Graphical abstract
Keywords: Anti-diabetic, Anti-inflammatory, Cytotoxicity, Secondary metabolites
Highlights
-
•
The results of the present study revealed the phytochemical composition and bio potentials of Tectaria paradoxa (Fee.) Sledge.
-
•
The extracts of T. paradoxa demonstrated dose dependent anti-inflammatory, anti-diabetic and cytotoxic activities.
-
•
The anti-inflammatory activity of the Tectarai paradoxa were as follows methanol > chloroform > acetone > petroleum ether.
-
•
The anti-diabetic properties of the Tectarai paradoxa were as follows methanol > acetone > chloroform > petroleum ether.•The cytotoxcity of the Tectarai paradoxa were as follows chloroform > petroleum ether > methanol > acetone.
Abstract
The present study was aimed to reveal the phytochemical composition and bio potentials of Tectaria paradoxa (Fee.) Sledge. The total phenolic, tannin, flavonoid, terpenoids, sterols content were determined. RBC membrane stabilization against heat induced haemolysis, In-vitro Alpha-amylase inhibitory assay and Brine Shrimp lethality bioassay was performed to determine the anti-inflammatory, anti-diabetic and cytotoxic activity. Among the tested extracts, methanolic extracts of T. paradoxa showed high amount of phenolics 351.43 ± 14.5 mg GAE/g, tannin 34.38 ± 1.02 mg GAE/g, flavonoids 1384.44 ± 50.92 mg QE/g, triterpenoids 130.5 ± 2.77 mg/g and acetone extracts of T. paradoxa displayed maximum amount of sterols 3.2 ± 0.2 mg/g. The extracts of T. paradoxa demonstrated dose dependent anti-inflammatory, anti-diabetic and cytotoxic activities. The anti-inflammatory activity of the T. paradoxa were as follows methanol > chloroform > acetone > petroleum ether. The anti-diabetic properties of the T. paradoxa were as follows methanol > acetone > chloroform > petroleum ether. The cytotoxicity of the T. paradoxa were as follows chloroform (LC50 = 25.52 μg/mL) > petroleum ether (LC50 = 36.99 μg/mL) > methanol (LC50 = 44.26 μg/mL) > acetone (LC50 = 55.9 μg/mL). The existence of phenolics, tannin, flavonoids, sterols and triterpenoids may be responsible for the observed biological activities. The results of the present study identified the pool of medicinal properties existence in T. paradoxa. Further studies on the isolation of active principles may bring out an alternative source for anti-inflammatory and anti-cancer drugs from T. paradoxa.
1. Introduction
Since the time immemorial the medicinal value of pteridophytes is known to man. The rhizome of Tectaria cicutaria has been used in Ayurveda for the treatment of various disorders [1,2]. The decoction of Tectaria cicutaria is employed for the treatment of various types of gynecological disorders as well as inflammatory conditions. Many researchers have confirmed the pharmacological activities of pteridophytes [[3], [4], [5], [6], [7], [8], [9], [10], [11]]. The metabolites phenolics, flavonoids, alkaloids and terpenoids are responsible for the biopotency of the ferns [[12], [13], [14], [15]]. Preeti and Namdeo [10] subjected Tectaria cicutaria rhizomes, to phytochemical analysis, anti-microbial activity and in-vitro anticancer activity and confirmed the presence of bioactive metabolites in the extracts. They observed the antimicrobial activity against Proteus vulgaris. The ethanolic extract of Tectaria cicutaria rhizomes showed excellent anticancer activity against Human Leukemia Cell Line (K562) with GI50 value 11.9 μg/mL. Castrejón-Arroyo et al., [8] evaluated the anti-inflammatory activity and antioxidant capacity, total phenolic and flavonoid contents of T. heracleifolia raw extracts. The T. heracleifolia raw extracts showed the anti-inflammatory activity with 52 % and 0.084 mg/ml was required to obtain a 50 % antioxidant effect (IC50). Pawar et al., [16] revealed the phytochemical profiles of Tectaria coadunata. Preeti and Namdeo [17] studied the anticancer action of Tectaria cicutaria in human cancer cell lines. Johnson et al., [18] have observed the inter-specific variation among the three Tectaria species using isoperoxidase analysis. But there is no report on the phytochemical composition and biological activities of Tectaria paradoxa (Fee.) Sledge. With this knowledge the present study was aimed to reveal the phytochemical composition and bio potentials of Tectaria paradoxa (Fee.) Sledge.
2. Materials and methods
2.1. Collection of materials
Healthy, disease free plant samples of Tectaria paradoxa (Fee.) Sledge were collected from high altitude semi-evergreen forest ranges of Tirunelveli district, Tamil Nadu, India.
The Tectaria paradoxa was identified using the standard flora and authenticated by Dr. M. Johnson. Tectaria paradoxa (Fee.) Sledge voucher specimen was deposited in Centre for Plant Biotechnology Herbarium, St. Xavier’s College (Autonomous), Palayamkottai, India. To remove the soil particles and other debris, the collected plants T. paradoxa were brought to the laboratory and washed well with running tap water for 10 min. The washed T. paradoxa were blotted on the blotting paper and spread out at room temperature under shade for a period of fifteen days. The shade dried T. paradoxa were ground to fine powder using tissue blender. The powdered T. paradoxa were then stored in refrigerator at 4 °C for further use.
2.2. Preparation of extracts
30 g of dried and powdered whole plant materials of T. paradoxa were extracted with 180 ml of petroleum ether (45 °C), chloroform (55 °C), acetone (52 °C) and methanol (75 °C) by using Soxhlet extractor for 8 h at a temperature not exceeding the boiling point of the solvent. All extracts were frozen and freeze dried. The powder was stored in an amber bottle and stored at 4 °C in a refrigerator for later biological activities. For quantitative analysis and biological activities, the extracts were disolved in DMSO (w/v) (5 mg of crude petroleum ether, chloroform, acetone and methanolic extracts of T. paradoxa were disolved in 5 ml of DMSO (mg/mL)).
2.3. Phytochemical analysis
The crude extracts were screened for the occurrence or absence metabolites by the standard method described by Harborne [19]. The total phenolic, tannin, flavonoid, terpenoids, sterols content were determined according to the method described by Siddhuraju and Becker [20], Zhishen et al., [21], Feng et al. [22], respectively.
2.4. Biological activities
RBC membrane stabilization against heat induced haemolysis was performed to determine the anti-inflammatory activity of T. paradoxa extracts [23,24]. In-vitro alpha-amylase inhibitory assay was carried out to determine the anti-diabetic properties of T. paradoxa extracts [25]. Brine Shrimp lethality bioassay was performed to examine the cytotoxic properties of T. paradoxa extracts [26]. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental studies [27].
To validate the observed results the statistical analysis was performed using SPSS 21 software. Pearson correlation test was performed between the metabolites concentration and biological activities. The correlation is significant at the 0.01 level (2-tailed). To determine the significance, the t - test was performed between the metabolites concentration and biological activities.
3. Results
Among the tested extracts, methanolic extracts of T. paradoxa showed high amount of phenolics 351.43 ± 14.5 mg GAE/g, tannin 34.38 ± 1.02 mg GAE/g, flavonoids 1384.44 ± 50.92 mg QE/g, triterpenoids 130.5 ± 2.77 mg/g and acetone extracts of T. paradoxa displayed maximum amount of sterols 3.2 ± 0.2 mg/g (Table 1). The total phenolics, tannin, flavonoids and triterpenoids contents of T. paradoxa extracts were as follows methanol > chloroform > acetone > petroleum ether. The extractable sterols of T. paradoxa were as follows acetone > methanol > petroleum ether > chloroform (Table 1).
Table 1.
Secondary Metabolites of Tectaria paradoxa.
| Metabolites | Methanol | Chloroform | Pet. Ether | Acetone |
|---|---|---|---|---|
|
Phenolics (mg GAE / g) |
351.43 ± 14.5 | 334.76 ± 7.95 | 288.89 ± 8.21 | 332.86 ± 7.14 |
| Flavonoids (mg QE / g) | 1384.44 ± 50.92 | 1104.44 ± 62.92 | 706.67 ± 10.72 | 1095.56 ± 59.66 |
| Sterols (mg / g) | 2.94 ± 0.07 | 2.27 ± 0.11 | 2.83 ± 0.19 | 3.2 ± 0.2 |
|
Tannin (mg GAE / g) |
34.38 ± 1.02 | 25.48 ± 1.37 | 5.3 ± 0.61 | 9.83 ± 0.65 |
| Triterpenoids (mg / g) | 130.5 ± 2.77 | 122.5 ± 2.25 | 102.33 ± 2.42 | 105.5 ± 4.17 |
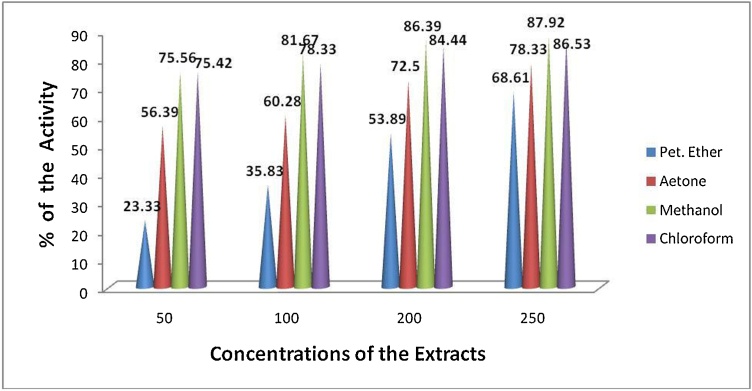
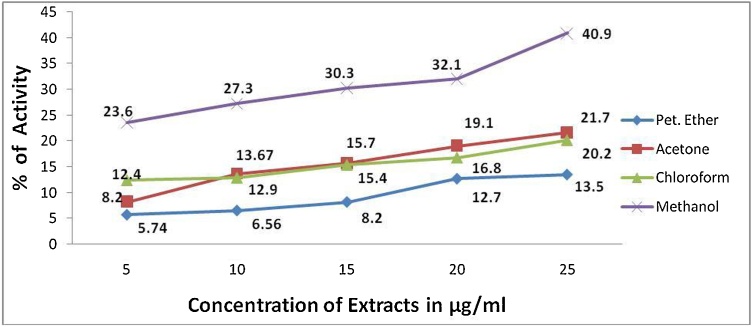
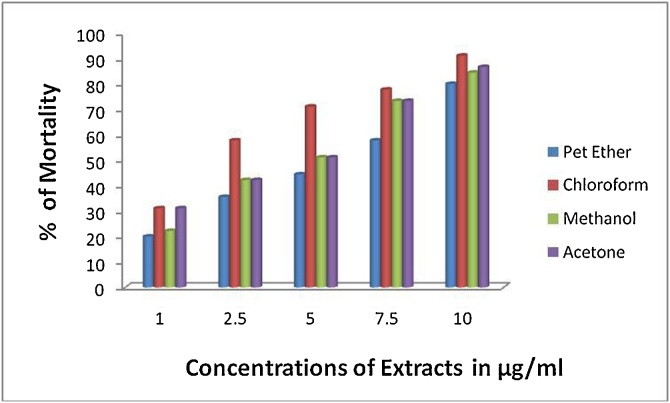
The biopotency of T. paradoxa extracts were determined by alpha glucosidase, heat induced haemolysis and brine shrimp biolethality bioassay. The extracts of T. paradoxa demonstrated dose dependent toxicity (brine shrimp lethality bioassay), anti-inflammatory and anti-diabetic activities (Fig. 1, Fig. 2, Fig. 3). The anti-inflammatory activity of the T. paradoxa were as follows methanol (t = 0.02) > chloroform (t = 0.001) > acetone (t = 0.001) > petroleum ether (t = 0.001) (Fig. 1). 100 μg/mL of standard indomethacin was dispalyed 71.43 % inhibition. A strong correlation (r = 0.998) between chloroform and petroleum ether extracts of T. paradoxa and anti-inflammatory activities was attained. Next to that r = 0.996 correlation coeeficient was obtained between acetone and anti-inflammatory activities. Correlation coeeficient of r = 0.967 was obtained between methanolic extracts of T. paradoxa and anti-inflammatory activities. The correlation is significant at the 0.01 level (2-tailed). The anti-diabetic properties of the T. paradoxa were as follows methanol (t = 0.000) > acetone (t = 0.003) > chloroform (t = 0.000) > petroleum ether (t = 0.004) (Fig. 2). 78 % of activity was observed in the standard acarbose at 500 μg/mL. A strong positive correlation (r = 0.973) was obtained between methanolic extracts of T. paradoxa and anti-diabetic activities, r = 0.987 for chloroform, r = 0.963 for acetone and r = 0.958 for petroleum ether. The cytotoxicity of the T. paradoxa were as follows chloroform (LC50 = 25.52 μg/mL; t = 0.003) > petroleum ether (LC50 = 36.99 μg/mL; t = 0.009) > methanol (LC50 = 44.26 μg/mL; t = 0.008) > acetone (LC50 = 55.9 μg/mL; t = 0.003) (Fig. 3). The standard plumbagin showed 100 % mortality of brine shrimp nauplii at 0.046 mg/mL. A strong positive correlation (r = 0.985) was observed between concentrations of methanolic extracts and cytotoxicity of T. paradoxa, r = 0.946 for chlorofom, r = 0.986 for acetone and r = 0.993 for petroleum ether. The studied extracts of T. paradoxa showed significant lethality against brine shrimp (Table 2; Fig. 3).
Fig. 1.
Anti-inflammatory Activity of T. paradoxa.
Fig. 2.
Anti-Diabetic Activity of T. paradoxa.
Fig. 3.
Cytotoxicity of T. paradoxa.
4. Discussion
Phenolic compounds and tannins are known to possess anti-inflammatory, anti-oxidant, anti-microbial, insecticidal, anti-diabetic [28], wound healing, anti-diuretic, anti-parasitic, cytotoxic and anti-neoplastic activities [29]. Flavonoids show anti-allergic, anti-inflammatory, anti-microbial and anticancer activity [30,31]. Steroids and saponins are the sub- groups of triterpenoids. Saponins possess antimicrobial and anti- inflammatory activity [32]. The results of the present study also confirm the presence of phenolics, tannin, flavonoids, sterols and triterpenoids with varied amount in the studied extracts of T. paradoxa. The existence of these metabolites may be responsible for the observed biological activities. T. paradoxa extracts showed anti-diabetic, anti-inflammatory and cytotoxic activities with varied frequencies. The varied frequency activities may be due to the variation in metabolites contents. The concentrations and frequency of activities are directly correlated. Brine Shrimp Lethality Bioassay (BSLB) has been successfully employed as a simple biological tool to identify the antitumour compounds / fractions / crude extracts of plants [33]. The BSL bioassay has good correlation with the human solid tumour cell lines [34]. The studied extracts of T. paradoxa can be considered as a promising candidate for a plant-derived anti-tumour compound. LC50 values < 1000 ppm are considered significant for crude extracts [35]. The cytotoxicity of the T. paradoxa were displayed less than LC50 values < 1000 ppm viz., chloroform (LC50 = 25.52 μg/mL) > petroleum ether (LC50 = 36.99 μg/mL) > methanol (LC50 = 44.26 μg/mL) > acetone (LC50 = 55.9 μg/mL). The crude extracts of plants with LC50 values <1000 μg/ml using BSLB are recognized to hold various physiologically active principles [36]. The existence of phytoconstitutents viz., alkaloids, phenolics and terpenoids in plant extracts has been associated with anticancer and cytotoxic activity [[35], [36], [37]]. The results of the present study suggested that T. paradaxa extracts treatment against Artemia salina induced a dose dependent lethal effect. The T. paradaxa extracts treated Artemia salina showed the morphological changes, which disrupted and affected the swimming ability, feeding, intestinal enlargement, deformation and loss of antennae in A. salina. The A. salina cultured in the control failed to show the morphological change. The exposure of T. paradaxa extracts may generate the reactive oxygen species (ROS) that may cause cytotoxicity. Similar kind of observations was observed in the aqueous and silver nanoparticles of O. chinensis [38]. Johnson et al. [36,38] and Nirmali et al. [39] employed in-vitro alpha amylase inhibitory activity to predict the antidiabetic potential of Sphaerostephanos unitus, Odontosoria chinensis and Adenanthera pavonina extracts respectively. In the present study also in-vitro alpha amylase inhibitory activity was adopted and identified the antidiabetic potetnials of T. paradoxa. The anti-inflammatory activity of Sphaerostephanos unitus, Odontosoria chinensis and Gardenia coronaria leaves extracts was assessed by in vitro HRBC membrane stabilization method [36,38,40]. Johnson et al. [36,38] employed the in-vitro alpha-amylase inhibitory assay and Brine Shrimp lethality bioassay to determine the toxicity and anti-diabetic properties of Sphaerostephanos unitus and Odontosoria chinensis. In the present study also in vitro HRBC membrane stabilization against heat induced haemolysis method and in-vitro alpha-amylase inhibitory assay and Brine Shrimp lethality bioassay are employed and determined the toxicity, anti-diabetic and anti-inflammatory properties of T. paradoxa. The results of the present study identified the pool of medicinal properties existence in T. paradoxa. Further studies on the isolation of active principles may bring out an alternative source for anti-inflammatory and anti-cancer drugs from Tectaria paradoxa.
CRediT authorship contribution statement
Manivannan V: Data curation, Investigation, Methodology. Johnson M: Data curation, Methodology, Supervision, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Upadhye A., Kumbhojkar M.S., Vartak V.D. Observations on wild plants used in folk medicine in the rural areas of the Kolhapur district. Ancient Sci Life. 1998;6:119–121. [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhari A.S., Raina P., Deshpande M.M. Evaluating the anti-inflammatory potential of Tectaria cicutaria L. Rhizome extract in - vitro as well as in - vivo. J. Ethnopharmacol. 2013;150:215–222. doi: 10.1016/j.jep.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Vasudeva S.M. Economic importance pteridophytes . Indian Fern J. 1999;16:2130–2152. [Google Scholar]
- 4.Rao T.K., Reddy K.N., Pattanaik C., Sudhakar Reddy C.H. Ethnomedicinal importance of pteridophytes used by Chenchus of Nallamalais, andhra pradesh. India. Ethnobotanical Leaflets. 2007;11:6–10. [Google Scholar]
- 5.Gupta S.K., Mitali G., Biswas R., Saha B.K., Das A.P., Mandal P. Evaluation of in - vitro antioxidant activity of methanolic extracts of some ferns from Mawsynram of Meghalaya. India. Int J Curr Sci. 2014;12 E 87-97. [Google Scholar]
- 6.Janakiraman N., Johnson M. Larvicidal potential of Cyathea species against Culex quinquefasciatus. Pharm. Biomed. Res. 2017;3:48–51. [Google Scholar]
- 7.Janakiraman N., Johnson M. Ethanol extracts of selected Cyathea species decreased cell viability and inhibited growth in MCF 7 cell line cultures. J. Acupunct. Meridian Stud. 2016;9:151–155. doi: 10.1016/j.jams.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Castrejón-Arroyo K.D.S., Sánchez-Córdova A.D.S., Jaqueline C.T., Sánchez-Ocampo P.M., Ariana A.H.H. Total phenolic and flavonoid contents, antioxidant and anti-inflammatory activities of Tectaria heracleifolia extracts. Mex. J. Biotechnol. 2016;1:42–50. [Google Scholar]
- 9.Jarial R., Shard A., Thakur S., Sakinah M., Zularisam A.W., Rezania S., Kanwar S.S., Singh L. Characterization of flavonoids from fern Cheilanthes tenuifolia and evaluation of antioxidant, antimicrobial and anticancer activities. J. King Saud Univ. - Sci. 2017 doi: 10.1016/j.jksus.2017.04.007. [DOI] [Google Scholar]
- 10.Preeti K., Namdeo J. Phytochemical screening and in-vitro anticancer activity of extracts of Tectaria cicutaria. IJPSR. 2018;9:3463–3468. [Google Scholar]
- 11.Johnson M., Ramakrishnan P., Perumal S., Shibila T. Anti-inflammatory activity of selected pteridophytes from western ghats. Int. J. Complement. Altern. Med. 2017;9:1–13. doi: 10.15406/ijcam.2017.09.00307. [DOI] [Google Scholar]
- 12.Ho R., Teai T., Bianchini J.P., Lafont R. Raharivelomanana P. Ferns: from traditional uses to pharmaceutical development, chemical identification of active principles. In: Fernandez H., Revilla M.A., Kumar A., editors. Working With Ferns: Issues and Applications. Springer; New York, USA: 2010. pp. 321–346. [Google Scholar]
- 13.Middleton E., Jr., Kandaswami C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne J.B., editor. The Flavonoids. Chapman & Hall; London, UK: 1994. pp. 619–652. [Google Scholar]
- 14.Rice-Evans C.A., Miller N.J., Paganga G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 15.Sakihama Y., Cohen M.F., Grace S.C., Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 16.Pawar S.G., Kamble S.Y., Patil S.R., Sawant P.S., Singh E.A. Preliminary phytochemical investigations of three species of traditional medicinal plants of tribal regions of maharashtra (India) Int. J. Pharmacogn. Phytochem. Res. 2016;8:742–745. [Google Scholar]
- 17.Preeti G.K., Namdeo R.J. In - vitro studies of the anticancer action of Tectaria cicutaria in human cancer cell lines: Go/G1 p53- associated cell cycle arrest-Part I. J. Tradit. Complement. Med. 2018;8:459–464. doi: 10.1016/j.jtcme.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson M., Irudaya Raj V., Rajkumar S.D. Isozymic variation studies on the selected species of Tectaria from India. J. Chem. Pharm. Res. 2010;2:334–338. [Google Scholar]
- 19.Harborne J.B. 3rd ed. Chapman Hall; New York, NY, USA: 1998. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 20.Siddhuraju P., Becker K. Studies on antioxidant activities of mucuna seed (Mucuna pruriens varutilis) extract and various non-protein amino/imino acids through in - vitromodels. J. Sci. Food Agric. 2003;83:1517–1524. doi: 10.1002/jsfa.1587. [DOI] [Google Scholar]
- 21.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 22.Feng Y.L., Chen H., Tian T., Chen D.O., Zhao Y.Y., Lin R.C. Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of Alismatis rhizoma. J Ethanopharmcol. 2014;154:386–390. doi: 10.1016/j.jep.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Shinde U.A., Phadke A.S., Nari A.M., Mungantiwar A.A., Dikshit V.J., Saraf M.N. Membrane stabilizing activity - a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil Fitoterapia. 1999;70:251–257. doi: 10.1016/S0367-326X(99)00030-1. [DOI] [Google Scholar]
- 24.Sakat S., Juvekar A.R., Gambhire M.N. In - vitro antioxidant and anti-inflammatory activity of methanol extract Ofoxalis corniculata Linn. International Journal of Pharma and Pharmacological Sciences. 2010;2:146–155. [Google Scholar]
- 25.Hara Y., Honda M. The inhibition of α-Amylase by tea polyphenols. Agric. Biol. Chem. 1990;54:1939–1945. doi: 10.1271/bbb1961.54.1939. [DOI] [Google Scholar]
- 26.Olaru O.T., Venables L., Maryana V.V., Nitulescu G.M., Denisa M., Spandidos D.A., Tsatsakis A.M. Anticancer potential of selected Fallopia adan species. Oncol. Lett. 2015;10:1323–1332. doi: 10.3892/ol.2015.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tveden-Nyborg P., Bergmann T.K., Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin. Pharmacol. Toxicol. 2018;123:233–235. doi: 10.1111/bcpt.13059. [DOI] [PubMed] [Google Scholar]
- 28.Gepdireman A., Mshvildadze V., Suleyman H., Elias R. Acute anti-inflammatory activity of four saponins isolated from Ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw oedema. Phytomedicine. 2005;12:440–444. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Fawole O.A., Amoo S.O., Ndhlala A.R., Light M.E., Finnie J.F., Van Staden J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J. Ethnopharmacol. 2010;127:235–241. doi: 10.1016/j.jep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Margina D., Olaru O.T., Ilie M., Gradinaru D., Guțu C., Sorina V., Anca D., Spandidos D.A., Tsatsakis A.M. Assessment of the potential health benefits of certain total extracts from Vitis vinifera, Aesculus hyppocastanum and Curcuma longa. Experminetal and Therapeutic medicine. 2015;10:1681–1688. doi: 10.3892/etm.2015.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margina D., Ilie M., Gradinaru D. Quercetin and epigallocatechin gallate induce inVitro a dose - dependent stiffening and hyperpolarizing effect on the cell membrane of human mononuclear blood cells. Int. J. Mol. Sci. 2012;13:4839–4859. doi: 10.3390/ijms13044839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandal P., Sinha Babu S., Mandal N. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia. 2005;76:462–465. doi: 10.1016/j.fitote.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin J.L. Crown-gall tumors in potato discs and brine shrimp lethality: Two simple bioassays for Higher plant screening and fractionation. In: Hostettmann K., editor. Vol. 6. Academic Press; London: 1991. pp. 1–32. (Methods Plant Biochemistry). [Google Scholar]
- 34.Anderson J.E., Goetz C.M., McLaughlin J.L. A blind comparison of simple bench-top bioassays and human tumor cell cytotoxicities as antitumor prescreens. Phytochem. Anal. 1991;2:107–111. doi: 10.1002/pca.2800020303. [DOI] [Google Scholar]
- 35.Meyer B.N., Ferrigni N.R., Putman J.E., Jacobson L.B., Nichols D.E., McLaughlin J.L. Brine Shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- 36.Johnson M., Xavier Madona C., Almeida R.S., Martins N., Coutinho H.D.M. In vitro toxicity, antioxidant, anti-inflammatory and antidiabetic potential of Sphaerostephanos unitus (L.) Holttum. Antibiotics. 2020;9(333) doi: 10.3390/antibiotics9060333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dushimemaria F., Preez C., Mumbengegwi D.R. Randomized anticancer and cytotoxicity activities of Guibourtia coleosperma and Diospyros chamaethamnus. Afr. J. Tradit. Complement. Altern. Med. 2017;14:1–7. doi: 10.21010/ajtcam.v14i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson M., Shibila T., Amutha S., Menezes I.R.A., Da Costa J.C.M., Sampaio N.F.L., Coutinho H.D.M. Synthesis of silver nanoparticles using Odontosoria chinensis (L.) J. Sm. And evaluation of their biological potentials. Pharmaceuticals. 2020;13:66. doi: 10.3390/ph13040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nirmali W.M., Punchihewa J.C., Wickramaratne D.B.M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016;16:466. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury A., Azam S., Jainul M.A., Faruq K.O., Islam A. Antibacterial Activities and In Vitro Anti-Inflammatory (Membrane Stability) Properties of Methanolic Extracts of Gardenia coronaria Leaves. Intnational Journal of Microbiology. 2014;410935(5) doi: 10.1155/2014/410935. [DOI] [PMC free article] [PubMed] [Google Scholar]