Abstract
Objective
We investigated the safety and effectiveness of a modified transabdominal approach for renal cell carcinoma (RCC) with a supradiaphragmatic inferior vena cava (IVC) tumor thrombus (TT).
Methods
Eight patients underwent radical nephrectomy with removal of a supradiaphragmatic IVC-TT through an abdominal incision using a transdiaphragmatic approach in Peking University Third Hospital from April 2015 to January 2018. We modified this technique using a Foley catheter balloon to avoid piggyback liver mobilization.
Results
All patients underwent successful operations. The median operative time was 7 hours 23 minutes. The median estimated blood loss was 2963 mL. All patients received a blood transfusion with a median blood infusion volume of 2162 mL. Two patients with Budd–Chiari syndrome developed postoperative ascites and hydrothorax due to non-watertight repair of the diaphragm. During a follow-up of 11 to 44 months, only one patient died of liver metastasis and four patients developed distant metastasis without recurrence in the IVC.
Conclusions
The modified transabdominal approach described herein has an encouraging safety profile and provides a surgical option for treatment of RCC with a supradiaphragmatic IVC-TT. More evidence concerning the beneficial role of this procedure will be elucidated in further studies.
Keywords: Renal cell carcinoma, supradiaphragmatic tumor thrombus, transabdominal approach, modified technique, inferior vena cava, nephrectomy
Introduction
Although enormous efforts have been made in the screening and diagnosis of renal cell carcinoma (RCC), the incidence of locally advanced RCC remains high. A tumor thrombus (TT) develops in up to 10% of patients with RCC, and less than 1% of these extend into the inferior vena cava (IVC) above the diaphragm. Supradiaphragmatic tumor thrombectomy is technically challenging and fraught with high risk and frequent complications.1 Recent studies have demonstrated that systemic and molecular targeted therapies produce minimal and limited clinical effects in reducing the tumor burden; thus, surgical resection remains the primary therapeutic regimen.2–4
In patients with a supradiaphragmatic IVC-TT, cardiopulmonary bypass (CPB) with or without deep hypothermic circulatory arrest (DHCA) is still the standard surgical regimen. Unfortunately, these approaches may increase the rates of perioperative morbidity, mortality, and certain known complications such as coagulation disorders and neurological complications; the perioperative mortality rate may be as high as 22%.1,5 Therefore, it is important to find alternative options for patients with a supradiaphragmatic IVC-TT to avoid the use of CPB and DHCA.
A newer technique that uses an abdominal incision has recently been described.6,7 The standard use of this technique and avoidance of sternotomy, CPB, and DHCA could potentially prevent major perioperative complications by avoiding major sternotomy and systemic heparinization. We used this technique in patients with RCC with a supradiaphragmatic TT in Peking University Third Hospital, and we modified the surgical approach by using a Foley catheter balloon to avoid piggyback liver mobilization. After retrospectively analyzing these patients’ data, we examined the safety and effectiveness of this technique and focused on the indications for its use in patients with a supradiaphragmatic TT.
Methods
Patients
In this retrospective study, we analyzed the data of patients who underwent radical nephrectomy with removal of a level IV TT through an abdominal incision using a transdiaphragmatic approach from April 2015 to January 2018. Sternotomy, CBP, and DHCA were not utilized in these patients.
All patients underwent preoperative computed tomography or magnetic resonance imaging. All clinical information, including the patient demographics, operative variables, and postoperative outcomes, were retrospectively reviewed. Complications occurring intraoperatively or within 90 days postoperatively were recorded. The clinical and radiological follow-up protocol consisted of quarterly follow-up for the first 2 years postoperatively, semiannual follow-up for an additional 2 years, and annual follow-up thereafter.
Surgical technique
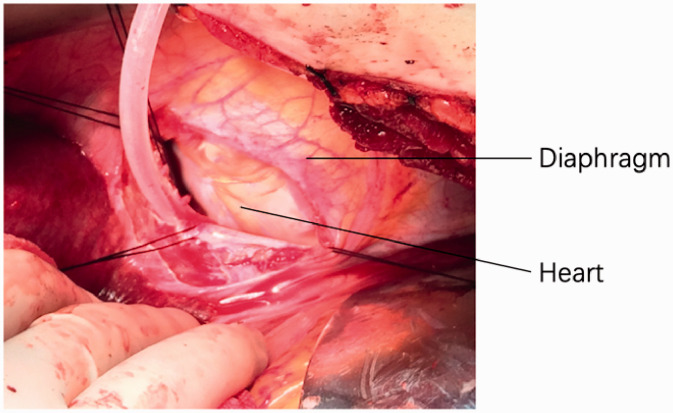
Intraoperative transesophageal echocardiography (TEE) was used to monitor the TT in real time. A modified Chevron incision was used; the kidney was mobilized laterally and posteriorly, and from the dorsal side of the kidney, the renal artery was identified, ligated, and divided. The infradiaphragmatic IVC was exposed and isolated by mobilizing the liver off the diaphragm without piggyback liver mobilization (in six patients) or totally mobilized by piggyback liver mobilization (in two patients).6 In six patients, the diaphragmatic central tendon was incised annularly around the IVC, and an extended transverse incision was performed if needed, until the supradiaphragmatic intrapericardial IVC was identified. The diaphragm was incised at the projection position of the right atrium, and a pericardiotomy was performed to expose the intrapericardial IVC and right atrium in the other two patients with an atrial TT (Figure 1). We then inserted a 16-Fr Foley catheter through the vena cava and carefully advanced the balloon of the Foley catheter into the cephalic portion of the TT under TEE monitoring. We injected 15 mL of water into the balloon and then gently “milked” the IVC-TT downward (in five patients) or pulled the IVC-TT down using the balloon of the Foley catheter (in three patients), assisted by two fingers to control the TT. The proximal and distal IVC to the TT, porta hepatis, and contralateral renal vein were clamped (the right renal artery was simultaneously clamped for the left renal tumor). The TT was then removed from the IVC. The vascular clamps on the IVC were changed to the portion below the major hepatic veins. Pringle’s maneuver was then released, and the hepatic blood supply and drainage recovered during suturing of the IVC incision.8
Figure 1.
Intraoperative view of the diaphragmatic incision and pericardiotomy, with heart seen.
Results
Eight patients underwent radical nephrectomy with complete extraction of the IVC-TT through an abdominal incision without the use of sternotomy, CBP, or DHCA. These eight patients (five men, three women) had a median age of 58.3 years (range, 17–73 years) and had four left and four right renal tumors. Their mean body mass index was 23.7 kg/m2 (range, 18.9–30.8 kg/m2). The patients’ demographics and operative variables are shown in Table 1.
Table 1.
Patients’ clinical parameters.
| Patient No. | Sex | Age (years) | BMI (kg/m2) | ECOG score | Side | Pathology | Pathological stage | Tumor size (cm) | Thrombus above diaphragm (cm) | Budd–Chiari syndrome | Diaphragm incision | Op. time (min) | Blood loss (mL) | Blood infusion (mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 73 | 30.8 | 1 | R | Clear cell type | T3cN0M0 | 7.0 | 1.3 | No | Central tendon | 387 | 1500 | 1200 |
| 2 | F | 67 | 23.8 | 1 | R | Clear cell type | T4N0M1 | 6.3 RCC, 8.7 RAM | 1.8 | No | Central tendon | 526 | 2000 | 1600 |
| 3 | M | 51 | 25.6 | 0 | L | Clear cell type | T3cN0M0 | 12 | 2.3 (1.6 in atrium) | Yes | Diaphragm+ pericardiotomy | 490 | 7000 | 5100 |
| 4 | M | 45 | 22.0 | 0 | L | Chromophobe cell type | T4N0M0 | 10.3 | 1.0 | Yes | Central tendon | 505 | 4500 | 3400 |
| 5 | M | 70 | 24.2 | 1 | R | Clear cell type | T3cN0M0 | 7.9 | 3.3 | No | Central tendon | 340 | 2000 | 1600 |
| 6 | F | 69 | 25.1 | 1 | L | Clear cell type | T3cN0M1 | 10.9 | 1.9 | No | Central tendon | 515 | 5000 | 3200 |
| 7 | M | 74 | 19.1 | 1 | L | Clear cell type | T4N0M0 | 8.5 | 1.1 | No | Central tendon | 340 | 900 | 400 |
| 8 | M | 17 | 18.9 | 0 | R | Nephroblastoma | T4N0M0 | 14.4 | 5.1 (2.7 in atrium) | No | Diaphragm+ pericardiotomy | 443 | 800 | 800 |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; F, female; IBS, intraoperative blood salvage; L, left; M, male; Op., operative; R, right; RAM, right adrenal metastasis; RCC, renal cell carcinoma.
Preoperative imaging revealed that all of the IVC-TTs were supradiaphragmatic and staged as level IV according to the Mayo criteria, and two TTs extended into the atrium. The median size of the renal mass was 9.7 cm (range, 6.3–14.4 cm). One patient had an 8.7-cm synchronous metastasis in the right adrenal gland, and the IVC-TT arose from the adrenal vein rather than the renal vein. Metastasis was present in two patients.
The median operative time was 7 hours 23 minutes (range, 5 hours 40 minutes to 8 hours 46 minutes). The median estimated blood loss was 2963 mL (range, 800–7000 mL), and all patients received a transfusion of packed red blood cells. The median blood infusion volume was 2162 mL (range, 400–5100 mL). Two patients had a large amount of intraoperative blood loss (7000 and 4500 mL), which might have been related to the development of Budd–Chiari syndrome. Histopathologic examination revealed clear cell RCC in six patients, chromophobe cell RCC in one patient, and a nephroblastoma in one patient.
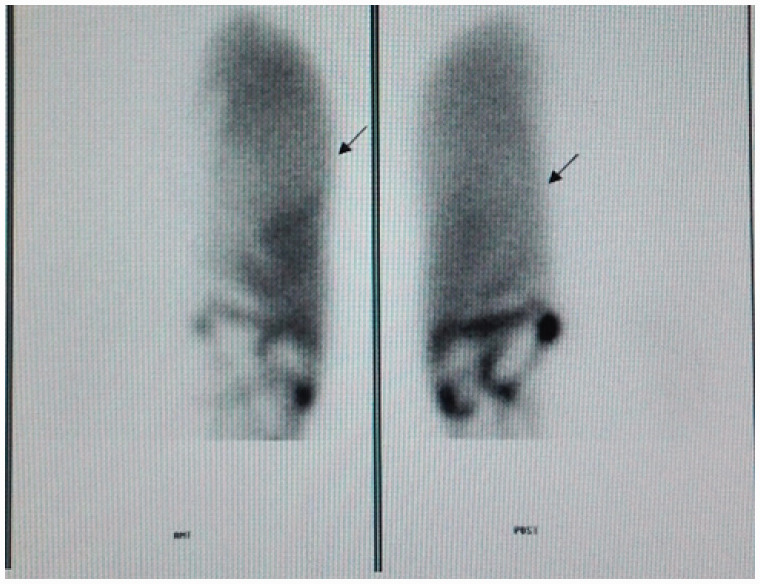
The median length of stay was 26 days (range, 7–69 days). Two patients with Budd–Chiari syndrome had massive ascites before and during the operation and subsequently developed postoperative hydrothorax (Clavien–Dindo grade III). In these patients, the division of the diaphragm led to a non-watertight repair, which in the presence of an increased amount of abdominal fluid caused the hydrothorax to develop. Radioisotope scintigraphy indicated a pleuroabdominal fistula in these two patients (Figure 2). A chest drainage tube was placed in two patients because of hydrothorax, which prolonged their hospital stays (61 and 69 days). Five of the eight patients developed postoperative liver function injury (Clavien–Dindo grade II), which rapidly resolved with the use of liver-protecting treatment.
Figure 2.
Radioisotope scintigraphy indicated a left-side pleuroabdominal fistula.
Three patients developed progressive disease, and one patient died of liver metastasis at 20 months postoperatively. One patient developed brain metastasis 1 year after surgery; this patient underwent gamma knife radiosurgery and was alive at the last follow-up at 40 months. One patient developed lung metastasis at 5 months postoperatively and was alive at the last follow-up at 11 months. One patient developed lung and liver metastasis at 10 months postoperatively and was still alive at the time of this writing (11 months postoperatively). One patient with postoperative lung metastasis was treated with axitinib postoperatively and was alive without progression at the 15-month follow-up. For the other three patients, no metastatic disease or death was reported after a follow-up of 18, 27, and 44 months, respectively. The prognoses of all eight patients are shown in Table 2.
Table 2.
Patients’ prognoses.
| Patient No. | Preoperative metastatic site | Preoperative therapy | Postoperative therapy | Recurrence or metastasis | Metastatic site | Recurrence time | Survival state | Survival time |
|---|---|---|---|---|---|---|---|---|
| 1 | None | No | No | No | None | None | Alive | 44 months |
| 2 | Ipsilateral adrenal gland | Sorafenib | Sorafenib+ sunitinib | Yes | Brain | 12 months | Alive | 40 months |
| 3 | None | Sunitinib | Sunitinib | No | None | None | Alive | 27 months |
| 4 | None | No | No | Yes | Liver | 20 months | Dead | 20 months |
| 5 | None | No | Sunitinib | No | None | None | Alive | 18 months |
| 6 | Lung | No | Axitinib | No | None | None | Alive | 15 months |
| 7 | None | No | Sorafenib | Yes | Lung and liver | 10 months | Alive | 11 months |
| 8 | None | No | No | Yes | Lung | 5 months | Alive | 11 months |
Discussion
Surgical resection remains the gold standard therapeutic method for patients with RCC with an upper-level IVC-TT. After curative resection, the 5-year survival rate of patients with an IVC-TT reportedly ranges from 34% to 72%.9–11 Once distant and lymph node metastases have been diagnosed, the 5-year survival rate of these patients is approximately 5% to 19%.9,10 For patients with metastasis, despite the limited survival rate, complete resection of the tumor remains the only option to achieve long-term survival and symptomatic control of symptoms, such as gross intermittent hematuria, flank pain, and repeated hospital admissions for blood transfusion.
Surgery for patients with a supradiaphragmatic TT frequently requires sternotomy, CPB, and DHCA to ensure that enough venous blood returns to the heart and creates a clear operative field. However, this approach is technically complex, involves prolonged operation times, and increases the morbidity and perioperative mortality.12 CPB requires complete heparinization that significantly increases the risk of hemorrhage and disseminated intravascular coagulation. Retroperitoneal hemorrhage occurs in 1% to 5% of patients who undergo an operation with CPB. Moreover, DHCA can cause kidney dysfunction and even acute renal failure. Although a multicenter clinical study showed that CPB may have no significant effect on the morbidity and perioperative mortality in patients with a high-level IVC-TT, we still believe that there are many advantages to avoiding CBP and sternotomy, such as early recovery and reduced complications (e.g., neurologic deficits and hematologic deficiencies).13 A recent multicenter retrospective study showed that operative complications occurred in 18% to 47% of patients with a high-level IVC-TT and that the perioperative mortality rate was as high as 22%.14
We have herein described eight patients who underwent supradiaphragmatic TT resection through dissection of the diaphragm or central tendon without application of sternotomy, CPB, or DHCA. The supradiaphragmatic intrapericardial IVC was exposed and isolated through dissection of the central tendon in six patients and through dissection of the diaphragm and pericardiotomy in the other two patients with an atrial thrombus. The latter approach required a larger incision in the diaphragm and provided better exposure to the pericardial vena cava and right atrium to control the cephalic end of the atrial TT. Intraoperative phrenic nerve injury should be avoided to prevent phrenic paralysis after surgery. For patients without an atrial TT, the IVC could be exposed through dissection of the central tendon alone. Both approaches have been used since the development of this technique in 2005.15 However, a disadvantage of this approach is that the diaphragmatic hiatus is technically difficult to close completely because of its close proximity to the IVC.
The largest group of patients to undergo this approach to date was reported by Ciancio et al.6 in 2010. Two of the 12 patients in that study died of arrhythmia and respiratory distress syndrome within 30 postoperative days. Major complications were reported in 3 of the 12 patients. In our study, no patients died within 90 postoperative days. Clavien–Dindo grade IIIa operative complications were observed in two of our eight patients, and they were treated with thoracentesis for postoperative hydrothorax. No obvious cardiac complications or postoperative hemorrhage occurred. Compared with CPB and DHCA, this approach can reduce the surgical trauma and the complication-associated mortality rate without affecting the surgical outcome.
The amount of blood loss in the present study varied greatly among the patients, ranging from 800 to 7000 mL. The transfusion and blood loss volumes of most patients were lower than the reported values, which can be attributed to the use of our modified procedure (i.e., the TT was “milked” downward or pulled down with the balloon of a Foley catheter) instead of piggyback liver mobilization, which probably decreased the blood loss. Two patients had a large amount of intraoperative blood loss that was most likely related to Budd–Chiari syndrome. Budd–Chiari syndrome is a rare disorder resulting from obstruction of the hepatic veins and the IVC, which can damage the hepatic and coagulation function and subsequently increase the intraoperative blood loss.16 When it is difficult to mobilize the liver because of hepatic congestion, manipulation should be very gently performed to prevent large amounts of blood loss.
The same two patients also developed postoperative hydrothorax. Using radioscopic scintigraphy, we found that these two patients had a pleuroabdominal fistula postoperatively. The radioactive tracer method revealed the pleuroabdominal fistulas, which can also be seen in patients undergoing peritoneal dialysis with congenital diaphragmatic access.17 A pleuroabdominal fistula is likely to occur in patients with ascites. These two patients also had a large amount of ascites preoperatively because of Budd–Chiari syndrome, and the peritoneal drainage of these patients exceeded 500 mL per day postoperatively because of lymphatic leakage. Ascitic fluid might enter the chest in such patients, causing hydrothorax through the diaphragmatic incision because of non-watertight repair and negative thoracic pressure. However, neither thoracotomy nor thoracoscopy was performed in these two patients, and we could not directly see the leakage. Although the ascites and hydrothorax in these two patients recovered spontaneously, it is possible that all patients with Budd–Chiari syndrome will develop hydrothorax; therefore, perhaps this method should not be employed in such patients because it increases their hospital stay, adversely affects resource use, and increases morbidity. Further investigation is needed through techniques such as thoracoscopy.
Piggyback liver mobilization was performed in Patients 2 and 7. In Patient 2, the TT entered the IVC through the right adrenal vein, and we needed to incise the segment of the IVC posterior to the liver to perform the tumor thrombectomy. In Patient 7, the bulkiness of the TT indicated adhesion to the wall of the IVC posterior to the liver. As a result, piggyback liver mobilization fully exposed the retrohepatic IVC and facilitated the tumor thrombectomy and suturing of the IVC incision. Ciancio et al.6 routinely performed piggyback liver mobilization and circumferential dissociation of the retrohepatic IVC in patients with a supradiaphragmatic TT. In some cases, the authors gently pulled the TT downward under the major hepatic vein to avoid blocking the first hepatic porta. In our study, piggyback liver mobilization was not routinely performed in the other six patients. The TT was gently “milked” downward (in three patients) or pulled down with the balloon of a Foley catheter (in three patients) under the guidance of TEE.
After removal of the TT, the IVC was re-occluded under the liver, and we rapidly removed the occlusion of the suprahepatic IVC and first hepatic porta; this also reduced the time of the first hepatic portal blockage and protected the liver function. After using our modified procedure, no tumor recurrence developed in the IVC during a median follow-up of 23.3 months postoperatively. We do not suggest that piggyback liver mobilization is routinely performed in all patients. First, piggyback liver mobilization is a technically difficult and high-risk procedure with a significant risk of injury to the IVC, major hepatic vein, and short hepatic vein. Second, when patients present with Budd–Chiari syndrome, the IVC-TT can cause liver congestion, and piggyback liver mobilization may increase the amount of bleeding and the risk of liver injury. However, if the TT is adhered to the vena cava wall or is large in size, it is not appropriate to pull the TT downward; piggyback liver mobilization is appropriate in such patients.
In the present study, we resected the supradiaphragmatic TT through dissection of the central tendon or diaphragm without application of sternotomy, CPB, or DHCA, which is referred to as Ciancio’s method.6 We further minimized intraoperative damage by avoiding piggyback liver mobilization. Based on our experience, the indications for avoiding piggyback liver mobilization are as follows.
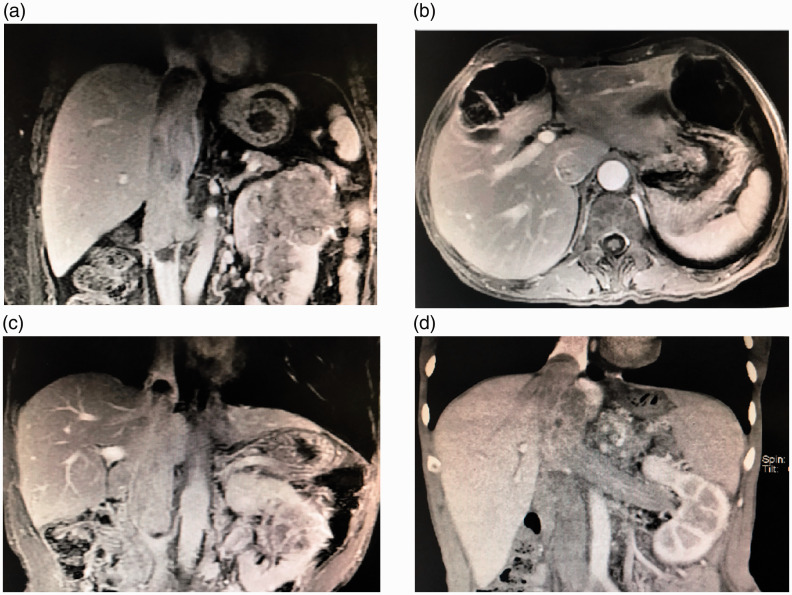
First, the diameter of the cephalic portion of the TT should be thinner than or equal to the caudal portion (Figure 3(a)). Second, features of wall invasion should not be observed in the hepatic segmental IVC, including lack of circulation around the TT (Figure 3(b)), an altered signal within the wall (matte, non-smooth with “burr sign”), thickening and edema bands of the vessel wall, and expansion and breaching of the IVC. Third, there should be no blood blots at the top of the TT (Figure 3(c)). Finally, patients with Budd–Chiari syndrome, especially those with preoperative ascites, should be cautioned when undergoing treatment with this method (Figure 3(d)).
Figure 3.
(a) The diameter of the cephalic portion of the tumor thrombus should be thinner than or equal to the caudal portion. (b) Magnetic resonance imaging showing circulation around the tumor thrombus. (c) Magnetic resonance imaging showing blood blots at the top of the tumor thrombus. (d) Computed tomography scan of an inferior vena cava tumor thrombus in a patient with Budd–Chiari syndrome.
The present study had a limited sample size and used retrospective methodology. Additionally, the safety and efficacy of the current modified surgical technique requires validation. Further studies and longer follow-up are required.
Conclusions
The present report described a single-institution initial experience of a modified surgical technique to remove a supradiaphragmatic TT through an abdominal incision without the use of sternotomy, CBP, or DHCA in China. However, patients with large amounts of ascites secondary to Budd–Chiari syndrome should be alerted to the possible development of hydrothorax postoperatively with the occurrence of a pleuroabdominal fistula. We found that this approach has an encouraging safety profile and provides a surgical option for patients with RCC and a supradiaphragmatic IVC-TT. More evidence concerning the beneficial role of this procedure will be elucidated in further studies.
Authors’ contributions
GLW performed some of the procedures and was a major contributor in writing the manuscript. HB analyzed and interpreted the patient data and was a major contributor in writing the manuscript. JFY assisted in the surgery and perioperative management. HXZ performed some of the procedures. XFH performed some of the procedures. CL analyzed the patient data. MQ collected the patient data. YT collected the patient data. DK interpreted the patient data regarding the supradiaphragmatic tumor thrombus and the transabdominal procedures. LLM modified the technique and performed all procedures. All authors read and approved the final manuscript.
Ethics approval
We obtained ethics approval to perform this retrospective study (Ethics number: M2018042; Committee name: Peking University Third Hospital Medical Science Research Ethics Committee).
Consent for publication
All patients provided written informed consent to undergo surgery.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004; 94: 33–41. [DOI] [PubMed] [Google Scholar]
- 2.Reese AC, Whitson JM, Meng MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol 2013; 31: 1305–1309. [DOI] [PubMed] [Google Scholar]
- 3.Cost NG, Delacroix SE, Jr, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 2011; 59: 912–918. [DOI] [PubMed] [Google Scholar]
- 4.Glazer AA, Novick AC. Long-term followup after surgical treatment for renal cell carcinoma extending into the right atrium. J Urol 1996; 155: 448–450. [PubMed] [Google Scholar]
- 5.Staehler G, Brkovic D. The role of radical surgery for renal cell carcinoma with extension into the vena cava. J Urol 2000; 163: 1671–1675. [PubMed] [Google Scholar]
- 6.Ciancio G, Shirodkar SP, Soloway MS, et al. Renal carcinoma with supradiaphragmatic tumor thrombus: avoiding sternotomy and cardiopulmonary bypass. Ann Thorac Surg 2010; 89: 505–510. [DOI] [PubMed] [Google Scholar]
- 7.Schneider M, Hadaschik B, Hallscheidt P, et al. Manual repositioning of intra-atrial kidney cancer tumor thrombus: a technique reducing the need for cardiopulmonary bypass. Urology 2013; 81: 909–914. [DOI] [PubMed] [Google Scholar]
- 8.Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908; 48: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaag MG, Toyen C, Russo P, et al. Radical nephrectomy with vena caval thrombectomy: a contemporary experience. BJU Int 2011; 107: 1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali AS, Vasdev N, Shanmuganathan S, et al. The surgical management and prognosis of renal cell cancer with IVC tumor thrombus: 15-years of experience using multi-specialty approach at a single UK referral center. Urol Oncol 2013; 31: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 11.Sidana A, Goyal J, Aggarwal P, et al. Determinants of out-comes after resection of renal cell carcinoma with venous involvement. Int Urol Nephrol 2012; 44: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 12.Klein EA, Kaye MC, Novick AC. Management of renal cell carcinoma with vena caval tumor thrombi via cardiopulmonary bypass and deep hypothermic circulatory arrest. Urol Oncol 1991; 18: 445–447. [PubMed] [Google Scholar]
- 13.Nguyen HG, Tilki D, Dall’Era MA, et al. Cardiopulmonary bypass has no significant impact on survival in patients undergoing nephrectomy and level III-IV inferior vena cava thrombectomy: multi-institutional analysis. J Urol 2015; 194: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abel EJ, Thompson RH, Margulis V, et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: a contemporary multicenter experience. Eur Urol 2014; 66: 584–592. [DOI] [PubMed] [Google Scholar]
- 15.Bassi P, Dal Moro F, Ciaccia M, et al. Transdiaphragmatic-intrapericardiac approach to supradiaphragmatic vena cava invasion secondary to renal cell carcinoma: a novel surgical approach. Urology 2005; 66: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell MC, Boitnott JK, Kaufman S, et al. Budd-Chiari syndrome: etiology, diagnosis and management. Medicine 1982; 61: 199–218. [DOI] [PubMed] [Google Scholar]
- 17.Szeto CC, Chow KM. Pathogenesis and management of hydrothorax complicating peritoneal dialysis. Curr Opin Pulm Med 2004; 10: 315–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.