Abstract
Type 1 diabetes arises from the autoimmune destruction of insulin-producing beta-cells of the pancreas, resulting in dependence on exogenously administered insulin to maintain glucose homeostasis. In this study, our aim was to identify genetic risk factors that contribute to progression from islet autoimmunity to clinical type 1 diabetes. We analyzed 6.8 million variants derived from whole genome sequencing of 160 islet autoantibody positive subjects, including 87 who had progressed to type 1 diabetes. The Cox proportional-hazard model for survival analysis was used to identify genetic variants associated with progression. We identified one novel region, 20p12.1 (TASP1; genome-wide P < 5 × 10–8) and three regions, 1q21.3 (MRPS21–PRPF3), 2p25.2 (NRIR), 3q22.1 (COL6A6), with suggestive evidence of association (P < 8.5 × 10–8) with progression from islet autoimmunity to type 1 diabetes. Once islet autoimmunity is initiated, functional mapping identified two critical pathways, response to viral infections and interferon signaling, as contributing to disease progression. These results provide evidence that genetic pathways involved in progression from islet autoimmunity differ from those pathways identified once disease has been established. These results support the need for further investigation of genetic risk factors that modulate initiation and progression of subclinical disease to inform efforts in development of novel strategies for prediction and intervention of type 1 diabetes.
Subject terms: Genome-wide association studies, Type 1 diabetes
Introduction
Type 1 diabetes is a complex autoimmune disorder whose etiology involves multiple genetic and environmental risk factors affecting up to 1 in 300 children1. The discovery of genetic variants associated with type 1 diabetes has accelerated greatly over the past years. Genome-wide association scan (GWAS) and fine-mapping efforts discovered over 40 risk loci2,3, with the majority of variants enriched in non-coding regions of the genome3.
The design of most genetic studies of type 1 diabetes typically involves comparison of cases (with varying duration of diabetes) with controls. The variants identified in such studies to be associated with type 1 diabetes reflect prevalent disease, and analyses thus preclude the factors that may be associated with the initiation of islet autoimmunity and the progression of subclinical disease4. The appearance of any of four beta-cell (islet) autoantibodies in the blood marks initiation of “islet autoimmunity” and is recognized as increasing risk for progression to type 1 diabetes5,6. The genetic contribution to initiation and progression of islet autoimmunity and clinical disease may be different (or overlapping), but this is not known.
The first region of the genome implicated in risk for type 1 diabetes, and the region with the greatest contribution to risk (~ one-half), includes the HLA genes on human chromosome 6p214,7. Genetic variation in HLA-DR and HLA-DQ genes appears to be critical in expression of islet autoantibodies and progression/risk to type 1 diabetes, with contributions from other type 1 diabetes-associated genes (PTPN22, UBASH3A, IFIH1, INS, PTPN2)8–11; however, in these studies only a small subset of genetic variants (those most strongly associated with type 1 diabetes risk from case–control studies) have been examined, overlooking the vast majority of the human genome. Identifying genetic risk factors that play a role in the preclinical period of type 1 diabetes can help devise therapies aiming to stop the autoimmune process and preserve function of remaining beta-cells. This report aims to identify novel genetic factors that play a role in progression from islet autoimmunity to clinical type 1 diabetes.
Results
Association analysis for progression from islet autoimmunity to type 1 diabetes
A total of 160 participants in Diabetes AutoImmunity Study in the Young (DAISY)12, all of whom were persistently positive for islet autoantibodies, were characterized using whole genome sequencing. A total of 87 (54.4%) of these participants progressed to clinical type 1 diabetes during the follow-up period. The age at time of islet autoimmunity was younger (3.64 years) than those who did not progress (8.49 years, P < 0.0001). The duration of islet autoimmunity in those who progressed to type 1 diabetes was significantly shorter (6.48 years, P < 0.0001) than the follow-up period in those remaining disease free (10.89 years). Additional characteristics of the 160 DAISY participants are shown in Table 1.
Table 1.
Demographics of 160 islet autoantibody positive DAISY participants.
| Progressors | Non-Progressors | P | |
|---|---|---|---|
| N = 87 | N = 73 | ||
| FDR, N (%) | 62 (71.26) | 44 (60.27) | 0.14* |
| Female, N (%) | 45 (51.72) | 38 (52.05) | 0.97* |
| NHW, N (%) | 79 (90.8) | 50 (68.49) | 0.0004* |
| HLA DR 3/4, N (%) | 40 (45.98) | 22 (30.14) | 0.04* |
| HLA DR, N (%) | |||
| 3/4 | 40 (45.98) | 22 (30.14) | 0.047† |
| 3/3 or 3/X | 16 (18.39) | 13 (17.81) | |
| 4/4 or 4/X | 26 (29.89) | 25 (34.25) | |
| X/X | 5 (5.75) | 13 (17.81) | |
| Age at seroconversion | |||
| Mean (SD) | 3.64 (3.09) | 8.49 (4.98) | < 0.0001‡ |
| Time from seroconversion | |||
| Mean (SD) | 6.48 (4.73) | 10.89 (5.89) | < 0.0001‡ |
Age at seroconversion: age of participant at time of seroconversion to persistent positivity for an autoantibody detected by either RBA or ECL methodology.
Time from seroconversion: time to T1D or last visit.
FDR first degree relative, NHW non-Hispanic white.
*Chi-square.
†Fisher’s exact.
‡Wilcoxon Rank Sum Test.
p < 0.05.
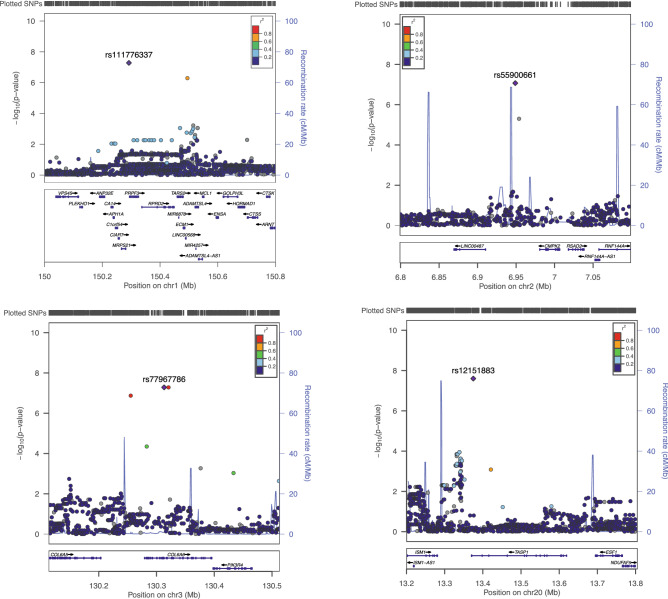
A total of 6,893,119 genetic variants (single nucleotide polymorphisms (SNPs) and small insertion or deletions (Indels)) were analyzed using the Cox proportional-hazard model for association with progression to type 1 diabetes. The genomic inflation factor (λ) compares the genome-wide distribution of the test statistic to that expected under the null distribution. Despite our relatively small population size, we observed λ = 1.03, supporting the absence of bulk inflation or excess false positive rate from the expected for this study. FFour independent regions in the genome showed evidence of association with progression to diabetes (Table 2, Fig. 1): one region attaining genome-wide significance, 20p12.1 (TASP; P < 5 x 10–8) and three regions with suggestive evidence (P < 8.5 × 10–8), 1q21.3 (MRPS21-PRPF3), 2p25.2 (NRIR), and 3q22.1 (COL6A6). None of these loci have been associated with type 1 diabetes in previous case–control analyses.
Table 2.
SNPs associated with progression to type 1 diabetes.
| Chra | SNP | Positionb (bp) | Allele | AFc | HR (95% CI) | P | Gened | Candidate genee |
|---|---|---|---|---|---|---|---|---|
| 1q21.3 | rs111776337 | 150,319,828 | T | 0.050 | 6.4 (3.3–12.6) | 5.23 × 10–8 | MRPS21, PRPF3 | MCL1 |
| 2p25.2 | rs55900661 | 6,808,849 | A | 0.078 | 4.6 (2.6–8.1) | 8.48 × 10–8 | NRIR | RSAD2 |
| 3q22.1 | rs77967786 | 130,594,375 | A | 0.056 | 5.5 (3.0–10.3) | 5.19 × 10–8 | COL6A6 | |
| 20p12.1 | rs12151883 | 13,394,404 | A | 0.059 | 6.5 (3.4–12.8) | 2.50 × 10–8 | TASP1 | NDUFAF5 |
aChr: chromosome.
bHuman genome assembly GRCh38/hg38.
cAF: allele frequency in DAISY cohort.
dGenes listed are based on proximity to lead SNP.
eCandidate genes identified based on functional annotation.
Figure 1.
Regional association plots for 1q21.3, 2p25.2, 3q22.1 and 20p12.1. SNPs in each locus were plotted using LocusZoom37. The most significant SNP associated with progression to type 1 diabetes at each locus is plotted (purple). Each circle on the plot represents a single SNP included in whole genome sequencing association test, the symbol color corresponds to the degree of linkage disequilibrium with the most significant SNP, colored purple.
Functional interpretation of genetic variants associated with progression
To gain insight into potential biological roles of the four loci (1q21.3, 2p25.2, 3q22.1 and 20p12.1) that showed evidence of association with progression to type 1 diabetes, we functionally annotated all SNPs with r2 > 0.6 (moderate linkage disequilibrium) with the lead SNP in each of the four novel regions, yielding 16 “credible SNPs” (a set of SNPs in each locus that have high probability of one being a causal variant, Supplementary Table 1). These SNPs were evaluated on their impact on gene function, gene expression and potential regulatory functions. Of the 16 credible SNPs, eleven were intergenic, four were intronic, and one resides in the 3′UTR sequence in COL6A6. Four of the SNPs were identified as cis-eQTLs (expression Quantitative Trait Loci, with the SNP-gene distance < 1 Mb).
In the 1q21.3 locus (MRPS21-PRPF3), the lead SNP rs111776337 is located in non-coding region of the genome and co-localizes with open chromatin landscape, in comparison to nearby SNP rs113588371 (r2 = 0.72), as seen in the majority of 127 tissue/cell types from ENCODE resource13. The location of the variant suggests a regulatory role in gene expression levels (eQTL). The eQTLGen Consortium database (https://www.eqtlgen.org/cis-eqtls.html) was queried for cis-eQTLs in whole blood. Although the nearest gene to rs111776337 is PRPF3, the variant is an eQTL for six genes: MCL1, APH1A, CTSK, BNIPL, MRPS21, CDC42SE1 (Supplementary Table 2). With the lead SNP playing a role in expression levels of multiple genes in whole blood, we focused on traits associated to blood phenotypes in the UK Biobank (https://www.nealelab.is/uk-biobank/) to determine whether rs111776337 is associated with any immune system-relevant phenotypes. rs111776337 is most strongly associated with reduced monocyte count (P = 1.07 × 10–8) and reduced percentage of monocytes (P = 1.96 × 10–8).
In the 2p25.2 locus, the nearest gene to lead SNP rs55900661 is a long non-coding RNA that resides ~ 19 kb downstream; this gene is the negative regulator of interferon response (NRIR). The functional annotations of eight credible SNPs in high linkage-disequilibrium (r2 > 0.6) suggests that rs55900661 is the strongest candidate, as it is the only SNP that is an eQTL to a gene immediately downstream of NRIR, radical S-adenosyl methionine domain containing 2 (RSAD2). RSAD2 functions in interferon gamma signaling and toll like receptor signaling pathways, and is also an adaptor molecule that plays a role in CD4 + T-cell activation and differentiation14, making it a strong candidate for an autoimmune disease. rs55900661 and associated SNPs in 3q22.1 and 20p12.1, results discussed below, did not show any significant association with blood or immune phenotypes available in the UK Biobank.
In the 3q22.1 locus, the lead SNP, is located in the COL6A6 gene (collagen, type VI, alpha 6 (uc003eni.4)). Alleles of this SNP do not affect expression levels of COL6A6 or neighboring genes in blood. In GTEx data, rs77967786 is classified as a splice eQTL (ENSG00000206384.10) in pituitary tissue (P = 2.0 × 10–33).
In the 20p12.1 locus, the lead SNP, rs12151883, is located in the last intron of TASP1 (Taspase 1). This gene functions in cleavage of the MLL protein, which is required for proper HOX gene expression. The rs2103987 credible SNP (r2 = 0.69 with lead SNP rs12151883) is an eQTL for Ubiquinone Oxidoreductase Complex Assembly Factor 5 (NDUFAF5).
Discussion
This study is the first in-depth analyses of the potential role of genetic variants in progression from islet autoimmunity to clinical type 1 diabetes at the genome-wide level using whole genome sequencing. Our study identified four novel regions that have not been previously associated with type 1 diabetes risk in genome-wide association studies. Functional mapping of the associated SNPs indicates pathways critical to response to viral infections and response to interferon signaling are contributing to progression to type 1 diabetes.
The most promising gene associated with regulatory function of the lead SNP rs111776337 in the 1q21.3 locus is MCL1, a key anti-apoptotic protein in human beta-cells15,16. Apoptosis is one mechanism the host utilizes to eliminate virus-infected cells and is the main form of cell death in type 1 diabetes17. Reduced expression of MCL-1 has been observed in islets from patients with type 1 diabetes infected with a diabetogenic enterovirus, suggesting MCL-1 expression levels play a role in the development of diabetes in humans18.
The most promising gene associated with the lead SNP rs55900661 in the 2p25.2 locus appears to be RSAD2, also referred to as VIPERIN (virus inhibitory protein, endoplasmic reticulum–associated, interferon-inducible), can be induced by interferon and is known to play a role in immune response to DNA and RNA viruses, including human cytomegalovirus, which has been implicated as a potential trigger for type 1 diabetes19. In mice, rsad2 facilitates T-cell receptor-mediated GATA3 activation and optimal Th2 cytokine production by modulating NFKB1 and JUNB activities14. Transcript analyses in human islets indicate that expression of several genes connected to antiviral response increases including IFIH1 (well established type 1 diabetes risk gene) and RSAD2 in virus infected islet cells20. The role of RSAD2 and progression to type 1 diabetes may be through its role in mounting an inflammatory response to viral infection of beta-cells.
NDUFAF5, is required for assembly of NADH-ubiquinone oxidoreductase complex (complex I) which is part of the mitochondrial respiratory chain that catalyzes the transfer of electrons from NADH to ubiquinone. To what extent metabolic dysregulation contributes to the breakdown of self-tolerance is still under investigation but there is evidence that mitochondrial metabolism plays an essential role for suppressive function of regulatory T-cells21.
This study has some limitations. First, the small sample size is underpowered to conduct the full-scale genome-wide analysis of variants contributing to progression of islet autoimmunity to diabetes and further limiting our ability follow-up findings focused on lead variants identified in case–control studies2,3. Steck et al.11, reported SNPs in INS (rs689, HR = 1.65, P = 0.03), UBASH3A (rs11203203, HR = 1.44, P = 0.04) and IFIH1 (rs1990760, HR = 1.47, P = 0.04) showed evidence of association with progression from islet autoimmunity to diabetes (P ≤ 0.04). While, these SNPs are not significant in our study, other than INS (HR = 1.00, P = 0.099), SNPs in IFIH1 (HR = 1.26, P = 0.20) and UBASH3A (HR = 1.71, P = 0.20) do have effects in the same direction. Second, variant calls in evolutionary divergent regions of the genome, including HLA, have poor performance, possibly masking important roles of this complex in the etiology of the disease. A final limitation is the representativeness of the sample, as the DAISY participants are selected for increased type 1 diabetes risk based upon HLA genotype. While this sampling provides an accelerated transition from genetic risk to islet autoimmunity and diabetes, it does not mirror the distribution of HLA genotypes seen in the general population.
At the same time, there are important strengths of the study. This is the first application of whole genome sequence analysis to progression from islet autoimmunity to type 1 diabetes, generating several plausible candidate genes for inspection. Second, the DAISY cohort represents an important and well-characterized cohort of subjects followed longitudinally for development of islet autoantibodies and type 1 diabetes. An earlier analysis in the DAISY cohort has shown a slower progression to multiple islet antibodies and type 1 diabetes among participants that develop islet autoimmunity later in adolescence or early adulthood22. However, several studies have demonstrated that the development of multiple islet antibodies is strongly predictive of progression to type 1 diabetes23–27. Among the 107 DAISY participants diagnosed with type 1 diabetes to date, 7.5% were older than 20 years of age at the time of diagnosis (unpublished data). Continued follow-up of these young adults with persistent islet autoimmunity will help us to answer the question of what happens to the non-progressors as they age and will allow analysis of fast progressors versus slow progressors.
In summary, we identified four risk regions that may play a role in progression to clinical diabetes from islet autoimmunity. The most associated genes and variants identified here are not those that have been seen in previous case–control studies of type 1 diabetes, suggesting that the genetic impact on progression to diabetes from islet autoimmunity may differ in key pathways from those identified once disease is established and support the need for follow-up studies to understand genetic risk factors that modulate progression of subclinical disease.
Methods
Study population
The DAISY study has followed two cohorts of young children at increased risk of type 1 diabetes (total N = 2547): a cohort of relatives of type 1 diabetes patients (siblings and offspring) enrolled by age 7, and a general population newborn cohort. The latter consists of children with type 1 diabetes susceptibility HLA-DR/DQ genotypes identified through screening of over 31,000 newborns at St. Joseph Hospital in Denver, Colorado. The details of screening and follow-up have been previously published28. Islet autoimmunity is defined by persistence of autoantibodies to insulin, GAD65, IA-2 and ZnT8. These autoantibodies were measured in the Immunogenetic Laboratory at the Barbara Davis Center using radiobinding assays (RBA)29. Additionally, autoantibodies to insulin, GADA, and IA-2A were measured using electrochemiluminescent (ECL) assays in participants with islet autoantibodies detected by RBA30,31. The age at seroconversion was defined by the first appearance of at least one islet autoantibody detected by RBA or ECL that persisted for at least two consecutive visits. Duration of islet autoimmunity was calculated from age of seroconversion to age of type 1 diabetes diagnosis (or age at last visit). Islet autoantibodies were measured at 9, 15, and 24 months and annually thereafter; autoantibody positive children were tested every 3–6 months. Type 1 diabetes onset was defined according to ADA criteria. DAISY participants included in this analysis were enrolled into the follow-up study by 7 years of age (enrollment criteria for the first-degree relative cohort) and were shown to be persistently positive for one or more islet autoantibody by RBA or ECL assay. This subset of participants are described here as “progressors”-those that progressed to clinical diagnosis of type 1 diabetes- and “non-progressors”-those that had not been diagnosed at the time of the last clinic visit or the last direct contact. Non-progressors were censored at the time of last direct contact: in-person or by phone, email, or text.
Informed consent was obtained from the parents of each study subject. The Colorado Multiple Institutional Review Board approved all study protocols. All experimental methods were carried out in accordance with relevant guidelines and regulations.
Whole genome sequencing
We sequenced 160 islet autoantibody positive DAISY participants on the Illumina HiSeq-X10 platform, yielding an average coverage of ~ 30-fold per base. Quality control checks were performed using FastQC32, and sequencing reads were aligned to the GRCh37 + decoy reference genome using BWA33 with default settings. We identified ~ 18.52 million SNPs and ~ 2.09 million indels using the reference model (gVCF-based) workflow for joint analysis using GATK-HaplotypeCaller34. Per sample variant calling metrics, determined using CollectVariantCallingMetrics tool, are provided in Supplementary Table 3.
Statistical analyses
Prior to statistical analysis, we filtered out variants with minor allele frequency < 0.05, call rate < 90% and/or HWE P < 10–10 in northern European samples, 6,893,119 variants remained for statistical analysis. Principal components (PCs) of ancestry were generated using KING35. Among the 160 samples, 12 pairs of full-siblings were identified, and the familial correlations among siblings were adjusted using the frailty function in R36 which added a simple random effects term to the Cox proportional-hazard model. In Cox proportional-hazard model for survival analysis, the time from seroconversion to either date of diabetes diagnosis or time of last contact was the time-to-event variable, the genotype at each variant was the independent variable, and the covariates to be adjusted included sex, age at seroconversion, first four PCs of ancestry, and the HLA haplotype groups (defined by DR3/4 as shown in Table 1).
Functional annotation
We conducted functional annotation using FUMA-v1.3.5e (https://fuma.ctglab.nl).
Supplementary information
Acknowledgements
The authors wish to thank the many participants in the DAISY cohort, and the many researchers and staff for their contributions. Funding This work was funded by NIH grants R01-DK32493, R01-DK032083, and R01-DK104351 as well as The Leona M. and Harry B. Helmsley Charitable Trust grants 2016PG-T1D047 and 2018PG-T1D017.
Author contributions
The study was conceptually designed by M.J.R. and S.S.R. DNA samples and phenotypic data were provided by M.J.R., A.K.S, B.I.F, J.M.N. and K.C.W. Variants from WGS data were called by A.R. and U.P. Statistical quality control methods were implemented by W.-M.C, Z.Z., A.R. Statistical analyses were performed by L.L, W-M.C., Z.Z. Bioinformatic analyses were performed by S.O–G, W-M.C., Z.Z. The paper was written by S.O–G., S.S.R. and M.J.R. All authors reviewed and contributed to the final paper. M.J.R. and S.S.R. are guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was funded by NIH grants R01-DK32493, R01-DK032083, and R01-DK104351 as well as The Leona M. and Harry B. Helmsley Charitable Trust Grants 2016PG-T1D047 and 2018PG-T1D017.
Data availability
All data used in the development of this manuscript is being deposited into dbGaP.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75690-6.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett JC, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onengut-Gumuscu S, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat. Genet. 2015;47:381–386. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 5.Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 6.Rewers M, et al. The environmental determinants of diabetes in the young (TEDDY) study: 2018 update. Curr. Diab. Rep. 2018;18:136. doi: 10.1007/s11892-018-1113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerup J, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2:864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 8.Steck AK, et al. Effects of non-HLA gene polymorphisms on development of islet autoimmunity and type 1 diabetes in a population with high-risk HLA-DR DQ genotypes. Diabetes. 2012;61:753–758. doi: 10.2337/db11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torn C, et al. Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes. 2015;64:1818–1829. doi: 10.2337/db14-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lempainen J, et al. Non-HLA gene effects on the disease process of type 1 diabetes: From HLA susceptibility to overt disease. J. Autoimmun. 2015;61:45–53. doi: 10.1016/j.jaut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Steck AK, et al. Improving prediction of type 1 diabetes by testing non-HLA genetic variants in addition to HLA markers. Pediatr. Diabetes. 2014;15:355–362. doi: 10.1111/pedi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rewers M, et al. Beta-cell autoantibodies in infants and toddlers without IDDM relatives: Diabetes autoimmunity study in the young (DAISY) J. Autoimmun. 1996;9:405–410. doi: 10.1006/jaut.1996.0055. [DOI] [PubMed] [Google Scholar]
- 13.Consortium, E. P An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu LQ, Cresswell P, Chin KC. Viperin is required for optimal Th2 responses and T-cell receptor-mediated activation of NF-kappaB and AP-1. Blood. 2009;113:3520–3529. doi: 10.1182/blood-2008-07-171942. [DOI] [PubMed] [Google Scholar]
- 15.Meyerovich K, et al. MCL-1 is a key antiapoptotic protein in human and rodent pancreatic beta-cells. Diabetes. 2017;66:2446–2458. doi: 10.2337/db16-1252. [DOI] [PubMed] [Google Scholar]
- 16.Colli ML, et al. Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim/Mcl-1 imbalance. PLoS Pathog. 2011;7:e1002267. doi: 10.1371/journal.ppat.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eizirik DL, Mandrup-Poulsen T. A choice of death—The signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 18.Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56:185–193. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- 19.Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2:1–4. doi: 10.1016/s0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 20.Marroqui L, et al. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic alpha and beta cells. Elife. 2015;4:e06990. doi: 10.7554/eLife.06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg SE, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–499. doi: 10.1038/s41586-018-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frohnert BI, et al. Late-onset islet autoimmunity in childhood: The Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 2017;60:998–1006. doi: 10.1007/s00125-017-4256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler AG, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietropaolo M, et al. Progression to insulin-requiring diabetes in seronegative prediabetic subjects: The role of two HLA-DQ high-risk haplotypes. Diabetologia. 2002;45:66–76. doi: 10.1007/s125-002-8246-5. [DOI] [PubMed] [Google Scholar]
- 25.Verge CF, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 26.Achenbach P, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53:384–392. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 27.Steck AK, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rewers M, et al. Newborn screening for HLA markers associated with IDDM: Diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 29.Bonifacio E, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J. Clin. Endocrinol. Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L, et al. Distinguishing persistent insulin autoantibodies with differential risk: Nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61:179–186. doi: 10.2337/db11-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao D, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62:4174–4178. doi: 10.2337/db13-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews S. FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 33.Li, H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEMarXiv:1303.3997v2 [q-bio.GN] (2013).
- 34.McKenna A, et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manichaikul A, et al. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2019). https://www.R-project.org/.
- 37.Pruim RJ, et al. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the development of this manuscript is being deposited into dbGaP.