Abstract
Objective
This study aimed to determine the chemical profile of Lantana camara leaf oil.
Methods
The essential oil was extracted from dried leaf samples using the Soxhlet extraction method. The oil was separated from the solvent and the bioactive compounds were identified using gas chromatography–mass spectroscopy (GC-MS) and Fourier transform infrared spectroscopy (FTIR). The identified peaks in the mass spectrum were matched with the database of the US National Institute of Standards and Technology (NIST) library.
Results
The FT-IR results indicated the presence of alcohols, carboxylic acids, phenols, alkanes, ketones, and primary amine compounds. GC-MS identified 43 compounds representing 95% of the total leaf essential oil components. Some of the major isolated compounds included a pyrrolizine; 1-dodecanol; 1,2-nonadecanediol; phytol; 1,3-dioxolane; 4-undecene, 9-methyl, (Z)-; 1-eicosanol; and imidazole.
Conclusions
The identified constituents of the extracted oil have established pharmacologic and insecticidal activities, and these compounds are also used in the drink, food, and cosmetic industries. This extract is highly valuable for the medical treatment of various ailments.
Keywords: Lantana camara, phytochemical profile, biological property, gas chromatography–mass spectroscopy, Fourier transform infrared spectroscopy, oil extract
Introduction
Plants are a source of many bioactive compounds that play a significant role in the treatment of various ailments.1 They are rich in secondary metabolites with outstanding biological activities that are suited to different applications and treatments. These secondary metabolites have a variety of structures and physicochemical properties.
Lantana camara (Verbenaceae) is a strong, evergreen, aromatic flowering shrub with a distinct fragrance2 The plant grows in tropical and subtropical regions of the world.3 In Ethiopia, L. camara is known locally as “Yewef kollo” and is used widely in traditional medicine.4 It was first introduced into Ethiopia for ornamental purposes because of its attractive fragrance and flower colors. L. camara can grow up to 4 m high under normal conditions, but it can grow as high as 13 m when supported. It grows well under a variety of agroclimatic conditions.5
L. camara is a medicinal plant that contains a large number of phytochemical compounds.6 The fresh leaves are crushed and boiled with water for the treatment of eczema, tinea, dermatitis, traumatic injuries, diarrhea and other bowel disorders, ulcers, cholera, and wound bleeding.7,8 Leaf powder and oil extract have been shown to exhibit antimicrobial, anti-inflammatory, antioxidant, antihypertensive, and antitussive effects.5 The plant roots and several other parts of the plant have been used to treat malaria, rheumatism, and certain skin ailments, among others.9 The therapeutic value of L. camara is attributable in the most part to bioactive compounds such as flavonoids, alkaloids, tannins, saponins, steroids, glycosides, phenyl-containing compounds, and triterpenoids.10
L. camara extracts are extremely toxic toward pests and exert a highly repellent effect on weevils in stored grains.11 In rural areas in Ethiopia, farmers have been using these extracts as insecticides during grain storage to prevent infestations by weevils and other insect pests. L. camara leaf extracts also possess larvicidal activity, while oil extracts from the flowers have repellent activity against mosquitoes.12
In recent years, chromatographic and spectroscopic analyses have played a key role in pharmaceutical and biomedical investigations.13 Researchers have used these methods for numerous studies of plants to analyze their physicochemical composition, particularly techniques such as gas chromatography–mass spectroscopy (GC-MS) and Fourier transform infrared spectroscopy (FTIR), which are highly sensitive. Even though the chemical composition of the essential oils extracted from L. camara growing in different countries has been reported, that of L. camara grown in Ethiopia has not been studied to date.
The objective of the present study was to analyze the volatile phytochemical constituents of oil extracted from L. camara leaves collected at Bezawit Palace, Ethiopia using chromatographic and spectroscopic techniques.
Materials and methods
Collection of plant materials
Healthy, mature L. camara leaves were collected from the forest at Bezawit Palace, Bahir Dar, Ethiopia in April 2019. The leaves were cleaned and then dried in the shade for 13 days. The leaves were subsequently ground to a powder using a grinder.
Extraction of Lantana camara oil
The Soxhlet extraction method14 was used to extract the oil from L. camara leaves. Approximately 50 g of the powdered L. camara leaf was placed into a cellulose thimble and put inside the Soxhlet apparatus. The oil extracting solvent comprised 500 mL of either methanol, ethanol, or ethyl acetate. Oil extraction was performed at the solvent boiling temperature and the extraction time was 5 to 6 hours. The solvent extract was concentrated with a rotary evaporator at reduced pressure to achieve maximum yield of the essential oil. The extracted oil was stored at 4°C in a refrigerator and dried under anhydrous Na2SO4 for further analysis.
Phytochemical investigation of oil extract
Phytochemical methods are used as a tool to study the different secondary metabolites present in plants that may be responsible for their medicinal properties.15 The oil extract and powdered leaf of L. camara were subjected to phytochemical screening to identify the presence of active compounds such as alkaloids, tannins, saponins, flavonoids, triterpenoids, steroids, glycosides, and phenols. The presence of bioactive compounds was determined using standard methods.
Alkaloids
The method used to identify alkaloids was described by Aguoru et al.16 The extracted leaf oil (0.2 g) was dissolved in 5 mL of 1% HCl and incubated in a water bath for approximately 2 minutes. The filtrate (1 mL) was treated with five drops of Dragendorff’s reagent. The formation of turbidity or an orange precipitate indicated the presence of alkaloids.
Saponins
A pinch of dried leaf powder was added to a test tube containing 2 to 3 mL of distilled water and the mixture was vigorously shaken. The presence of saponins was indicated by foam formation.17
Tannins
The method implemented was described by Idris et al.18 Extracted leaf sample (0.5 g) was dissolved in 5 mL of distilled water, then boiled gently and cooled. The solution (1 mL) was put into a test tube and three drops of ferric chloride solution were added. The sample was observed for a color change to blue-black, green, or blue-green, which indicated the presence of tannins.
Terpenes/terpenoids
The Salkowski test was used to test for the presence of terpenes.19 The extracted leaf oil was mixed in 2 mL of chloroform, and 3 mL of concentrated H2SO4 were carefully added to form a layer. The sample was observed for a color change to reddish-brown at the interface, which indicated the presence of terpenes/terpenoids.
Steroids
Acetic anhydride (2 mL) was added to 0.5 g essential oil leaf extract, followed by the addition of 2 mL of H2SO4. The sample was observed for a color change to blue, which indicated the presence of steroid rings.20
Flavonoids
Diluted ammonia solution (5 mL) was added to the aqueous filtrate of the leaf extract, followed by the addition of concentrated H2SO4. The sample was observed for a color change to yellow, which indicated the presence of flavonoids.20
Glycosides
To 5 mL of the plant extract, 2 mL of glacial acetic acid and one drop each of FeCl3 and concentrated H2SO4 were added.21 Browning of the interface indicated the presence of glycosides.
FT-IR analysis
FT-IR analysis of the solvent-extracted oil was performed using a Shimadzu FTIR-8400s Fourier transform infrared spectrophotometer (Kyoto, Japan). The FTIR spectrum was used to identify the functional groups of the active components based on the peaks observed in the infrared region. The extracted oil was encapsulated in a 100-mg potassium bromide (KBr) pellet to prepare a translucent sample disk and the analysis was carried out by scanning the samples across the wavenumber range of 400 to 4,000 cm−1.
GC-MS analysis
GC-MS analysis was performed on a 5975C Series GC/MSD instrument (Agilent Technology, Santa Clara, CA, USA) coupled with an HP-5ms fused silica capillary column (30 m × 0.25 mm × 0.25 µm). The GC oven temperature was first raised from 60°C to 120°C at a rate of 10°C/minute, then increased at a rate of 3°C/minute to 175°C, 5°C/minute to 205°C, 0.8°C/minute to 210°C, and then 5°C/minute to 280°C. The temperature was then held at 280°C for 5 minutes, giving a total runtime of 55.583 minutes. The temperature of the split injector was set at 240°C and the split ratio was 1:10. The essential oil (1 µL) was injected into the GC. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/minute. The mass spectrometer was operated in full-scan mode with an ionization energy of 70 eV and an interface temperature of 280°C. The MS source temperature and MS quadruple temperature were 230°C and 150°C, respectively. The scan range was from m/z 30 to 550. Identification of each component was carried out by matching the retention times and mass spectra with those in the US National Institute of Standards and Technology (NIST) mass spectra database.22 Relative abundance (% area) calculations were based on the ratio between the peak area of each compound and the sum of the peak areas of all compounds.23
Results and Discussion
Phytochemical analysis
Preliminary phytochemical screening tests are important for the identification of bioactive principles and may subsequently guide drug discovery and improvement. In the present study, several phytochemical constituents of L. camara were identified. Primary phytochemical screening of L. camara leaves confirmed the presence of steroids, flavonoids, tannins, glycerol, and saponins, while alkaloids were present only in the methanol and ethanol extracts. The results of the phytochemical analyses are presented in Table 1. Such phytochemicals may provide new avenues for the development of new classes of pharmaceutical, biopesticidal, insecticidal, and antimicrobial agents.24 Previously, various organic extracts of L. camara leaves were reported to contain steroids, flavonoids, tannins, and fixed oil.25 These phytochemical compounds are the top candidates conferring medicinal value to this plant. Indeed, the most abundant compounds found in all solvent extracts in the present study, including several flavonoids, glycosides, terpenoids, and alkaloids isolated from this plant, have been reported to exert diverse biological activities.26
Table 1.
Phytochemical analysis of Lantana camara leaf oil.
| Phytochemical | Positive indicator |
Solvent extract |
||
|---|---|---|---|---|
| Methanol | Ethanol | Ethyl acetate | ||
| Steroids | Blue color | +++ | ++ | + |
| Terpenoids | Reddish-brown color | + | − | − |
| Alkaloids | Orange precipitate | + | ++ | − |
| Flavonoids | Yellow precipitate | +++ | ++ | ++ |
| Tannins | Greenish-black color | ++ | + | + |
| Glycosides | Brown interface | ++ | + | ++ |
| Saponins | Froth/foam formation | +++ | ++ | + |
+++, high; ++, moderate; +, weak; −, absent.
FT-IR analysis
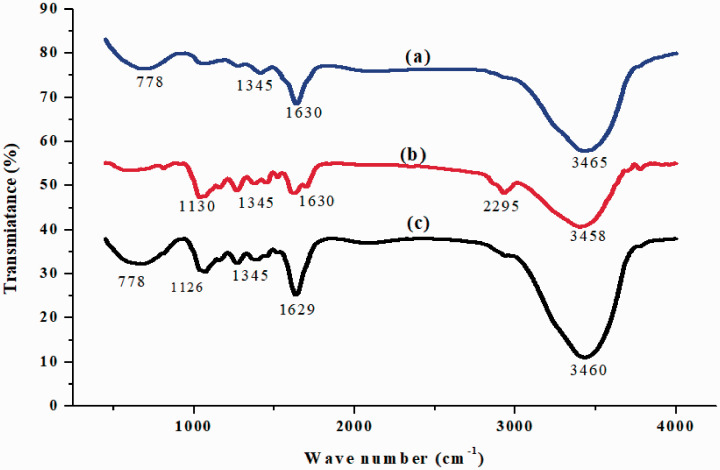
FT-IR spectroscopy revealed the presence of several functional groups such as phenols, amines, alcohols, alkenes, carboxylic acids, aliphatic compounds, carbonyl compounds, and esters. Representative FT-IR spectra of the methanol, ethanol, and ethyl acetate extracts are shown in Figure 1. Bands were observed at 3465, 3458, and 3460 cm−1, which were related to the vibration of the stretching hydroxy (-OH) groups. The bands found at 1629 cm−1 and 1630 cm−1 might have resulted from the stretching vibration of the C=C groups, which include cyclic structures with a ring resonance bond that gives increased stability, and the vibration of the C=O groups of the flavonoids and lipids. The band at 1345 cm−1 could be related to the CH3 and CH2 groups of the flavonoids and aromatics. Here, the vibration would be the bending vibrations of C-H and the stretching vibration of the aromatics. The bands at 1250 cm−1 and 1247 cm−1 were related to the stretching vibration of the carboxyl group (O-H and C-O stretch), i.e., the stretching of the COOH groups in flavonoids and lipids. Bands at 1126 cm−1 and 1130 cm−1 were related to C-O stretching in the ester groups. The band at 778 cm−1 was due to C-C stretching vibration.27 The band at 2295 cm−1 might have been related to C-H stretching vibration of the methyl and methoxy groups28 and to stretching vibration of the O-H groups in carboxylic acid.29 These findings were consistent with those of earlier studies.30,31. The FT-IR spectra of all the oil extracts appeared similar. However, careful examination revealed several significant differences, including in the number of peaks obtained. Overall, FT-IR analysis of the methanol, ethanol, and ethyl acetate leaf extracts of L. camara revealed the presence of proteins, oils, fats, phenolic compounds, flavonoids, saponins, tannins, and carbohydrates as the major functional groups that are likely responsible for various medicinal properties of L. camara.
Figure 1.
Fourier transform–infrared spectra of (a) methanol, (b) ethyl acetate, and (c) ethanol Lantana camara leaf extracts.
Chemical compounds identified in the leaf oil
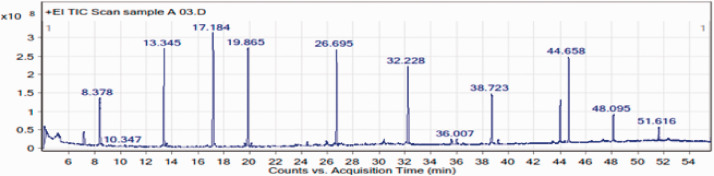
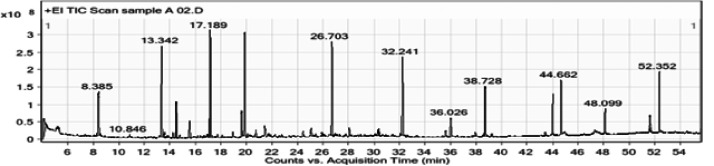
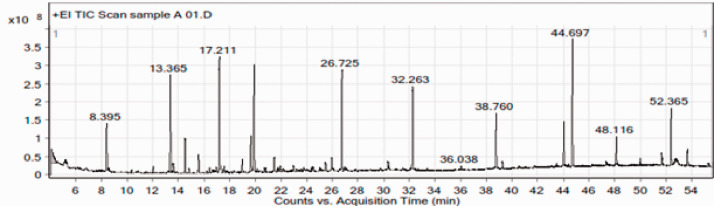
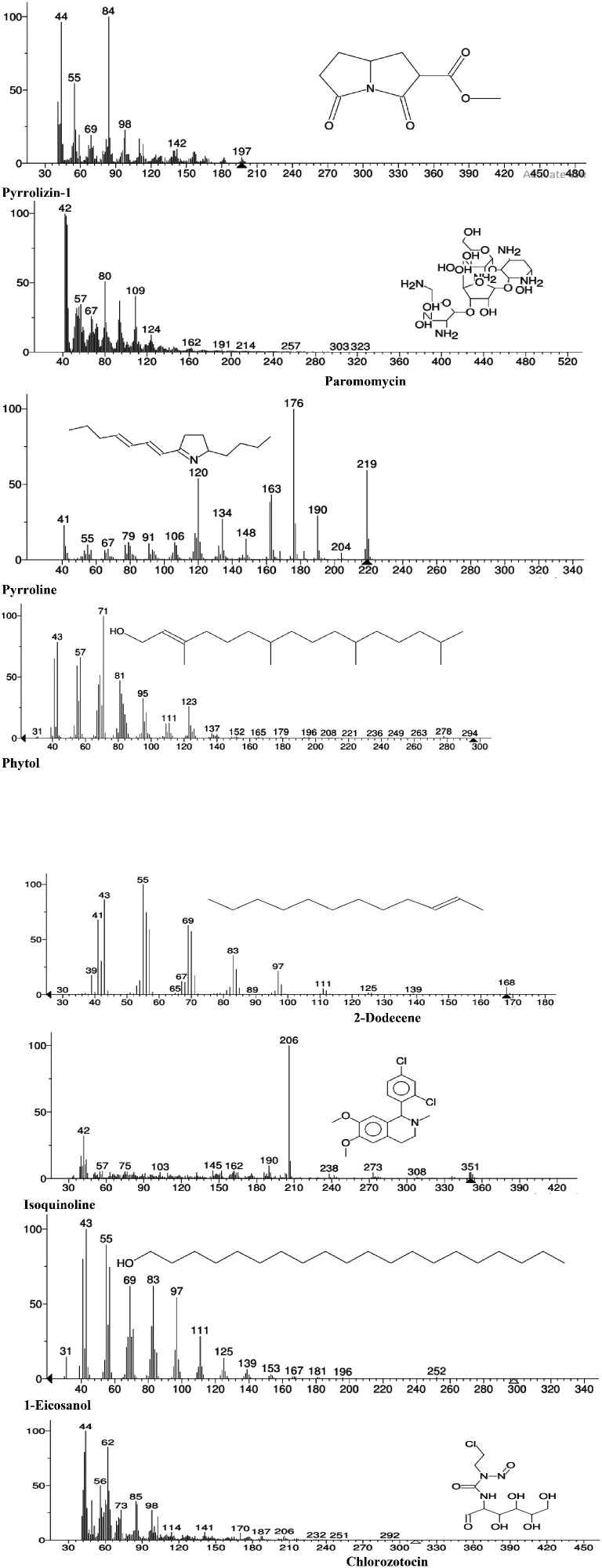
The chemical composition of the leaf oil was further investigated using GC-MS, and representative chromatographs of the ethyl acetate, ethanol, and methanol extracts are presented in Figures 2, 3, and 4, respectively. The identified peaks and their contents are described in Tables 2, 3, and 4. GC-MS analysis resulted in the identification of 43 unique constituents in the leaf oil of L. camara. The oil was shown to be a complex mixture of numerous compounds, many of which are present in low quantities. The identified compounds comprised more than 95% of the total extracted oils. The percentage peak areas were taken to represent the proportion of each compound relative to the total. The major chemical components of the oil comprised a pyrrolizine, paromomycin, a pyrroline, phytol, 2-dodecene, isoquinoline, 1-eicosanol, and chlorozotocin (Figure 5). The biological properties of select components of L. camara essential oil based on the findings of previous studies are presented in Table 5. These include insecticidal and antimicrobial properties.
Figure 2.
Gas chromatography–mass spectrometry chromatogram of an ethyl acetate extract of Lantana camara leaves.
Figure 3.
Gas chromatography–mass spectrometry chromatogram of an ethanol extract of Lantana camara leaves.
Figure 4.
Gas chromatography–mass spectrometry chromatogram of a methanol extract of Lantana camara leaves.
Table 2.
Major chemical constituents of Lantana camara leaf oil when using ethyl acetate for extraction.
| No. | Retention time (minutes) | Compound | Peak area | Area % | Molecular weight | Molecular formula |
|---|---|---|---|---|---|---|
| 1 | 8.32 | 4-Piperidinemethanamine | 59.99 | 11.58 | 114 | C6H14N2 |
| 2 | 9.06 | 1-(1-Propenyl)-piperidine | 1.45 | 0.28 | 125 | C8H15N |
| 3 | 13.34 | 1-Azabicyclo[5.1.0]octane | 81.57 | 15.74 | 111 | C7H13N |
| 4 | 14.50 | 4-[(4-methoxyphenyl)methyl]-1,2-oxazole-3,5-diamine | 50.20 | 9.69 | 219 | C11H13N3O2 |
| 5 | 14.57 | 4-Undecene, 3-methyl-, (Z)- | 2.01 | 0.39 | 168 | C12H24 |
| 6 | 15.50 | Amphetamine | 17.20 | 3.32 | 135 | C9H13N |
| 7 | 17.11 | 2,5-Di-tert-butylaniline | 87.40 | 16.87 | 205 | C14H23N |
| 8 | 19.84 | E-2-Tetradecen-1-ol | 100.00 | 19.3 | 212 | C14H28O |
| 9 | 21.40 | 1,2,5-Oxadiazol-3-amine | 6.91 | 1.33 | 85 | C2H3N3O |
| 10 | 26.60 | 13-Methyltetradecanal | 63.60 | 12.27 | 226 | C15H30O |
| 11 | 32.20 | Hexadecanal | 33.02 | 6.37 | 240 | C16H32O |
| 12 | 38.70 | Imidazole, 2-amino-5-[(2-carboxy)vinyl]- | 11.40 | 2.20 | 153 | C6H7N3O2 |
| 13 | 43.80 | 4-(3-Pyridyl)-4-oxo-butyramide | 1.21 | 0.23 | 250 | C12H18N2O2Si |
| 14 | 52.33 | 2-(6,7-Dimethoxy-2-oxo-3,4-dihydro-1H-quinolin-4-yl)acetic acid | 2.266 | 0.44 | 265 | C13H15NO5 |
No., number.
Table 3.
Major chemical constituents of Lantana camara leaf oil when using ethanol for extraction.
| No. | Retention time (minutes) | Compound | Peak area | Area % | Molecular weight | Molecular formula |
|---|---|---|---|---|---|---|
| 1 | 8.37 | Acetic acid, trifluoro-, 3,7-dimethyloctyl ester | 48.45 | 6.97 | 254 | C12H21F3O2 |
| 2 | 9.80 | Chlorozotocin | 54.00 | 7.77 | 313 | C9H16CIN3O7 |
| 3 | 10.34 | Pyrrolizin-1,7-dione-6-carboxylic acid, methyl(ester) | 1.01 | 0.15 | 197 | C9H11NO4 |
| 4 | 11.48 | Pyrimidin-2,4-dione,1,2,3,4-tetrahydro-5-methyl-1-[2-hydroxymethyl-3-dimethylamino]tetrahydrofur-5-ylPyrimidin-2,4-dione | 1.09 | 0.16 | 269 | C12H19N3O4 |
| 5 | 13.35 | 1-Undecene, 9-methyl- | 69.20 | 9.96 | 168 | C12H24 |
| 6 | 14.57 | 4-Undecene, 3-methyl-, (Z)- | 2.01 | 0.29 | 168 | C12H24 |
| 7 | 15.40 | beta-Quinoline | 25.70 | 3.70 | 129 | C9H7N |
| 8 | 17.18 | 2,5-Di-tert-butylaniline | 100.00 | 14.39 | 205 | C14H23N |
| 9 | 19.87 | N, N'-Tetramethylenebis | 81.86 | 11.78 | 396 | C10H24N2O6S4 |
| 10 | 22.70 | Paromomycin | 2.17 | 0.31 | 615 | C23H45N5O14 |
| 11 | 26.69 | Z-2-Acetoxy-12-tetradecenitrile | 77.00 | 11.08 | 279 | C17H29NO2 |
| 12 | 26.70 | 2-Dodecene | 86.00 | 12.38 | 168 | C12H24 |
| 13 | 32.20 | Octadecane, 1-(ethenyloxy)- | 65.50 | 9.43 | 296 | C20H40O |
| 14 | 32.21 | 1-Hexacosanol | 75.29 | 10.83 | 382 | C26H54O |
| 15 | 36.00 | 1,5-Dinitro-3,7-diazabicyclo[3.3.1]nonane | 5.61 | 0.81 | 216 | C7H12N4O4 |
No., peak number.
Table 4.
Major chemical constituents of Lantana camara leaf oil when using methanol for extraction.
| No. | Retention time (minutes) | Compound | Peak area | Area % | Molecular weight | Molecular formula |
|---|---|---|---|---|---|---|
| 1 | 8.34 | 4-Undecene, 5-methyl-, (E)- | 43.29 | 5.16 | 168 | C12H24 |
| 2 | 8.40 | 1,8-Nonadien-3-ol | 47.00 | 5.60 | 140 | C9H16O |
| 3 | 13.37 | Pyrrolizin-1,7-dione-6-carboxylic acid, methyl(ester) | 68.00 | 8.10 | 197 | C9H11NO4 |
| 4 | 14.50 | 1-Pentanamine, N-(phenylmethylene)- | 27.00 | 3.22 | 175 | C12H17N |
| 5 | 15.50 | 2-Oxobicyclo[2.2.1]heptane-1-carbonitrile | 12.30 | 1.46 | 135 | C8H9NO |
| 6 | 17.21 | 2,5-Di-tert-butylaniline | 99.40 | 11.84 | 205 | C14H23N |
| 7 | 19.60 | Cyclohexanamine, N-(phenylmethylene)- | 28.00 | 3.33 | 187 | C13H17N |
| 8 | 19.80 | Cycloundecane, (1-methylethyl)- | 87.10 | 10.37 | 196 | C14H28 |
| 9 | 19.84 | Imidazole, 2-amino-5-[(2-carboxy) vinyl]- | 37.40 | 4.45 | 153 | C6H7N3O2 |
| 10 | 20.00 | 1-Docosene | 73.10 | 8.71 | 308 | C22H44 |
| 11 | 21.40 | 4,7,10-Hexadecatrienoic acid, methyl ester | 11.40 | 1.36 | 264 | C17H28O2 |
| 12 | 26.70 | 1-Dodecanol | 77.60 | 9.24 | 186 | C12H26O |
| 13 | 30.35 | Propanenitrile, 3-[1-[3-(1-pyrrolidinyl) propynyl | 4.41 | 0.53 | 260 | C16H24N2O |
| 14 | 32.20 | 1-Eicosanol | 42.50 | 5.06 | 298 | C20H42O |
| 15 | 36.03 | Phytol | 3.06 | 0.36 | 296 | C20H40O |
| 16 | 44.70 | Pyrroline, 5-butyl-2-[1,3-heptadienyl]- | 100.0 | 11.92 | 219 | C15H25N |
| 17 | 48.10 | 1,2-Nonadecanediol | 17.30 | 2.06 | 300 | C19H40O2 |
| 18 | 48.30 | 4-Acetyloxyimino-6,6-dimethyl-3-methylsulfanyl-4,5,6,7 | 2.70 | 0.32 | 341 | C15H19NO4S2 |
| 19 | 51.10 | 6-Ethoxy-7-methoxy-1-(3-nitro-phenyl | 2.39 | 0.28 | 328 | C18H20N2O4 |
| 20 | 52.30 | 1,3-Dioxolane | 55.69 | 6.63 | 74 | C3H6O2 |
No., peak number.
Figure 5.
Mass spectra of the major chemical compounds in the oil extract of the Ethiopian Lantana camara leaf.
Table 5.
Biological properties of individual components of Lantana camara essential oil.
| No. | Compound | Biological properties | Reference |
|---|---|---|---|
| 1 | Paromomycin | Antibiotic | 32 |
| 2 | Pyrroline | Flavoring agent | 33 |
| 3 | 2-Dodecene | Antibacterial | 34 |
| 4 | 1-Eicosanol | Emollient for cosmetics | 35 |
| 5 | Phytol | Insecticidal, pest repellent, anti-inflammatory agent | 36 |
| 6 | Imidazole | Antifungal, antiprotozoal, antibacterial, antitubercular | 37 |
| 7 | Pyrimidine | Antiviral, anticancer, antimalarial, antifungal, antithyroid | 38 |
| 8 | Pyrrolizine | Antimicrobial | 39 |
| 9 | Amphetamine | Antibiotic, antibacterial | 40 |
| 10 | Isoquinoline | Insecticidal, antifungal | 41 |
| 11 | Chlorozotocin | Anticancer | 42 |
No., peak number.
The extracts of L. camara leaf oil prepared using different solvents were qualitatively similar, although they did differ markedly in terms of the relative concentrations of the compounds present. The chemical composition of the essential oils extracted from Ethiopian L. camara agreed quite well with that previously reported in the literature,43 with some differences in the relative quantities of the volatile compounds. Notably, there have previously been major differences reported in the chemical composition of L. camara leaf oils extracted from plants in certain countries.44 The observed differences in the quality and composition of the extracted oil between studies may be attributable to factors such as genetic, climate, topographical, and seasonal variations. Among the crucial compounds identified in the present study, phytol, azabicyclo-containing compounds, and 2-dodecene have been reported in the essential oils extracted from L. camara leaves in other studies.25,45
The leaves of L. camara are aromatic and contain highly volatile components that confer the essential oil with many medicinal and other properties. The extracted essential oil was bright yellow. Several of the isolated compounds, namely a pyrroline, phytol, 1-dodecanol, paromomycin, 1-hexacosanol, and amphetamine are known for their pharmaceutical applications.46 For example, paromomycin has been reported as having pain-relieving and anti-inflammatory properties, and it exhibits antifungal activity against dermatophytes. It is also well known for its use as a preservative in drugs, foods, and cosmetics.47 Dodecene oil, which is rich in dodecene (> 73%), has been reported to show antioxidant, antibacterial, and insecticidal activities.48 Besides the medicinal value of L. camara oil, the sustained demand for synthetic flavorings and fragrances for use in the pharmaceutical, food, and cosmetic industries makes this essential oil valuable for exploitation in these industries as well.
Conclusions
The Soxhlet solvent extraction method was applied to the leaves of L. camara harvested in Ethiopia. The leaves were found to be rich in phytol, 4-undecene, 1-eicosanol, 1-docosene, imidazole, and pyrroline. This oil extract may possess antimicrobial, insecticidal, insect repellent, and cytotoxic activities as reported for most pyrroline- and phytol-rich oils. The wide spectrum of volatile components in L. camara oil suggests that it may be useful for application as an antimicrobial for food preservation, as an insect repellent, and also as a flavoring agent in pharmaceutical and cosmetics products. The present study showed that the phytochemical composition of the L. camara extracts varied depending on the solvent used for extraction. In particular, the total flavonoid and terpenoid content varied extensively with different solvents. Methanol extracts of L. camara leaves contained more unique compounds than extracts obtained with the other solvents. Thus, this study affords a good foundation for further investigations of the biochemical and phytochemical functions of the various compounds identified, and the findings propose that the methanol leaf extract of L. camara, with its numerous bioactive compounds, is a promising source of lead compounds for future drug development. However, further studies are required to fully evaluate the potential of this oil.
Acknowledgments
The author would like to thank the Faculty of Chemical and Food Engineering, Bahir Dar Institute of Technology for providing funding and access to all the materials required for the successful completion of this study.
Footnotes
Declaration of conflicting interest: The author declares that there is no conflict of interest.
Funding: This work was supported by the Faculty of Chemical and Food Engineering, Bahir Dar Institute of Technology.
ORCID iD: Adane Adugna Ayalew https://orcid.org/0000-0002-5899-8737
References
- 1.Pagare S, Bhatia M, Tripathi N, et al. Secondary metabolites of plants and their role: Overview. Curr Trends Biotechnol Pharm 2015; 9: 294–305. [Google Scholar]
- 2.Sonibare OO, Effiong I. Antibacterial activity and cytotoxicity of essential oil of Lantana camara L. leaves from Nigeria. Afr J Biotechnol 2008; 7: 2618–2620. [Google Scholar]
- 3.Rajashekar Y, Ravindra KV, Bakthavatsalam N. Leaves of Lantana camara Linn (Verbenaceae) as a potential insecticide for the management of three species of stored grain insect pests. J Food Sci Technol 2014; 51: 3494–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemu SC, Terefe AA. Impact of invasion: A case study on the ecological and socioeconomic impact of Lantana camara (L.) in Abay Millennium Park (AMP), Bahir Dar, Ethiopia. J Ecol Nat Environ 2015; 7: 132–145. [Google Scholar]
- 5.Sousa EO, Almeida TS, Menezes IRA, et al. Chemical composition of essential oil of Lantana camara L. (Verbenaceae) and synergistic effect of the aminoglycosides gentamicin and amikacin. Rec Nat Prod 2012; 6: 144–150. [Google Scholar]
- 6.Sasidharan S, Chen Y, Saravanan D, et al. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 2011; 8: 1–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Kalita S, Kumar G, Karthik L, et al. A review on medicinal properties of Lantana camara Linn. Res J Pharm Technol 2012; 5: 711–715. [Google Scholar]
- 8.Shimelis D, Benti D, Challi D. Effect of zinc supplementation in treatment of acute diarrhea among 2-59 months children treated in Black Lion Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev 2008; 22: 187–190. [Google Scholar]
- 9.Silva TSC, Suffredini IB, Ricci EL, et al. Antinociceptive and anti-inflammatory effects of Lantana camara L. extract in mice. Rev Bras Plantas Med 2015; 17: 224–229. [Google Scholar]
- 10.Saikia AK, Sahoo RK. . Chemical composition and antibacterial activity of essential oils of Lantana camara L. Middle East J Sci Res 2011; 8: 599–602. [Google Scholar]
- 11.Murugesan S, Senthilkumar ND, Rajasugunasekar D. Chemical constituents and toxicity assessment of the leaf oil of Lantana camara Linn from Tamilnadu regions. Asian J Plant Sci Res 2016; 6: 32–42. [Google Scholar]
- 12.Dua VK, Pandey AC, Dash AP. Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian J Med Res 2010; 131: 434–439. [PubMed] [Google Scholar]
- 13.Siddiqui MR, AlOthman ZA, Rahman N. Analytical techniques in pharmaceutical analysis: A review. Arab J Chem 2017; 10: S1409–S1421. [Google Scholar]
- 14. Yue Y, Qiu ZD, Qu XY, et al. Discoursing on Soxhlet extraction of ginseng using association analysis and scanning electron microscopy. J Pharm Anal 2018; 8: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayodele AE, Shokefun EO, Akinloye AJ. Systematic importance of leaf anatomical characters in some species of Microcos Linn. Section Eumicrocos Burret, in Nigeria. Am J Plant Sci 2016; 7: 108–117. [Google Scholar]
- 16.Aguoru CU, Pilla C, Olasan JO. Phytochemical screening of Xylopia aethiopica with emphasis on its medicinally active principles. J Med Plants Res 2016; 7: 1–5. [Google Scholar]
- 17.Vaghasiya Y, Dave R, Chanda S. Phytochemical analysis of some medicinal plants from Western region of India. Res J Med Plant 2011; 5: 567–576. [Google Scholar]
- 18.Idris S, Ndukwe GI, Gimba CE. Preliminary phytochemical screening and antimicrobial activity of seed extracts of Persea americana (avocado pear). BAJOPAS 2009; 2: 173–176. [Google Scholar]
- 19.Rahman AHMM, Sarker AK. Investigation of medicinal plants at Katakhali Pouroshova of Rajshahi district, Bangladesh and their conservation management. Appl Ecol Env Res 2015; 3: 184–192. [Google Scholar]
- 20.Borokini TI, Omotayo FO. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plants Res 2012; 6: 1106–1118. [Google Scholar]
- 21.Ayoola GA, Coker HAB, Adesegun SA, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res 2008; 7: 1019–1024. [Google Scholar]
- 22.US National Institute of Standards and Technology (NIST). Mass Spectrometry Data Center, https://chemdata.nist.gov/ (2020, accessed 2 October 2020).
- 23.Topi D. Volatile and chemical compositions of freshly squeezed sweet lime (Citrus limetta) juices. J Raw Mater Process Foods 2020; 1: 22–27. [Google Scholar]
- 24.Sharma B, Kumar P. Bioefficacy of Lantana camara L. against some human pathogens. Indian J Pharm Sci 2009; 71: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurade NP, Jaitak V, Kaul VK, et al. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm Biol 2010; 48: 539–544. [DOI] [PubMed] [Google Scholar]
- 26.Prasad A, Purohit S. Evaluation of the morphological abnormalities in the 4th instar larva of Helicoverpa armigera (Hub.) on application of leaf extract of Lantana camara. WJZ 2009; 4: 253–255. [Google Scholar]
- 27.Ingawale GS, Goswami-Giri AS. Isolation and characterization of bioactive molecule from Lantana camara. Asian J Research Chem 2014; 7: 339–344. [Google Scholar]
- 28.Oliveira RN, Mancini MC, Passos TM, et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Revise Mat 2016; 21: 767–779. [Google Scholar]
- 29.Vardin H, Tay A, Ozen B, et al. Authentication of pomegranate juice concentrate using FTIR spectroscopy and chemometrics. Food Chem 2008; 108: 742–748. [DOI] [PubMed] [Google Scholar]
- 30.Kalita S, Kumar G, Karthik L, et al. Phytochemical composition and in vitro hemolytic activity of Lantana camara L. (Verbenaceae) leaves. Pharmacologyonline 2011; 1: 59–67. [Google Scholar]
- 31.Ragavendran P, Sophia D, Arul Raj C, et al. Functional group analysis of various extracts of Aerva lanata (L.,) by FTIR spectrum. Pharmacologyonline 2011; 1: 358–364. [Google Scholar]
- 32.Wiwanitkit V. Interest in paromomycin for the treatment of visceral leishmaniosis (kala-azar). Ther Clin Risk Manag 2012; 8: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajay Kumar K, Lokeshwari DM, Chandramouly M, et al. Synthetic strategies and significance of pyrroline analogs. Res J Pharm Technol 2013; 6: 137–142. [Google Scholar]
- 34.Zellagui A, Gherraf N, Rhouati S, et al. Chemical composition and antibacterial activity of the essential oils of Ferula vesceritensis Coss et Dur. leaves, endemic in Algeria. Org Med Chem Lett 2012; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee S, Karmakar A, Azmi SA, et al. Antibacterial activity of long-chain primary alcohols from Solena amplexicaulis leaves. Proc Zool Soc 2018; 71: 313–319. [Google Scholar]
- 36.Olofsson P, Hultqvist M, Hellgren LI, et al. Phytol: A chlorophyll component with anti-inflammatory and metabolic properties.
- 37.Verma A, Joshi S, Singh D. Imidazole: having versatile biological activities. J Chem 2013; 1–13. [Google Scholar]
- 38.Yerragunta V, Patil P, Anusha V, et al. Pyrimidine and its biological activity: a review. PharmaTutor 2013; 1: 39–44. [Google Scholar]
- 39.Al-Rubaye AF, Kaizal AF, Hameed I. Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int J Pharmacogn Phytochem Res 2017; 9: 537–552. [Google Scholar]
- 40.Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol 2013; 27: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balewski L, Sączewski F, Gdaniec M, et al. Synthesis and fluorescent properties of novel isoquinoline derivatives. Molecules 2019; 24: 4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mossman BT, Ireland CM, Filipak M, et al. Comparative interactions of streptozotocin and chlorozotocin with DNA of an insulin-secreting cell line (RINr). Diabetologia 1986; 29: 186–191. [DOI] [PubMed] [Google Scholar]
- 43.Passos JL, Barbosa LCA, Demuner AJ, et al. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules 2012; 17: 11447–11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rana VS, Prasad D, Blazquez MA. Chemical composition of the leaf oil of Lantana camara. J Oil Res 2005; 17: 198–200. [Google Scholar]
- 45.Nerio LS, Olivero-Verbel J, Stashenko EE. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J Stored Prod Res 2009; 45: 212–214. [Google Scholar]
- 46.Abirami P, Rajendran A. GC-MS analysis of Tribulus terrestris. Asian J Plant Sci Res 2011; 1: 13–16. [Google Scholar]
- 47.Jawonisi IO, Adoga GI. Chemical constituents of essential oil of Lantana camara Linn. leaves. British J Pharma Toxic 2013; 4: 155–157. [Google Scholar]
- 48.Hemalatha P, Elumalai D, Vignesh A, et al. Bio-efficacy of essential oils of Lantana camara Aculeata, against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. Int J Pure Appl Zool 2014; 2: 329–338. [Google Scholar]