Abstract
Background
Triple-negative breast cancer (TNBC) is associated with higher aggressiveness and mortality than hormone-positive breast cancer because of the lack of approved therapeutic targets. Patients with TNBC who attain a pathological complete response (pCR) after neoadjuvant chemotherapy have improved survival. Platinum-based agents show promising activity in TNBC; however, their use remains controversial. We conducted a meta-analysis to assess the role of platinum-based agents in neoadjuvant chemotherapy in patients with TNBC.
Methods
We performed an extensive literature search of the Pubmed, Embase, and Cochrane databases. We calculated pooled odds ratios (OR) with 95% confidence intervals (CI) for the identified studies.
Results
Eight randomized controlled trials with 1345 patients were included in the analysis. The addition of platinum-based agents improved pCR compared with neoadjuvant therapy based on anthracyclines, cyclophosphamide, taxanes, and fluorouracil (49.1% vs. 35.9%; OR: 1.87, 95% CI: 1.23–2.86). Hematological adverse events were similar in both groups, except for more thrombocytopenia in the platinum-based group (OR: 7.96, 95% CI: 3.18–19.93).
Conclusion
The addition of platinum-based agents to neoadjuvant chemotherapy improved pCR rates in patients with TNBC, with a slight increase in hematological toxicities. Platinum-based agents might thus be an accessible and economically viable option in patients with TNBC.
Keywords: Triple-negative breast cancer, neoadjuvant therapy, platinum-based agent, pathological complete remission, thrombocytopenia, adverse event
Introduction
Breast cancer is one of the most prevalent malignant tumors among women worldwide. Triple-negative breast cancer (TNBC) is a specific subtype characterized by the absence of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression. In contrast with other subtypes, TNBC is more aggressive and associated with higher mortality, due to a lack of approved therapeutic targets.1
Chemotherapy is currently the only acknowledged systemic treatment option for TNBC. Despite its aggressiveness, TNBC is highly chemosensitive, especially in the neoadjuvant setting, with higher pathological complete response (pCR) rates after neoadjuvant chemotherapy compared with hormone-positive breast cancer.2–4 Additionally, patients with TNBC who attain pCR have improved survival.4,5 Pursuing higher pCR rates has thus become an important goal of neoadjuvant chemotherapy for TNBC. As cytotoxic DNA-damaging agents, platinum-based agents (i.e. carboplatin and cisplatin) can cause DNA strand breaks and consequent cell apoptosis.6 More than half of all TNBCs have some degree of homologous DNA recombination defects,7 suggesting that they might be more sensitive to platinum damage. Based on this biological rationale, several studies have evaluated the impact of adding platinum-based agents to standard chemotherapy; however, the results are conflicting.8–10 Meanwhile, current recommendations in several international breast cancer guidelines are also disputed.11–14
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to provide up-to-date evidence on the controversial role of platinum-based agents in neoadjuvant chemotherapy in patients with TNBC.
Methods
Search strategy
An extensive literature search of the PubMed, Embase, and Cochrane databases was performed from their inception to 2 February 2020, with no language restriction, using the following MeSH terms and/or text words: ‘breast neoplasm’, ‘breast cancer’, ‘breast carcinoma’, ‘breast tumor’, ‘mammary cancer’, ‘breast malignant tumor’, ‘neoadjuvant therapy’, ‘neoadjuvant treatment’, ‘neoadjuvant therapy’, ‘cisplatin’, ‘carboplatin’, ‘platinum’, and ‘Platidiam’. If duplicate trials were identified, the most recent and complete article was included.
Inclusion and exclusion criteria
Eligible studies had to fulfill the following inclusion criteria: (1) RCTs containing at least two treatment groups of platinum-based neoadjuvant chemotherapy and platinum-free neoadjuvant chemotherapy; (2) adult (18 years and above) TNBC patients; and (3) reported pCR as outcome. The exclusion criteria were as follows: (1) studies with incomplete or missing information; (2) reviews and case reports; (3) inflammatory breast cancer; (4) metastatic breast cancer; and (5) involving drugs other than anthracyclines, cyclophosphamide, taxanes, and fluorouracil.
Two authors independently carried out the literature searches and identified eligible articles based on the above criteria. Disagreements were resolved by discussion and consensus, or by a third investigator.
Data extraction
The following information was extracted from each article by two investigators independently: (1) study information, including the first author’s name and year of publication; and (2) trial design, including study design arms, therapy regimens, number of patients in each arm, and adverse events.
Risk of bias assessment
The risk of bias of the eligible studies was assessed using the Cochrane Collaboration bias assessment tool for systematic review of interventions. Selection bias (parameters of details of random sequence generation and allocation concealment), performance bias (blinding for participants and personnel), detection bias (blinding for outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. The risk of bias was stratified as high, low, or unclear.
Publication biases
Publication bias was evaluated by funnel plots and measured using Egger’s and Begg’s tests with Stata version 15.1 software (Stata, College Station, TX, USA). We also executed t-tests to determine the significance of the intercept, with a P value <0.05 deemed to be statistically significant.
Statistical analysis
The meta-analysis was conducted via Review Manager (version 5.3.5; the Cochrane Collaboration, Oxford, UK). Pooled odds ratio (ORs) with corresponding 95% confidence intervals (CI) were used to calculate dichotomous variables. Between-study heterogeneity was assessed using the χ2-based Q statistic. A random-effect model was used if the P value was < 0.10 in the Q-test, otherwise a fixed-effect model was applied. Classic forest plots were used to present the meta-analysis results, and statistical significance was set at P < 0.05. Sensitivity analyses were used to estimate the influence of individual studies on the overall effect.
Results
Search results
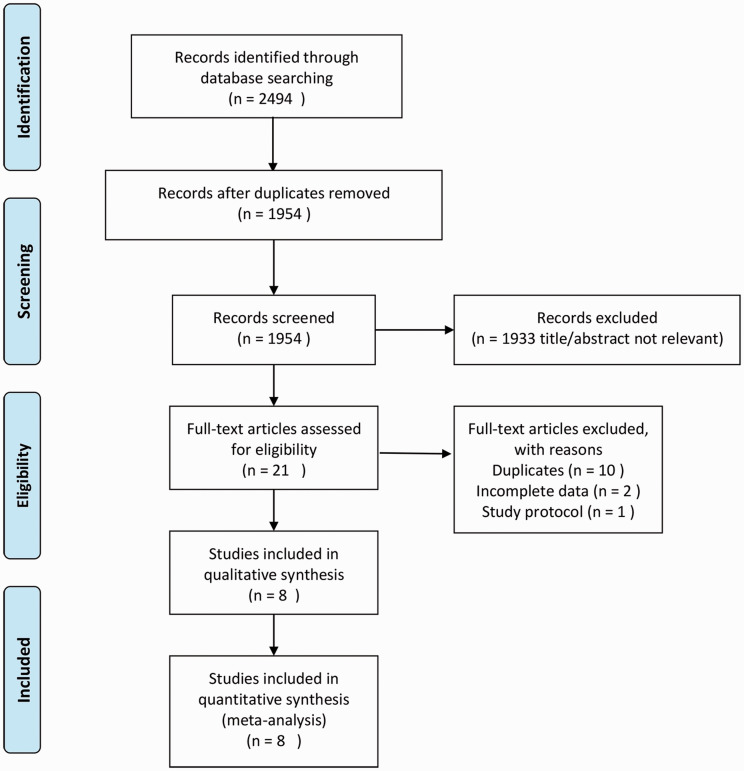
Based on the predefined search strategy, 2494 records were initially identified for evaluation. After checking the titles, abstracts, and full-texts of the records based on the above inclusion and exclusion criteria, eight studies and 1345 patients were finally eligible for the meta-analysis. The PRISMA flow chart is shown in Figure 1. The characteristics of the eight included studies are summarized in Table 1.
Figure 1.
Flow-chart of the literature search.
Table 1.
Characteristics of eligible studies considered in this meta-analysis.
| Study | Year | Type of study |
Regimen |
Number of patients |
Hematological adverse events |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Platinum | Control | Platinum | Control | Anemia | Neutropenia | Thrombocytopenia | Febrile neutropenia | |||
| Aguilar Martinez et al.17 | 2015 | RCT | Cis + P→Cis + Dox | P → FAC | 30 | 31 | No | No | No | No |
| Alba et al.8 | 2012 | RCT | EC→DCb | EC→D | 48 | 46 | Yes | Yes | Yes | Yes |
| Ando et al.18 | 2014 | RCT | PCb→CEF | P→CEF | 37 | 38 | No | No | No | No |
| Loibl et al.15 | 2018 | Subgroup in RCT | PCb→AC | P→AC | 160 | 158 | Yes | Yes | Yes | Yes |
| Schneeweiss et al.9 | 2019 | RCT | PM(Cb) | iddEPC | 203 | 200 | No | No | No | No |
| Sikov et al.16 | 2015 | Subgroup in RCT | wPCb→ddAC | wP→ddAC | 113 | 108 | No | Yes | Yes | Yes |
| Zhang et al.10 | 2016 | RCT | PC | EP | 47 | 44 | No | Yes | Yes | No |
| Zhao et al.19 | 2014 | RCT | TC | TE | 38 | 44 | Yes | Yes | Yes | No |
A, adriamycin; C, cyclophosphamide; Cb, carboplatin; Cis, cisplatin; D, docetaxel; Dox, doxorubicin; E, epirubicin; M, non-pegylated liposomal doxorubicin; P, paclitaxel; RCT, randomized controlled trial; T, taxol; wP, weekly paclitaxel; idd, intense dose-dense.
Two of the eight RCTs involved more than two neoadjuvant chemotherapy regimen groups.15,16 Data for the eligible groups were extracted and they were considered as separate studies in the final analysis. Another study included all four subtypes of breast cancer,9 but only the data pertaining to TNBC patients were considered in this meta-analysis. A total of 1345 patients were included from the final selected studies, of whom 676 received platinum-based neoadjuvant chemotherapy and 669 received platinum-free chemotherapy.
Only two studies reported all four types of hematological adverse events (neutropenia, anemia, thrombocytopenia, and febrile neutropenia),8,15 and three studies reported none.9,17,18 pCR was defined as the absence of residual invasive disease on evaluation of the resected breast specimens and resected lymph nodes (ypT0/is ypN0) in all the included studies except one,19 which defined pCR as the absence of invasive cancer and in situ cancer in the breast and axillary nodes (ypT0 ypN0).
Quality assessment
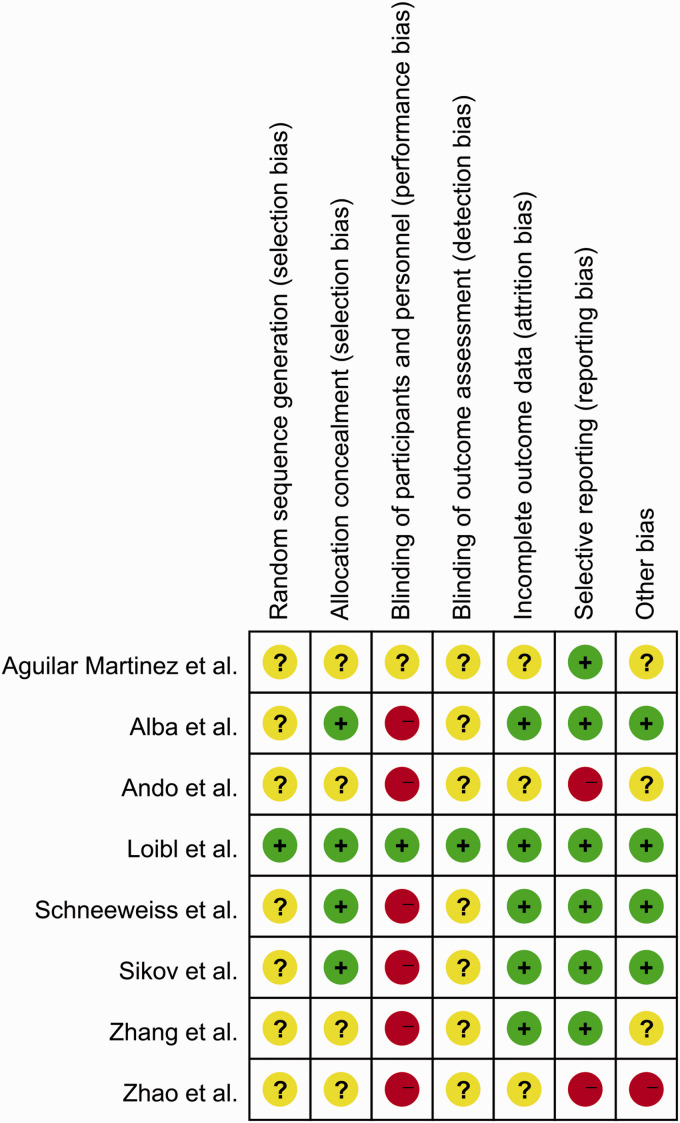
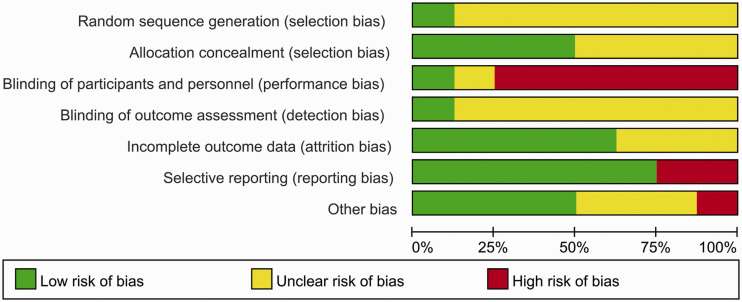
The risks of bias for the eight included studies were appraised according to the Cochrane risk of bias tool. All studies randomly allocated patients to the treatment arms, but only one study15 referred to the specific randomization methods (Figure 2). Four studies were performed with allocation concealment, according to the centralization method.8,9,15,16 Only one study announced that all trial personnel and participants were masked to the assignment throughout the study course.15 Two studies had no registration information.18,19 Overall, these characteristics suggested moderate risks of study-design bias (Figure 3).
Figure 2.
Quality assessment for risk of bias for the included randomized controlled trials.
Figure 3.
Risk of bias for the included randomized controlled trials.
pCR rates
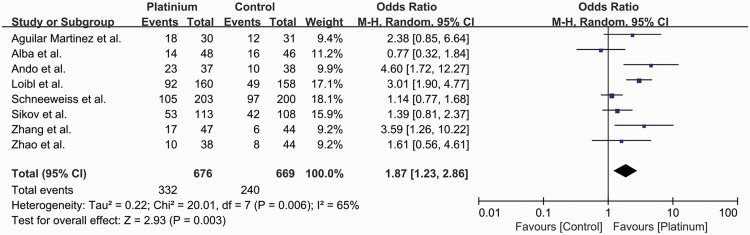
Among all eight studies, 572 of 1345 (42.5%) patients achieved pCR after neoadjuvant treatment, including 332 out of 676 (49.1%) in the platinum-based chemotherapy group and 240 out of 669 (35.9%) in the control group (OR 1.87, 95% CI 1.23–2.86, P = 0.003) (Figure 4). An absolute 13.2% increase in pCR rate was observed in patients treated with carboplatin-based neoadjuvant chemotherapy. The studies had high heterogeneity (I2 = 65%, P = 0.006) and were evaluated using a random-effect model.
Figure 4.
Odds ratios for pathological complete response in patients treated with platinum-based vs. platinum-free (control) neoadjuvant chemotherapy in the included randomized controlled trials.
CI, confidence interval; M-H, Mantel–Haenszel; df, degrees of freedom.
Sensitivity analysis
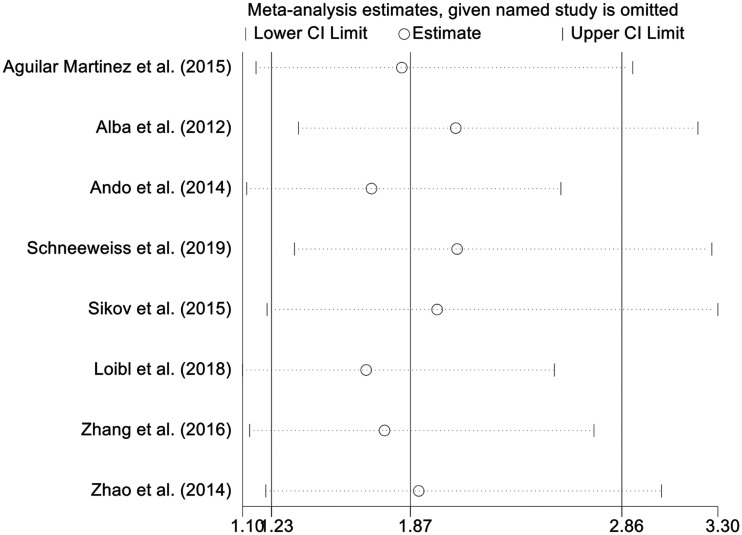
Sensitivity analysis was conducted by excluding each study sequentially. The results showed stable pooled OR estimates (Figure 5).
Figure 5.
Forest plot showing odds ratios of pathological complete response after removing each study (random-effects model with 95% confidence interval).
CI, confidence interval.
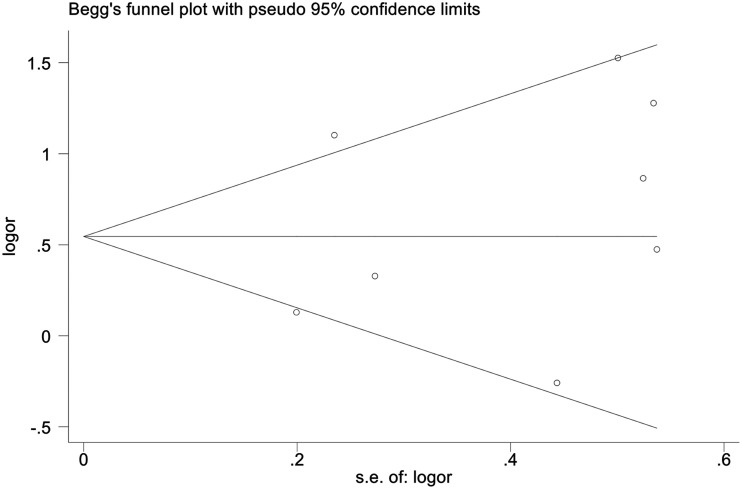
Publication bias
No publication bias was detected by funnel plot (Figure 6).
Figure 6.
Funnel plots for publication bias.
SE, standard error; OR, odds ratio.
Grade 3 and 4 hematological adverse events
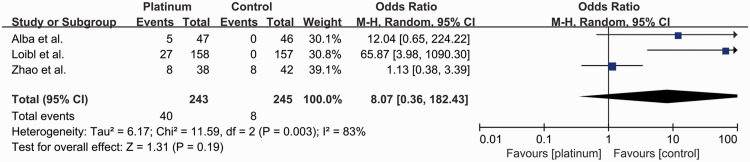
Three studies reported the rates of grade 3 and 4 anemia8,15,19 (Figure 7). Overall, 48 out of 488 patients (9.8%) developed grade 3 and 4 anemia, including 40 out of 243 (16.5%) in the platinum-based group and eight out of 245 (3.3%) in the control group (OR: 8.07, 95% CI: 0.36–182.43). There was no significant difference between the groups, with significant heterogeneity (I2 = 83%, P = 0.003).
Figure 7.
Odds ratios for grade 3–4 anemia in patients treated with platinum-based vs. platinum-free (control) neoadjuvant chemotherapy in the included randomized controlled trials.
CI, confidence interval; M-H, Mantel–Haenszel; df, degrees of freedom.
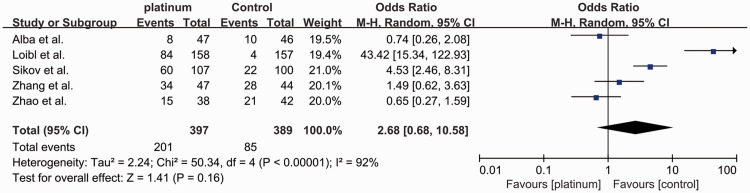
Five studies reported the rates of grade 3 and 4 neutropenia8,10,15,16,19 (Figure 8). A total of 286 out of 786 patients (36.4%) developed grade 3 and 4 neutropenia, including 201 out of 397 (50.6%) in the platinum-based group and 85 out of 389 (21.9%) in the control group (OR: 2.68, 95% CI: 0.68–10.58). There was no significant difference between the groups, with significant heterogeneity (I2 = 92%, P < 0.00001).
Figure 8.
Odds ratios for grade 3–4 neutropenia in patients treated with platinum-based vs. platinum-free (control) neoadjuvant chemotherapy in the included randomized controlled trials.
CI, confidence interval; M-H, Mantel–Haenszel; df, degrees of freedom.
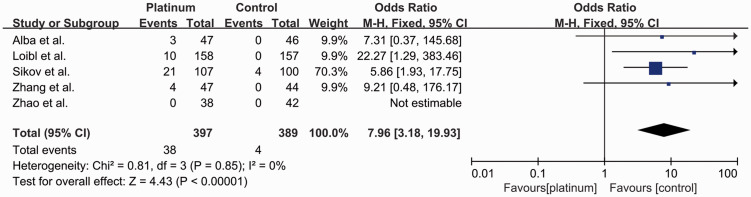
Five studies reported the rates of grade 3 and 4 thrombocytopenia8,10,15,16,19 (Figure 9). Overall, 42 out of 786 patients (5.3%) developed grade 3 and 4 thrombocytopenia, including 38 out of 397 (9.6%) in the platinum-based group and four out of 389 (1.0%) in the control group (OR: 7.96, 95% CI: 3.18–19.93). The incidence was significantly higher in the platinum-based group (P < 0.001), with no significant heterogeneity (I2 = 0%).
Figure 9.
Odds ratios for grade 3–4 thrombocytopenia in patients treated with platinum-based vs. platinum-free (control) neoadjuvant chemotherapy in the included randomized controlled trials.
CI, confidence interval; M-H, Mantel–Haenszel; df, degrees of freedom.
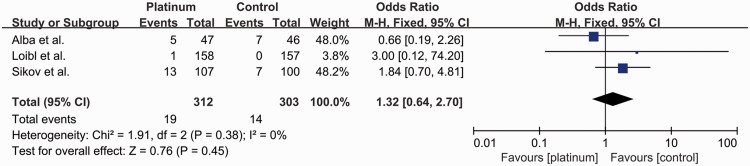
Three studies reported the rates of febrile neutropenia8,15,16 (Figure 10). A total of 33 out of 615 patients (5.4%) developed febrile neutropenia, including 19 out of 312 (6.1%) in the platinum-based group and 14 out of 303 (4.6%) in the control group (OR: 1.32, 95% CI: 0.64–2.70). There was no significant difference between the groups, with no significant heterogeneity (I2 = 0%).
Figure 10.
Odds ratio for febrile neutropenia in patients treated with platinum-based vs. platinum-free (control) neoadjuvant chemotherapy in the included randomized controlled trials.
CI, confidence interval; M-H, Mantel–Haenszel; df, degrees of freedom.
Discussion
We conducted a systematic review and meta-analysis to compare the outcomes of patients with TNBC treated with platinum-based and non-platinum-based neoadjuvant chemotherapy, to assess the role of platinum-based agents in the neoadjuvant setting. With no evidence of publication bias, the results revealed that the inclusion of platinum-based agents in the neoadjuvant chemotherapy regimen significantly increased the pCR rate, with a slight increase in hematological toxicities.
By binding to nucleophilic sites on DNA, platinum-based compounds cause double-stranded DNA breaks, which are preferentially repaired by homologous recombination. The proteins encoded by BRCA1 and BRCA2 are crucial components of the homologous recombination-mediated repair process.6,7 Up to two thirds of BRCA1-related breast cancers, as well as 7.5% of BRCA2-related breast cancers, are TNBCs. Even sporadic breast cancers that arise in the absence of a BRCA germline mutation may present with features of DNA-repair deficiency.20 These features suggest that TNBCs may be particularly susceptible to platinum-based agents, as supported by the results of several studies.21–23 Similarly, the present meta-analysis revealed that patients with TNBC would benefit from the addition of platinum-based agents, with a significant increase of 13.2% in the pCR rate in patients treated with carboplatin-based neoadjuvant chemotherapy (OR: 1.87, 95% CI: 1.23–2.86 P = 0.003).
Regarding adverse events, Poggio et al.21 conducted a similar study in 2018 and found that the increase in pCR rates was achieved at the cost of worse hematological toxicities. However, the current meta-analysis found no significant differences between the two groups, except for significantly higher rates of grade 3 and 4 thrombocytopenia. Compared with the previous study,21 none of the eight trials included in the present meta-analysis included drugs other than anthracyclines, cyclophosphamide, taxanes, and fluorouracil (such as bevacizumab and gemcitabine). Moreover, given that hormone receptor status might influence the risk of adverse events, data for adverse events from two studies,9,18 which were also included in the previous meta-analysis, were not included in the present analysis of adverse events. The current study thus implemented stricter inclusion criteria and its results might therefore be more reliable.
This meta-analysis had some important limitations that should be considered when interpreting the results. First, seven of the included studies were open-label studies. Second, non-hematologic toxicities, such as nervous system disorders and gastrointestinal disorders, which are important factors affecting treatment decisions, were not evaluated in our study. Third, there was a lack of long-term follow-up in the included studies, and the effects of the increased pCR rates associated with the addition of platinum-based agents on long-term outcomes, such as disease-free and overall survival, remain unknown. Future research should thus pay more attention to the design of RCTs, such as strict blinding and allocation concealment, to ensure balance between the intervention groups and to prevent potential selective bias. Moreover, disease-free and overall survival times and quality of life should also be included as outcomes in future studies.
Increasing numbers of drugs are available to treat TNBC, such as veliparib and bevacizumab; however these are difficult to obtain and financially prohibitive for most patients. Anthracyclines, taxanes, cyclophosphamide, fluorouracil, and platinum-based drugs are the most widely used, easily available, and low-cost drugs. Compared with the previous meta-analysis,21,22 the current meta-analysis found that the above drugs alone could achieve better clinical effect, i.e. similar pCR rates but a lower incidence of hematologic toxicities such as anemia and neutropenia. However, compared with standard anthracycline- and taxane-based therapies, the possible added gonadotoxic impact of platinum-based chemotherapy remains an unexplored issue, and should be taken into account during the treatment decision-making process, especially in young patients.24
In conclusion, our results showed that adding platinum to neoadjuvant chemotherapy based on anthracyclines, cyclophosphamide, taxanes, and fluorouracil, without the inclusion of any other drugs, could improve pCR rates in patients with TNBC, with a slight increase in hematological toxicities. Neoadjuvant therapy including platinum-based agents might thus be a viable option for patients with TNBC in terms of drug accessibility and affordability.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Zhen-Yu Li https://orcid.org/0000-0001-9926-8055
References
- 1.Bagegni NA, Tao Y, Ademuyiwa FO. Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the National Cancer Database. PLoS One 2019; 14: e0222358. DOI: 10.1371/journal.pone.0222358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13: 2329–2334. DOI: 10.1158/1078-0432.Ccr-06-1109. [DOI] [PubMed] [Google Scholar]
- 3.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26: 1275–1281. DOI: 10.1200/jco.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. DOI: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 5.Von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 2012; 366: 299–309. DOI: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 6.Sikov WM. Assessing the role of platinum agents in aggressive breast cancers. Curr Oncol Rep 2015; 17: 3. DOI: 10.1007/s11912-014-0428-7. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P. Update on the treatment of early-stage triple-negative breast cancer. Curr Treat Options Oncol 2018; 19: 22. DOI: 10.1007/s11864-018-0539-8. [DOI] [PubMed] [Google Scholar]
- 8.Alba E, Chacon JI, Lluch A, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat 2012; 136: 487–493. DOI: 10.1007/s10549-012-2100-y. [DOI] [PubMed] [Google Scholar]
- 9.Schneeweiss A, Möbus V, Tesch H, et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto—GBG 84): A randomised phase III trial. Eur J Cancer 2019; 106: 181–192. DOI: 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Yin Y, Mo H, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 2016; 7: 60647–60656. DOI: 10.18632/oncotarget.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 1194–1220. DOI: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 12.Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2017; 28: 1700–1712. DOI: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 2017; 35: 203–217. DOI: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Jiang ZF, Li JB. Development of guidelines and clinical practice for breast cancer. Zhonghua Wai Ke Za Zhi 2020; 58: 85–90. DOI: 10.3760/cma.j.issn.0529-5815.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 2018; 19: 497–509. DOI: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 16.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33: 13–21. DOI: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar Martinez MC, Arce-Salinas C, Alvarado-Miranda A, et al. Randomized phase II trial to evaluate the safety and efficacy of neoadjuvant cisplatin in combination with taxanes-anthracyclines vs taxanes-anthracyclines alone in locally advanced triple negative breast cancer. J Clin Oncol 2015; 33: e12024. [Google Scholar]
- 18.Ando M, Yamauchi H, Aogi K, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat 2014; 145: 401–409. DOI: 10.1007/s10549-014-2947-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Li JF, Chu GW. Neoadjuvant chemotherapy regimens for patients with triple-negative breast cancer: TE versus TC. J Pract Oncol 2014; 29: 576–579. [Google Scholar]
- 20.Winter C, Nilsson MP, Olsson E, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol 2016; 27: 1532–1538. DOI: 10.1093/annonc/mdw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol 2018; 29: 1497–1508. DOI: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 22.Pandy JGP, Balolong-Garcia JC, Cruz-Ordinario MVB, et al. Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer 2019; 19: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan X, Ma F, Fan Y, et al. Platinum-based chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis of randomized-controlled trials. Anticancer Drugs 2015; 26: 894–901. DOI: 10.1097/cad.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 24.Perachino M, Poggio F, Lambertini M. Call for assessing treatment-induced gonadotoxicity of platinum-based chemotherapy in early breast cancer. Breast Cancer Res Treat 2020; 183: 239–240. DOI: 10.1007/s10549-020-05767-3. [DOI] [PubMed] [Google Scholar]