Correction to: Archives of Toxicology (2019) 93:1095–1139 https://doi.org/10.1007/s00204-019-02400-1
(1) In the original publication, the starting point in time for the three feeding trials was incorrectly given. The first sentence in the second paragraph of the “Rat feeding trials” subchapter in the Materials and methods section (page 1098) should read:
“The first 90-day feeding trial and the chronic toxicity/carcinogenicity study were started 2–3 weeks and the second 90-day feeding trial was started 1–2 weeks after delivery of the animals at the animal testing facility”.
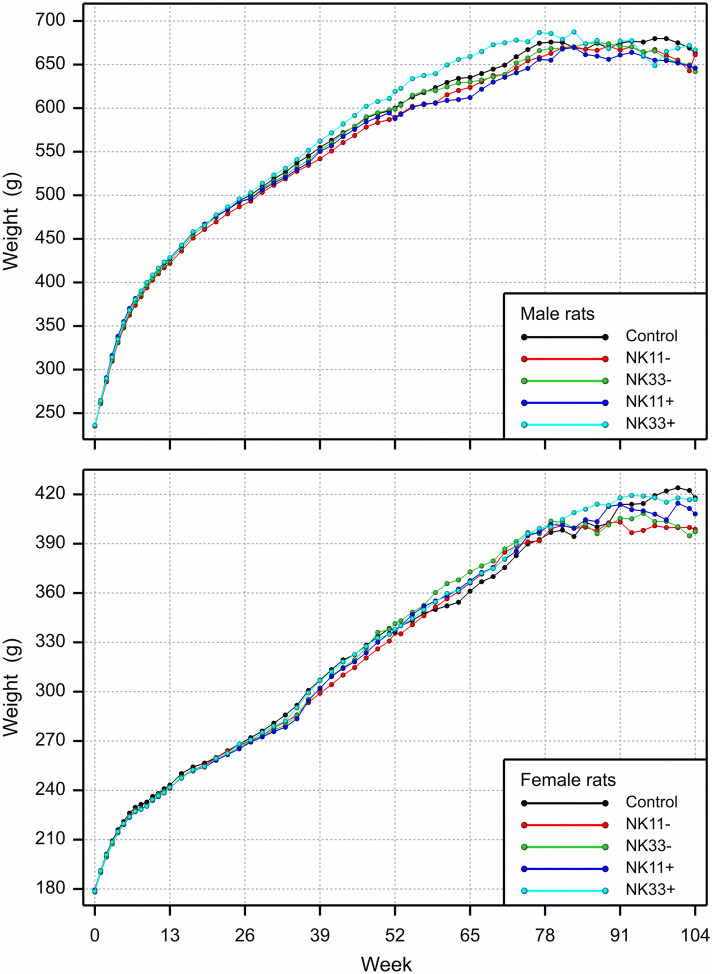
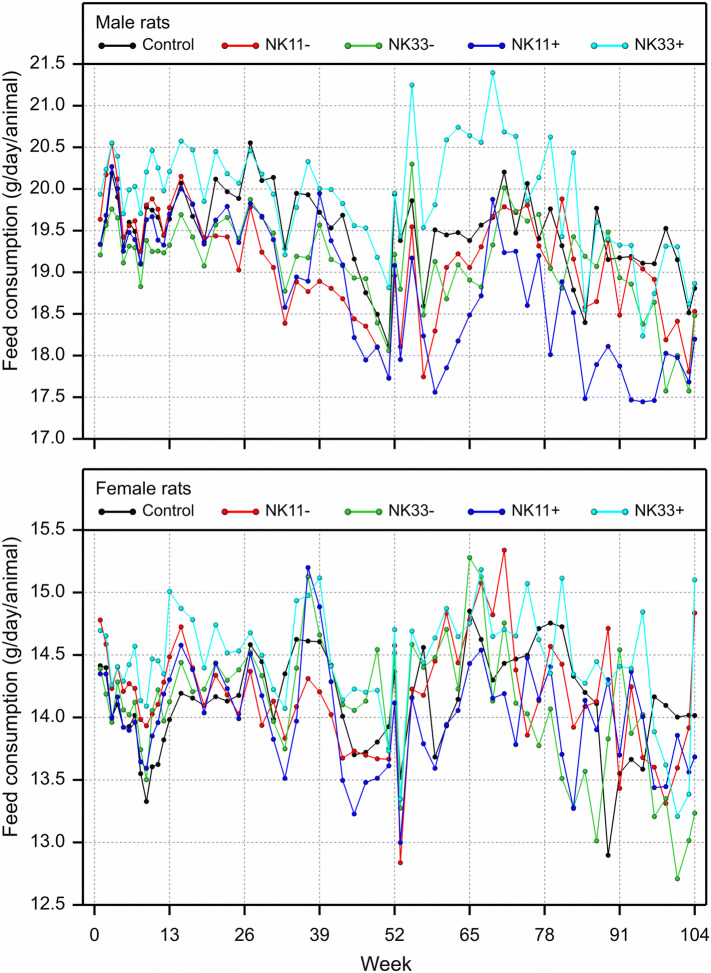
(2) In the original publication, Figs. 5 and 6 (page 1118) showed, for all points in time, the mean body weight and the food consumption data, respectively, of only the male and female rats that survived until the end of the chronic toxicity/carcinogenicity study. The figure legends refer, however, to another option, where all available data are used to calculate mean body weights and feed consumptions, and these legends describe the figures that we intended to publish. The current Figs. 5 and 6 should, therefore, be replaced by the accompanying new Figs. 5 and 6.
Fig. 5.
Mean body weight of all male and female rats surviving until the points in time plotted in the combined chronic toxicity/carcinogenicity feeding trial with GM maize NK603 at an inclusion rate of 11 and 33% in the diet
Fig. 6.
Mean feed consumption of all male and female rats surviving until the points in time plotted in the combined chronic toxicity/carcinogenicity feeding trial with GM maize NK603 at an inclusion rate of 11 and 33% in the diet
Consistent with these new figures, two changes in the text have to be made as follows:
(I) The second and third sentences in the second paragraph of the “Combined chronic toxicity/carcinogenicity feeding trial with the GM maize NK603 at an inclusion rate of 11 and 33% in the diets” subchapter in the Results section on page 1117 should be substituted by the following sentence:
“In weeks 52–78, the male rats fed the NK603 + Roundup diet at an inclusion rate of 33% showed a higher mean body weight and feed consumption when compared to the corresponding control group, while the mean body weight and feed consumption of the female rats were similar in all five experimental groups (Figs. 5 and 6)”.
(II) In the Discussion section, the third sentence in the second paragraph of page 1134 should be substituted by the following sentence:
“The increased mortality observed between the 12th and 24th month of the feeding trial in male rats fed the 33% NK603 + Roundup diet coincided with an increase in the body weight and feed consumption”.
Please note that these revisions do not affect in any way the overall conclusions on the lack of any NK603 maize-related adverse effects. Finally, we would like to thank Dr. Graham Tobin, formerly the Technical Director for Envigo Teklad diets and Global QA Director at Harlan, for his extremely helpful comments.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.