acute viral infection in COVID-19 patients [1]. A robust generation and a dynamic pattern of IgA, IgM and IgG antibodies can be detected 2–3 weeks following the first symptoms of COVID-19 [2, 3]. An early robust antibody response in patients hospitalized with severe COVID-19 was reported in survivors, versus a weak antibody production in non-survivors [4, 5]. However, antibody responses to SARS-CoV-2 in critically ill patients is largely unknown.
We investigated the antibody response to SARS-Cov-2 Spike-1 protein in adult patients (n = 19) admitted to the intensive care unit (ICU) at a tertiary care hospital in Uppsala, Sweden. Plasma samples were collected at two different time points; (a) early, day 0–3 and (b) late, day 10–13, and concentrations of IgA, IgG and IgM antibodies were quantified by FluoroEnzymeImmunoassay (FEIA), Phadia AB, Uppsala, Sweden.
The median age of our cohort was 57 years, and 93% were males. The most common co-morbidities were obesity (93%), hypertension (42%) and diabetes mellitus type-2 (32%). The median COVID-19 day at ICU admission was 10 (6–14) days, the median length of ICU stay was 18 (11–38) days, and 30 days mortality was 21% (Table 1). In our cohort, a IgA, IgG and IgM antibody response could be detected as early as day 0–3 post-ICU admission, and this increase in antibodies was persistent up to day 10–13 (Fig. 1). A significant change in antibody concentrations over time was detected in patients who survived till day 30 in comparison with those who did not (Fig. 1). No associations were seen between antibody levels and patient age, or any other clinical or laboratory parameters. At both early and late timepoints, plasma concentrations of IgA, IgG and IgM antibodies tend to be higher in patients who survived compared to those who had died at 30 days (Fig. 1). This suggests that SARS-CoV-2 antibody response, similar to other virus illnesses, is important for virus protection and recovery. A limitation of the present dataset is the relatively low number of patients.
Table 1.
Clinical characteristics of COVID-19 patients (n = 19) with at least 10 days length-of-stay in intensive care unit (ICU)
| Variables | |
|---|---|
| Cohort characteristics | Median (range) |
| Age, years, median (range) | 57 (26–76) |
| Male, n (%) | 18 (93) |
| COVID-19 days, median (range) | 10 (6–14) |
| Length-of-stay in ICU, median (range) | 18 (11–38) |
| 30-day mortality, n (%) | 4 (21) |
| Comorbidities | n (%) |
| Obesity (BMI > 25) | 18 (93) |
| Diabetes mellitus | 6 (32) |
| Hypertension | 8 (42) |
| Pulmonary disease | 4 (21) |
| Cardiovascular disease | 3 (16) |
| Clinical parameters | Median (range) |
| Fever (> 38 °C), n (%) | 15 (79) |
| Mean arterial pressure, mmHg, median (range) | 94 (68–137) |
| Heart rate, min−1, median (range) | 92 (67–116) |
| Respiratory rate, min−1, median (range) | 31 (15–50) |
| SAPS3 score, median (range) | 49 (39–63) |
| Laboratory parameters | |
| SOFA score | 7 (3–9) |
| CRP mg/L | 241 (131–476) |
| Ferritin μg/L | 676 (104–3960) |
| Lactate mmol/L | 1.1 (0.8–1.7) |
SAPS3 simplified acute physiology score-3, SOFA score sequential organ failure assessment score, CRP C-reactive protein
Fig. 1.
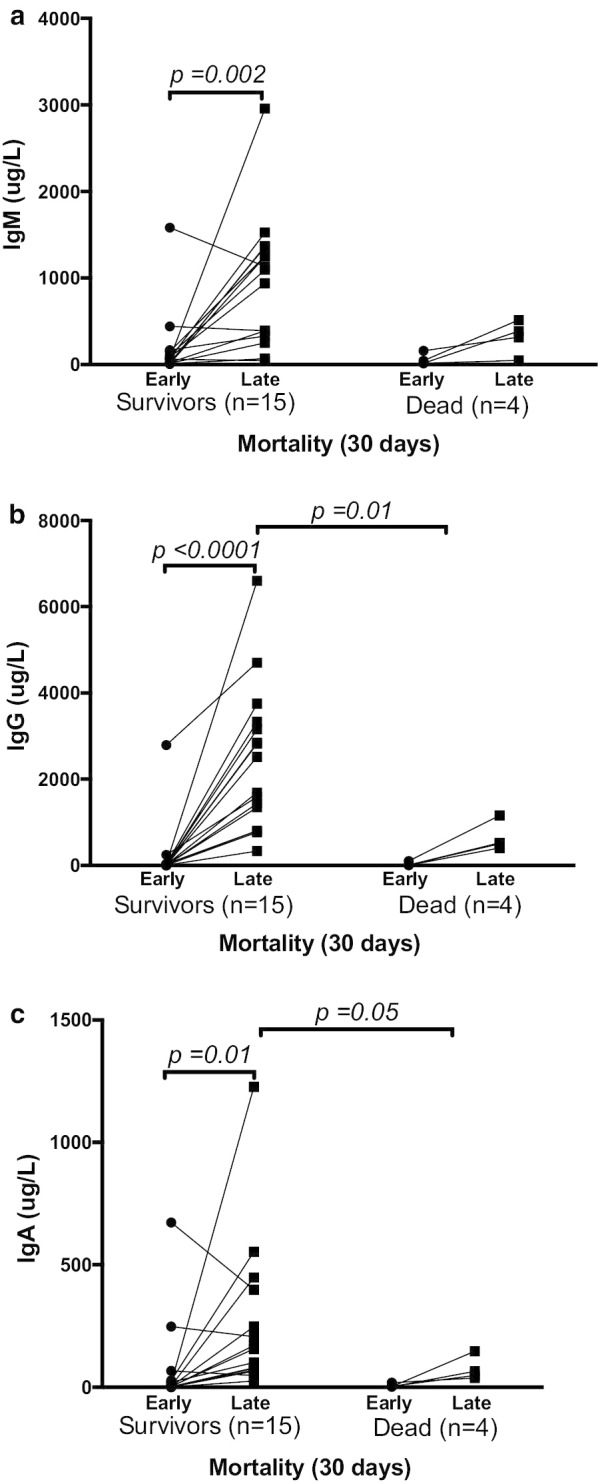
Plasma concentrations of IgM (a), IgG (b) and IgA (c) antibodies measured on ICU day 0–3 (early) and on ICU day 10–13 (late), in patients who survived (n = 15) of COVID-19 versus those who died (n = 4) within 30 days. Each data point on the graph represents individual values, differences were considered statistically significant when P < 0.05. Change over time within groups was determined by Wilcoxon signed rank test and between groups were determined by Mann–Whitney test. Plasma concentrations of all three antibody isotypes changed over time and were significantly higher on day 10–13 for IgG and IgA in patients who survived COVID-19 than in those who died
To our knowledge, this study provides the earliest evidence that an early and potent antibody response may contribute to infection clearance and improved prognosis in patients critically ill with COVID-19.
Acknowledgements
The authors thank research nurses Joanna Wessbergh and Elin Söderman, and the biobank assistants Erik Danielsson and Philip Karlsson for their expertise in compiling the study.
Authors’ contributions
All authors participated in conception and design of the study. All authors had access to the data. SA drafted the manuscript. All authors contributed to manuscript revision. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Uppsala University. The study was funded by the SciLifeLab/KAW national COVID-19 research program project Grant to MH (KAW 2020.0182), and the Swedish Research Council to RF (2014-02569 and 2014-07606).
Availability of data and materials
Data in the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The presented data are part of a study approved by the National Ethical Review Agency (EPM; No. 2020-01623). Informed consent was obtained from the patient, or next of kin if the patient was unable to give consent. The Declaration of Helsinki and its subsequent revisions were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu X, Wang J, Xu X, Liao G, Chen Y, Hu C-H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Z, Chen H, Xue M, Huang H, Zheng P, Luo W, et al. Characteristics and roles of severe acute respiratory syndrome coronavirus 2-specific antibodies in patients with different severities of coronavirus 19. Clin Exp Immunol. 2020;7(24):3404. doi: 10.1111/cei.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longchamp A, Longchamp J, Croxatto A, Greub G, Sanchez B, Delaloye J. Serum antibody response in critically ill patients with COVID-19. Intensive Care Med. 2020;46(10):1921–1923. doi: 10.1007/s00134-020-06171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fourati S, Hue S, Pawlotsky J-M, Mekontso-Dessap A, de Prost N. SARS-CoV-2 viral loads and serum IgA/IgG immune responses in critically ill COVID-19 patients. Intensive Care Med. 2020;46(9):1781–1783. doi: 10.1007/s00134-020-06157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Guo J, Xu G, Cai G, Chen D, Shen Y. Detection of IgG antibody during the follow-up in patients with COVID-19 infection. Crit Care. 2020;24(1):448. doi: 10.1186/s13054-020-03138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in the current study are available from the corresponding author on reasonable request.


