Abstract
Objective
To evaluate the prognostic accuracy of d-dimer levels for advanced non-small-cell lung cancer (NSCLC).
Methods
This retrospective cohort study included 651 patients initially diagnosed with advanced NSCLC. Patients with d-dimer levels ≥0.5 mg/L were included in the high d-dimer group, whereas patients with lower levels were included in the normal group. Cumulative survival was estimated using Kaplan–Meier curves and compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model.
Results
The median plasma d-dimer level in the study cohort was 0.61 ± 0.49 mg/L. d-dimer levels were elevated in 60.98% of patients, and 80.1% of such patients had adenocarcinoma. Univariate and multivariate analyses identified d-dimer content as an independent factor for the prognosis of NSCLC (hazard ratio [HR] = 1.54, 95% confidence interval [CI] = 1.19–1.98). Kaplan–Meier analysis revealed that high plasma d-dimer levels were associated with shorter overall survival (HR = 1.48, 95% CI = 1.19–1.84). In addition, the receipt of <2 lines of treatment was associated with a higher risk of death than the receipt of >2 lines.
Conclusion
The present results imply that pretreatment plasma d-dimer levels could represent a prognostic factor for advanced NSCLC.
Keywords: D-dimer, overall survival, prognosis, non-small-cell lung cancer, cohort study, coagulation
Introduction
Lung cancer remains leading cause of cancer-related mortality worldwide, being responsible for approximately 1.76 million deaths per year.1 Non-small-cell lung cancer (NSCLC), accounting for 80% to 85% of all lung cancer cases, is usually diagnosed when the disease has progressed to an advanced stage. In addition, the overall survival (OS) of patients with NSCLC remains poor, with a 5-year survival rate of 3.5% to 29.6% despite the tremendous improvement in medical technologies regarding NSCLC.2 However, various factors affect the prognosis of patients with NSCLC, including tumor heterogeneity, physical performance, and responses to treatment. Therefore, identifying the key factors affecting patient prognosis is of great significance for decision making regarding the clinical treatment of NSCLC.
As the most frequent tool in assessment of prognosis of various cancers, the TNM staging system mainly focuses on the anatomical description of tumor development, but its utility in clinical practice is limited because it ignores the effects of oncogenes, the status of the immune system, and the nutritional status. In addition, the TNM staging system mainly depends on the interpretation of imaging tests, which may result in inconsistency between the size of the radiological solid component and the scope of pathological infiltration.3 Thus, development of alternative biomarkers is urgently needed. Recently, published data described some biomarkers that could represent independent prognostic factors for lung cancer. Meanwhile, plasma-based detection has been widely investigated because of its unique advantages, including non-invasiveness, quickness, and repeatability.4,5
The occurrence and development of malignant tumors can destroy the balance of the blood coagulation and anticoagulation systems through a variety of mechanisms, leading to a hypercoagulable state.6 As a product of fibrin degradation, d-dimer levels can reflect the degree of hypercoagulability in the body, and its concentration can be detected in blood samples. Previous studies found that high d-dimer levels were associated with poorer OS in several cancers, including lung, gastric, prostate, and breast cancers.7 Some studies reported that plasma d-dimer levels can serve as a predictor and prognostic factor for patients with small-cell lung cancer.8–10 However, studies on patients with NSCLC are limited. This study investigated the independent correlation of plasma d-dimer levels with OS in patients with advanced NSCLC.
Patients and methods
Study design
A retrospective cohort study was conducted to address the relationship between plasma d-dimer levels and OS among patients with NSCLC. The target independent variable was the plasma level of d-dimer at baseline. The dependent variable was OS (dichotomous variable: 1 = death; 0 = stability or survival).
Study population
The process of data collection was non-selective and consecutive. The data of patients with advanced NSCLC who were admitted to Guangxi Medical University Affiliated Cancer Hospital (Nanning, Guangxi, China) between December 2010 and October 2018 were collected. The study was approved by the Medical Ethics Committee of Guangxi Medical University Affiliated Cancer Hospital, and the protocol complied with the Declaration of Helsinki (Approval number LW2020032, approval date: 2020.05.29). To protect the privacy of patients, all data were anonymized, and thus, the requirement for informed consent was waived. All studies were conducted in accordance with relevant guidelines and regulations.
Patients with a histologically or cytologically confirmed diagnosis of advanced NSCLC were enrolled for further screening, and their clinical data including sex, age, smoking history, Eastern Cooperative Oncology Group performance status (ECOG-PS), pathology, and differentiation were recorded.
The inclusion criteria of this study were as follows: (1) stage IIIB or IV NSCLC at the initial diagnosis; (2) the availability of complete follow-up data; and (3) no history of chemotherapy, radiotherapy, or other treatments prior to diagnosis. Meanwhile, the exclusion criteria of the study were as follows: (1) malignant diseases other than NSCLC; (2) diseases and conditions such as cerebral infarction, coronary heart disease, pulmonary embolism, and venous thrombosis or the receipt of antithrombotic or anticoagulant therapy; (3) end-stage renal or liver disease; and (4) intracranial infection.
Variables
Plasma d-dimer levels were measured at baseline and recorded as a categorical variable. In brief, venous blood samples were collected from patients, preserved in Vacutainer citrate test tubes for centrifugation (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), and then analyzed using the nephelometry immunoassay (Siemens Healthcare Diagnostics Products GmbH, Erlangen, Germany). Patients with d-dimer levels ≥0.5 mg/L were included in the high d-dimer group, whereas those with lower levels were included in the normal group. Meanwhile, OS was recorded as the time from the date of diagnosis to that of death or the last follow-up.
In this study, we included the following covariates: (1) demographic data; (2) variables that may impact plasma d-dimer levels or OS according to previous literature; and (3) variables that may impact OS according to our clinical experiences. The following variables were used to construct the adjusted model: age, sex, smoking history, ECOG-PS, clinical stage, pathology, degree of differentiation, epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK)/c-ros oncogene 1 kinase (ROS1) mutation status, number of metastatic organs (obtained at baseline), number of lines of treatment, and the first-line treatment approach. Each adjusted variable was changed to a categorical variable mainly based on the significance of clinical guidance, previous literature, or the balance of data between the groups.
The TNM system of the 7th version of the American Joint Committee on Cancer was used for staging. Patients’ physical status was scored using ECOG-PS. Patients who had had smoked no more than 100 cigarettes in their lifetime were defined as non-smokers. Smokers were defined as current smokers or individuals who quit smoking within 1 year before diagnosis. Tumor histology was classified according to the 3rd edition of the World Health Organization Classification of tumors.
Follow-up procedure
The second author was in charge of follow-up. At each follow-up visit, imaging and laboratory data were reviewed to evaluate the efficacy of treatment. Patients were followed up every two cycles before progression and every 2 months after progression. The cutoff date for participant follow-up was October 2019. Follow-up data were stored in the hospital’s electronic medical record system.
Statistical analysis
Continuous variables with a normal distribution were presented as the mean ± SD. Categorical variables were expressed as frequencies or as percentages. The chi-squared test or Fisher’s exact test was used to assess categorical variables. Multivariate analyses were performed using the Cox proportional hazards model and adjusted for statistically significant variables in univariate analysis. The subgroup analysis and interaction test were performed to further assess the robustness of our study findings. Cumulative OS was estimated using Kaplan–Meier curves and compared using the log-rank test. All analyses were performed with the statistical software packages in R (http://www.R-project.org) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA). P < 0.05 (two-sided) denoted statistical significance.
Results
Baseline characteristics of selected patients
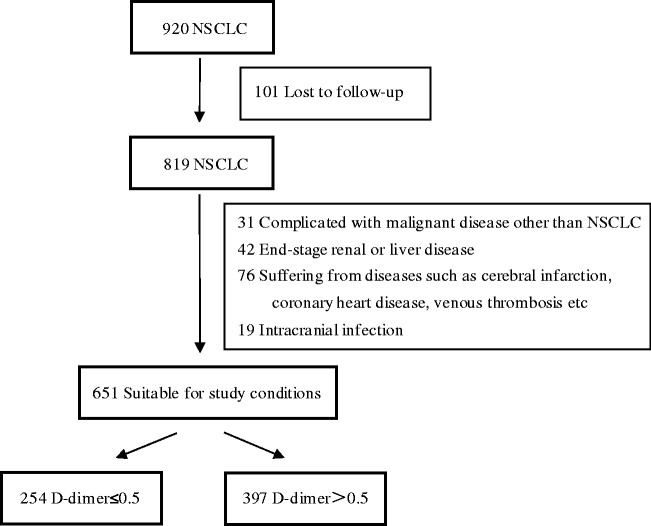
In total, 920 patients qualified for enrollment in this study. After applying the inclusion and exclusion criteria, 651 participants were selected for the final analysis (Figure 1). The baseline characteristics of the selected patients according to their d-dimer levels are listed in Table 1. Briefly, d-dimer levels were normal in 254 patients (39.02%) and elevated in 397 patients (60.98%). In the high d-dimer group, 90.2% of patients had advanced diseases, and the pathological diagnosis was adenocarcinoma in 80.1% of patients. There were significant differences in age, ECOG-PS, clinical stage, the EGFR/ALK/ROS1 mutation status, the number of metastatic organs, and the first-line treatment approach (all P < 0.05) between the normal and high d-dimer groups. No statistically significant differences were detected for sex, smoking history, pathology, the degree of differentiation, or the number of treatment lines between the groups.
Figure 1.
Flowchart of the study.
NSCLC, non-small-cell lung cancer.
Table 1.
Clinical characteristics of patients with normal and high d-dimer levels.
| Variables | Normal d-dimer group (N = 254) | High d-dimer group (N = 397) | P | |
|---|---|---|---|---|
| Age (years) | 0.0270 | |||
| ≤60 | 62.2 | 53.4 | ||
| >60 | 37.8 | 46.6 | ||
| Sex | 0.8570 | |||
| Male | 66.9 | 66.2 | ||
| Female | 33.1 | 33.8 | ||
| Smoking history | 0.2460 | |||
| Never smoked | 48 | 51.9 | ||
| Previous smoker | 39.8 | 33.5 | ||
| Current smoker | 12.2 | 14.6 | ||
| ECOG-PS | <0.0010 | |||
| <2 | 96.5 | 88.2 | ||
| ≥2 | 3.5 | 11.8 | ||
| Clinical stage | <0.0010 | |||
| IIIB | 23.2 | 9.8 | ||
| IV | 76.8 | 90.2 | ||
| Histological diagnosis | 0.0520 | |||
| Adenocarcinoma | 73.2 | 80.1 | ||
| Squamous cell carcinoma | 24 | 16.4 | ||
| Other | 2.8 | 3.5 | ||
| Degree of differentiation | 0.3840 | |||
| Poorly differentiated | 40.2 | 35.3 | ||
| Moderately differentiated | 19.3 | 18.1 | ||
| Well-differentiated | 20.5 | 21.2 | ||
| Unknown | 20.1 | 25.4 | ||
| Mutation status | <0.0010 | |||
| No | 82.7 | 66.2 | ||
| EGFR/ALK/ROS1 mutation | 17.3 | 33.8 | ||
| Number of metastatic organs | <0.0010 | |||
| ≤2 | 66.5 | 40.3 | ||
| >2 | 33.5 | 59.7 | ||
| Number of treatment lines | 0.3480 | |||
| <2 | 67.3 | 63.7 | ||
| ≥2 | 32.7 | 36.3 | ||
| Treatment approach | <0.0010 | |||
| None | 11.4 | 15.1 | ||
| Chemotherapy | 59.4 | 42.6 | ||
| Tyrosine kinase inhibitors | 18.9 | 31.2 | ||
| Other | 10.2 | 11.1 |
Data are presented as percentages.
ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1 kinase.
Univariate analysis
The results of univariate analyses are listed in Table 2. The median d-dimer level among all patients was 0.61 ± 0.49 mg/L. In univariate survival analysis, age, sex, smoking history, clinical stage, pathology, the degree of differentiation, the EGFR/ALK/ROS1 mutation status, the number of metastatic organs, the number of treatment lines, and the first-line treatment approach were significantly associated with OS (all P < 0.05). In addition, female sex (hazard ratio [HR] = 0.76, 95% confidence interval [CI] = 0.60–0.96, P = 0.0225), moderately differentiated tumors (HR = 0.86, 95% CI = 0.64–1.15, P = 0.3088), well-differentiated tumors (HR = 0.30, 95% CI = 0.22–0.42, P < 0.0001), EGFR/ALK/ROS1 mutations (HR = 0.42, 95% CI = 0.31–0.56, P < 0.0001), ≥2 treatment lines (HR =0.78, 95% CI = 0.63–0.98, P = 0.0320), treatment with chemotherapy (HR = 0.63, 95% CI = 0.46–0.87, P = 0.0043), treatment with tyrosine kinase inhibitors (HR = 0.49, 95% CI = 0.34–0.70, P < 0.0001), and the use of other treatment approaches (HR =0.51, 95% CI = 0.31–0.87, P = 0.0047) were predictive of longer OS. By contrast, high d-dimer levels (HR = 1.55, 95% CI = 1.23–1.95, P = 0.0002), age > 60 years (HR = 1.29, 95% CI = 1.03–1.60, P =0.0238), prior smoking (HR = 1.33, 95% CI = 1.05–1.68, P = 0.0197), current smoking (HR = 1.60, 95% CI = 1.15–2.22, P = 0.0051), clinical stage IV lesions (HR = 1.46, 95% CI = 1.06 to 2.00, P = 0.0191), squamous cell carcinoma (HR = 1.30, 95% CI = 0.99–1.71, P =0.0565), other histologies (HR = 1.76, 95% CI = 1.01–3.07, P = 0.0478), and >2 metastatic organs (HR = 1.34, 95% CI = 1.08–1.67, P = 0.0080) were associated with worse OS. Meanwhile, ECOG-PS was not considered predictive of OS because of the imbalance between the study groups.
Table 2.
Univariate analysis of patient survival.
| Variables | HR (95% CI) | P-value | |
|---|---|---|---|
| d-Dimer | 0.61 ± 0.49 mg/L | 1.55 (1.23, 1.95) | 0.0002 |
| Age (years) | |||
| ≤60 | 370 (56.84%) | 1 | |
| >60 | 281 (43.16%) | 1.29 (1.03, 1.60) | 0.0238 |
| Sex | |||
| Male | 433 (66.51%) | 1 | |
| Female | 218 (33.49%) | 0.76 (0.60, 0.96) | 0.0225 |
| Smoking history | |||
| Never smoked | 328 (50.38%) | 1 | |
| Previous smoker | 234 (35.94%) | 1.33 (1.05, 1.68) | 0.0197 |
| Current smoker | 89 (13.67%) | 1.60 (1.15, 2.22) | 0.0051 |
| ECOG-PS | |||
| <2 | 595 (91.40%) | 1 | |
| ≥2 | 56 (8.60%) | 1.38 (0.95, 2.01) | 0.0867 |
| Clinical stage | |||
| IIIB | 98 (15.05%) | 1 | |
| IV | 553 (84.95%) | 1.46 (1.06, 2.00) | 0.0191 |
| Histological diagnosis | |||
| Adenocarcinoma | 504 (77.42%) | 1 | |
| Squamous cell carcinoma | 126 (19.35%) | 1.30 (0.99, 1.71) | 0.0565 |
| Others | 21 (3.23%) | 1.76 (1.01, 3.07) | 0.0478 |
| Degree of differentiation | |||
| Poorly differentiated | 242 (37.17%) | 1 | |
| Moderately differentiated | 121 (18.59%) | 0.86 (0.64, 1.15) | 0.3088 |
| Well-differentiated | 136 (20.89%) | 0.30 (0.22, 0.42) | <0.0001 |
| Unknown | 152 (23.35%) | 1.96 (1.42, 2.70) | <0.0001 |
| Mutation status | |||
| No | 473 (72.66%) | 1 | |
| EGFR/ALK/ROS1 mutation | 178 (27.34%) | 0.42 (0.31, 0.56) | <0.0001 |
| Sum of metastasis organs | |||
| ≤2 | 329 (50.54%) | 1 | |
| >2 | 322 (49.46%) | 1.34 (1.08, 1.67) | 0.0080 |
| Sum of treatment lines | |||
| <2 | 424 (65.13%) | 1 | |
| ≥2 | 227 (34.87%) | 0.78 (0.63, 0.98) | 0.0320 |
| Treatment approach | |||
| None | 89 (13.67%) | 1 | |
| Chemotherapy | 320 (49.16%) | 0.63 (0.46, 0.87) | 0.0043 |
| Tyrosine kinase inhibitors | 172 (26.42%) | 0.49 (0.34, 0.70) | <0.0001 |
| Other | 70 (10.75%) | 0.51 (0.31, 0.81) | 0.0047 |
Data are presented as the mean ± SD or n (%).
HR, hazard ratio; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1 kinase.
Results of unadjusted and adjusted Cox proportional hazard analysis
The HRs and 95% CIs of the unadjusted and adjusted Cox proportional hazard models are listed in Table 3. In the unadjusted model (crude model), elevated d-dimer levels increased the risk of death by 55% compared with normal d-dimer levels (HR = 1.55, 95% CI = 1.23–1.95, P =0.0002). In the fully adjusted model, high d-dimer levels remained associated with a significantly higher risk of death (HR = 1.54, 95% CI = 1.19–1.98, P = 0.0010).
Table 3.
Relationship between d-dimer levels and survival in different models.
| Variable | Crude model | P | Model 1 | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| d-Dimer levels ≤0.5 mg/L | 1 | 1 | ||
| d-dimer levels >0.5 mg/L | 1.55 (1.23, 1.95) | 0.0002 | 1.54 (1.19, 1.98) | 0.0010 |
| N | 651 | 651 |
Crude model: no adjustments.
Model l: adjusted for age, sex, smoking history, clinical stage, histological diagnosis, degree of differentiation, mutation status, number of metastatic organs, number of treatment lines, and treatment approach.
HR, hazard ratio; CI, confidence interval.
Subgroup analysis
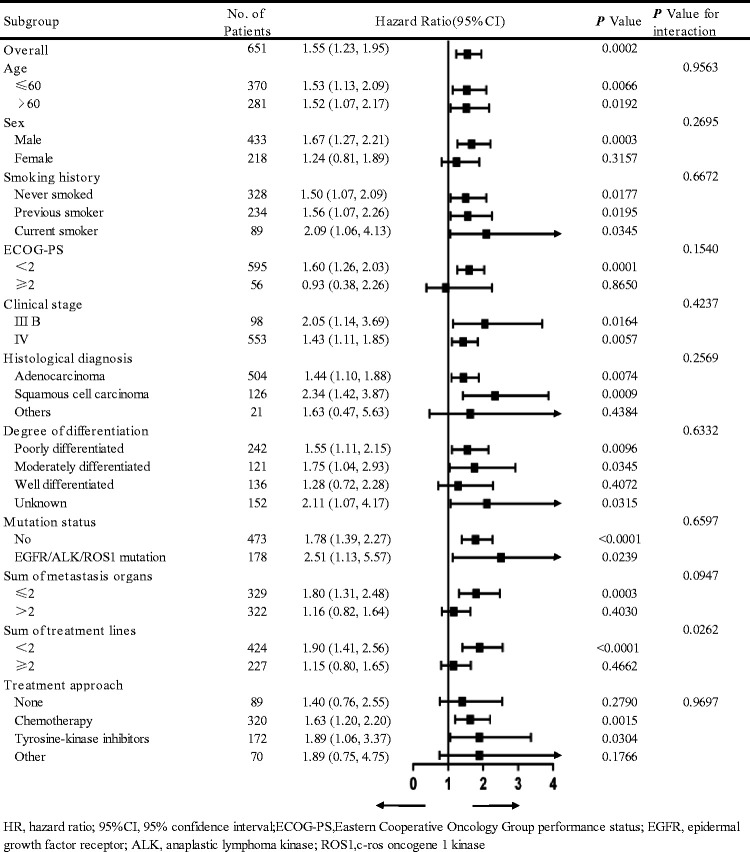
Age, sex, smoking history, ECOG-PS, clinical stage, pathology, the degree of differentiation, the EGFR/ALK/ROS1 mutation status, the number of metastatic organs, the number of treatment lines, and the first-line treatment approach were used as the stratification variables to observe the trends of effect sizes (Figure 2). In this analysis, the relationship between d-dimer levels and risk of death remained stable in most subgroups. Additionally, there was a significant interaction (P for interaction) between the number of treatment lines and d-dimer levels concerning the risk of death. In the high d-dimer level group, patients who received <2 lines of treatment had a higher risk of death than those who received ≥2 lines of treatment (HR = 1.90 [95% CI = 1.41–2.56] vs. HR = 1.15 [95% CI = 0.80–1.65], P for interaction = 0.0262).
Figure 2.
Subgroup analysis of the relationship between high d-dimer levels and outcomes
CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1 kinase.
Kaplan–Meier survival analysis
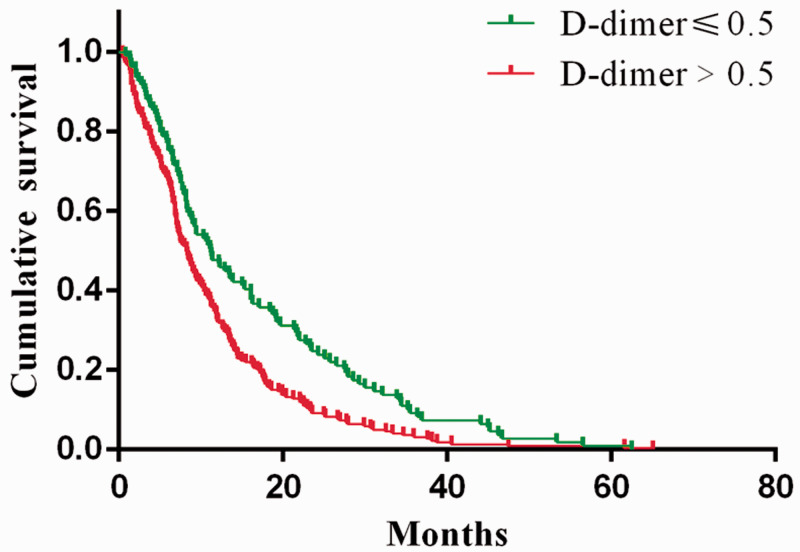
As presented in Figure 3, patients with high d-dimer levels had significantly shorter median OS than those with normal d-dimer levels (8.3 months vs. 11.2 months; HR = 1.48, 95% CI = 1.19–1.84, log-rank P = 0.0006).
Figure 3.
Kaplan–Meier estimates of survival probabilities in the normal and high d-dimer level group
OS, overall survival; HR, hazard ratio; CI, confidence interval.
Discussion
The present study assessed the prognostic value of d-dimer in patients with advanced NSCLC with no history of treatment with standard anticancer therapies. These patients reflected the natural course of advanced NSCLC, making them worthy of study. Our findings indicated that pretreatment serum d-dimer levels represent an independent prognostic factor for OS among patients with advanced NSCLC.
A substantial amount of data have established the imbalance between blood coagulation and anticoagulation in patients with malignant tumors.11–13 However, the underlying mechanisms have not been fully elucidated, and per several studies, it may be related to tissue factors,14 coagulants,15,16 colony-stimulating factors,17 platelet activation markers,18,19 inflammatory cytokines,20 DNA, and RNA21 produced by tumor cells that can activate the coagulation system and subsequently trigger the amplification of the coagulation cascade through multiple signaling pathways. The rupture of thrombin converts plasma fibrinogen into insoluble fibrin, thereby increasing d-dimer levels in plasma. Surprisingly, there is a complex two-way relationship between the imbalanced blood coagulation system and cancer. Activation of the clotting system accelerates thrombosis, whereas the resulting embolism has emerged as the second leading cause of cancer-related death.22 At the same time, the components of the coagulation and fibrinolysis systems act on different stages of tumor growth, infiltration, and metastasis by promoting the movement and invasion of tumor cells, supporting the development of tumor stroma, and inducing tumor angiogenesis, eventually leading to poor prognoses among patients with cancer.23–25
Antoniou et al.26 detected d-dimer levels in plasma from 47 patients with advanced lung cancer before, during, and after chemotherapy and detected dynamic changes in its levels in response to treatment. In 78 patients with lung cancer (60 patients with NSCLC and 18 patients with small-cell lung cancer),27 multivariate analysis illustrated that the plasma d-dimer level was an independent predictor of prognosis, suggesting that elevated plasma d-dimer levels are related to decreased survival rates and poor responses to treatment. In the present study, we specified that pretreatment plasma d-dimer levels affected the risk of death in a large cohort of patients with advanced NSCLC.
Our study observed no significant correlations of plasma d-dimer levels with several baseline variables, including sex, smoking history, pathology, differentiation, or the number of treatment lines. However, we found that high plasma d-dimer levels were significantly correlated with age >60 years, ECOG-PS ≥2, stage IV cancer, EGFR/ALK/ROS1 mutations, and >2 metastatic organs. Interestingly, we also noted that 90.2% of patients with elevated with d-dimer levels had stage IV cancer, indicating that pretreatment d-dimer levels are associated with tumor invasion and metastasis. In univariate analysis, age, sex, smoking history, clinical stage, pathology, differentiation, the EGFR/ALK/ROS1 mutation status, the number of metastatic organs, the number of treatment lines, and the first-line treatment approach were cited as independent prognostic factors for OS. In this study, compared with normal d-dimer levels, subgroup analysis illustrated that the correlation between high d-dimer levels and the risk of death remained stable after stratification using covariates. The subsequent analysis indicated that the increased risk of death associated with high d-dimer levels was affected by the number of treatment lines. In other words, tumor progression and changes of the treatment strategy reduced the influence of pretreatment d-dimer levels in plasma on OS among patients. Thus, it is important to detect plasma d-dimer levels dynamically during the treatment of NSCLC.
Ge et al.28 studied the prognostic value of plasma d-dimer levels at different time points before and after chemotherapy in 82 patients with advanced NSCLC and found that progression-free survival was significantly shorter in patients with high d-dimer levels than in those with normal levels (P = 0.0210). However, multivariate analysis identified no relationship between pretreatment d-dimer levels and the risk of death, completely contradicting our results. In our study, OS was significantly shorter in patients with elevated d-dimer levels. After evaluating 1931 patients with NSCLC, Wang et al.29 also found that d-dimer levels had a potent, independent, and negative effect on prognosis (HR = 1.245, P < 0.001). In contrast to the two previously published papers, the EGFR/ALK/ROS1 mutation status, first-line treatment approach, and number of treatment lines were also used as adjusting factors to reflect the prognostic relationship between d-dimer levels and the risk of death more accurately in this study. However, the prior studies mainly focused on exploring the effect of d-dimer levels in the first-line setting. The receipt of second- and third-line chemotherapy and/or targeted therapy after first-line chemotherapy may have a significant impact on OS, especially in patients harboring driving mutations.
In this study, high d-dimer levels were negatively correlated with the risk of death in patients with advanced NSCLC in the fully adjusted model. Thus, the routine detection of d-dimer levels before treatment may provide valuable prognostic information that may aid clinicians in decision-making processes for the treatment of advanced NSCLC.
The clinical implications of this study were as follows: (1) pretreatment plasma d-dimer levels are independently correlated with the risk of death in patients with advanced NSCLC, and thus, they could serve as a clinical monitoring index and facilitate early decision making; (2) the findings of this study should be helpful for future research to establish diagnostic or predictive models of OS; and (3) early antithrombotic management in patients with advanced NSCLC and elevated d-dimer levels may lead to better clinical outcomes, although further clarification is needed.
The strengths of this study were as follows: (1) as an observational study, potential confounding factors could not be excluded, and thus, we used strict statistical adjustment to minimize residual confounders; and (2) the effect modifier factor analysis improved the use of the data and yielded stable conclusions in different subgroups.
Some limitations must be addressed: (1) this study was subject to the limitations inherent to retrospective analyses of observational data from a single center; (2) we could not fully describe the effects of coagulation and fibrinolysis in NSCLC, and whether other fibrin degradation products have the same effect remains to be determined; (3) although we used an adjusted Cox regression model, some selection bias may have remained, including the use of anticoagulants in the course of the disease; and (4) the validity of the results was compromised by the lack of standardized d-dimer assays, and the conclusions of the article should be interpreted carefully.
In summary, pretreatment d-dimer levels in plasma appear to have value for predicting the prognosis of advanced NSCLC, and such a finding should be validated in the future in perspective studies with larger cohorts.
Acknowledgements
The authors thank all the staff members at our institution.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding
This study was funded by the National Key Clinical Specialist Construction Programs of China (No. 2015-6), Guangxi Medicine and Health Appropriate Technology Promotion Project of China (grant number: S201630), and 139 Talent Planning Project of Guangxi Health Commission of China (grant number: 201903030).Authors’ contributions
Conceptualization: Qianfei Liu, Jianbo He, Aiping Zeng, Shaozhang Zhou. Methodology: Qianfei Liu, Jianbo He, Aiping Zeng, Ruiling Ning, Liping Tan. Formal analysis and investigation: Qianfei Liu, Jianbo He, Shaozhang Zhou. Writing – original draft preparation: Qianfei Liu, Jianbo He. Writing - review and editing: Qianfei Liu, Aiping Zeng, Shaozhang Zhou. Funding acquisition: Aiping Zeng, Shaozhang Zhou. Resources: Jianbo He, Ruiling Ning, Liping Tan. Supervision: Aiping Zeng, Shaozhang Zhou. All authors read and approved the final manuscript.
ORCID iDs
Qianfei Liu https://orcid.org/0000-0002-4755-9755
Shaozhang Zhou https://orcid.org/0000-0002-5471-4231
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019; 37: 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattori A, Takamochi K, Oh S, et al. New revisions and current issues in the eighth edition of the TNM classification for non-small cell lung cancer. Jpn J Clin Oncol 2019; 49: 3–11. [DOI] [PubMed] [Google Scholar]
- 4.Coate LE, John T, Tsao MS, et al. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol 2009; 10: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 5.Pennell NA, Arcila ME, Gandara DR, et al. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book 2019; 39: 531–542. [DOI] [PubMed] [Google Scholar]
- 6.Abdol Razak NB, Jones G, Bhandari M, et al. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel) 2018; 10: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012; 97: 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Yu H, Wu C, et al. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother 2016; 81: 210–217. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Mei X, Wu H, et al. D-dimer level is related to the prognosis of patients with small cell lung cancer. Ann Transl Med 2017; 5: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Jia Y, Jia Y, et al. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int J Clin Oncol 2018; 23: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 11.Agorogiannis EI, Agorogiannis GI. Coagulation, angiogenesis, and venous thromboembolism in cancer. Lancet 2002; 359: 1440. [DOI] [PubMed] [Google Scholar]
- 12.Yeh E, Chang HM. Cancer and Clot: Between a Rock and a Hard Place. J Am Coll Cardiol 2017; 70: 939–941. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Berg YW, Osanto S, Reitsma PH, et al. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 2012; 119: 924–932. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes CJ, Morinaga L, Alves JJ, Jr, et al. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev 2019; 28: 180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol 2009; 27: 4848–4857. [DOI] [PubMed] [Google Scholar]
- 16.Piccioli A, Falanga A, Baccaglini U, et al. Cancer and venous thromboembolism. Semin Thromb Hemost 2006; 32: 694–699. [DOI] [PubMed] [Google Scholar]
- 17.Hisada Y, Mackman N. Mouse models of cancer-associated thrombosis. Thromb Res 2018; 164: S48–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedl J, Preusser M, Nazari PM, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017; 129: 1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma BK, Flick MJ, Palumbo JS. Cancer-Associated Thrombosis: A Two-Way Street. Semin Thromb Hemost 2019; 45: 559–568. [DOI] [PubMed] [Google Scholar]
- 20.Gil-Bernabe AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012; 119: 3164–3175. [DOI] [PubMed] [Google Scholar]
- 21.Key NS, Khorana AA, Mackman N, et al. Thrombosis in Cancer: Research Priorities Identified by a National Cancer Institute/National Heart, Lung, and Blood Institute Strategic Working Group. Cancer Res 2016; 76: 3671–3675. [DOI] [PubMed] [Google Scholar]
- 22.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5: 632–634. [DOI] [PubMed] [Google Scholar]
- 23.McEachron TA, Pawlinski R, Richards KL, et al. Protease-activated receptors mediate crosstalk between coagulation and fibrinolysis. Blood 2010; 116: 5037–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawar NR, Buzza MS, Antalis TM. Membrane-Anchored Serine Proteases and Protease-Activated Receptor-2-Mediated Signaling: Co-Conspirators in Cancer Progression. Cancer Res 2019; 79: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwaan HC, Lindholm PF. Fibrin and Fibrinolysis in Cancer. Semin Thromb Hemost 2019; 45: 413–422. [DOI] [PubMed] [Google Scholar]
- 26.Antoniou D, Pavlakou G, Stathopoulos GP, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer 2006; 53: 205–210. [DOI] [PubMed] [Google Scholar]
- 27.Altiay G, Ciftci A, Demir M, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007; 19: 494–498. [DOI] [PubMed] [Google Scholar]
- 28.Ge LP, Li J, Bao QL, et al. Prognostic and predictive value of plasma D-dimer in advanced non-small cell lung cancer patients undergoing first-line chemotherapy. Clin Transl Oncol 2015; 17: 57–64. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther 2015; 8: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]