Abstract
Objective
To study the characteristics of headache attributed to COVID-19 infection and predictors of its severity.
Methods
A cross-sectional study involved 172 individuals who had headache due to COVID-19 infection. A detailed analysis of such headache was done through a face-to-face interview. Patients with any other form of secondary headache were excluded. Labs, including lymphocytic count, C-reactive protein, D-dimer and ferritin and chest imaging, were made available.
Results: The
majority of our patients had a diffuse headache (52.9%). It was pressing in 40.7%, with median intensity of 7 (assessed by visual analogue scale) and median frequency of 7 days/week. Patients with preexisting primary headache (52.9%) had significantly more frequent COVID-19 related headache than those without (47.1%) (p = 0.001). Dehydrated patients (64.5%) had more frequent COVID-19 related headache than those who were not dehydrated (35.5%) (p = 0.029). Patients with fever (69.8%) had significantly higher frequency and intensity of COVID-19 related headache compared to those without fever (30.2%) (p = 0.003, 0.012). Patients with comorbidities (19.8%) had significantly higher frequency and intensity of headache than those without comorbidities (80.2%) (p = 0.006, 0.003). After multiple linear regression, primary headache disorders, dehydration and comorbidities were considered predictors of frequency of COVID-19 related headache. Meanwhile, fever and dehydration were predictors of pain intensity.
Conclusion
Healthcare providers of COVID-19 patients need to be aware of frequency and intensity predictors of COVID-19 related headache: Primary headache disorders, fever, dehydration, and comorbidities.
Keywords: COVID-19 related headache, primary headache disorders, VAS, fever, dehydration
Introduction
Coronavirus disease 2019 (COVID-19) is an escalating crisis all over the world caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). It was first identified in December 2019 in Wuhan, China (2). The World Health Organization (WHO) declared the 2019–20 coronavirus outbreak a Public Health Emergency of International Concern (PHEIC) on 30 January 2020, and a pandemic on 11 March 2020 (3,4).
Common symptoms include headache, fever, malaise, muscle and joint pains, dyspnea, cough, and loss of smell and taste (5,6). Less common symptoms include abdominal pain, nausea, vomiting and diarrhea (7). The time from exposure to onset of symptoms ranges from 5–14 days (8). While the majority of cases have mild symptoms, some cases may progress to acute respiratory distress syndrome (ARDS).The highest proportion of severe cases occurs in adults over 60, and in those with certain underlying chronic co-morbidities such as diabetes, cerebrovascular and cardio-vascular diseases (9,10).
Multiple studies confirmed that headache was a frequently reported symptom in patients infected with SARS-COV-2. However, there was a great diversity in its frequency, severity, character and duration (11). Borges do Nascimento et al. (12) found that headache was the most common neurological symptom in patients with COVID, as it was observed in 12% of confirmed cases. In another study, headache was reported to be the second most common neurological symptom after dizziness and was present in 13% of the patients (13).
Much concern was directed towards clarifying the criteria of headache attributed to systemic infection. Headache is usually diffuse and bilateral, but in some cases, it may be fronto-temporal or occipital with associated retroocular pain. The intensity of headache is variable and it may increase by coughing, straining or head movement. It may be associated with conjunctival injection, nausea, vomiting, photophobia or phonophobia. Fever was considered one of the most significant predictors of frequency and intensity of such a type of headache (14).
In some susceptible individuals, systemic infections may be a provoking factor for an underlying primary headache disorder. Consequently, caution must be given when trying to distinguish whether a patient’s headache is an infection triggering pre-existing primary headache attack, or is an acute headache attributable to a systemic viral infection (14).
The International Classification of Headache Disorders, third edition (ICHD-3) requires a confirmed diagnosis of systemic viral infection in the absence of meningitic or encephalitic involvement for the diagnosis of headache attributed to systemic viral infections (15).
The aim of this work was to study the headache characteristics in patients infected with COVID-19, and to detect predictors of its frequency and intensity in relation to history of pre-existing primary headache disorder, medical comorbidities, fever, dehydration, steroids intake, laboratory biomarkers and severity of chest imaging findings of SARS-COV-2 infection.
Methods
Study design and participants
This is a cross-sectional study, carried out on 172 patients suffering from COVID-19 related headache in the period from 1 April 2020 to 1 June 2020. Patients were recruited from two quarantine hospitals in two Egyptian governorates, Cairo and Beni-Suef.
The diagnosis of COVID-19 infection and grading of its severity were made according to the clinical management of COVID-19, released by the World Health Organization (WHO) (16). The polymerase chain reaction (PCR) testing of a nasopharyngeal sample should have tested positive for COVID-19.
The grading of COVID-19 severity was stratified into mild, moderate, or severe according to WHO classification (16) as follows: Mild COVID-19: Patients with COVID-19 infection without evidence of viral pneumonia or hypoxia. Moderate COVID-19: Patients with clinical signs of pneumonia (fever, cough, dyspnea, tachypnea) but no signs of severe pneumonia, including SpO2 ≥90% on room air. Severe COVID-19: Patients with clinical signs of pneumonia plus one of the following: Respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air (16).
The treatment protocol used in the two centers was in accordance with the treatment guidelines for national institutes of health (NIH); the nation's medical research agency (17) steroids were used in treatment protocol of moderate cases that required supplemental oxygen and in severe cases that required mechanical ventilation.
Any adult patient (age ≥18 years) suffering from headache related COVID-19 infection according to the ICHD-III criteria of acute headache attributed to systemic viral infection (9.2.2.1) (15) was eligible. Patients with any other form of secondary headache (from detailed neurological and medical history, fundus examination or brain imaging) were excluded. Patients with evidence of intracranial infections (from history, neurological examination, brain imaging or CSF analysis), or patients with altered mental state, structural lesion in brain imaging, or participating in clinical trials during the recruitment period were excluded.
Out of 230 patients with headache due to COVID-19, 58 were excluded (16 declined to participate, four had abnormal brain imaging, 10 had secondary headache and 28 were participating in other clinical trials) (Figure 1).
Figure 1.
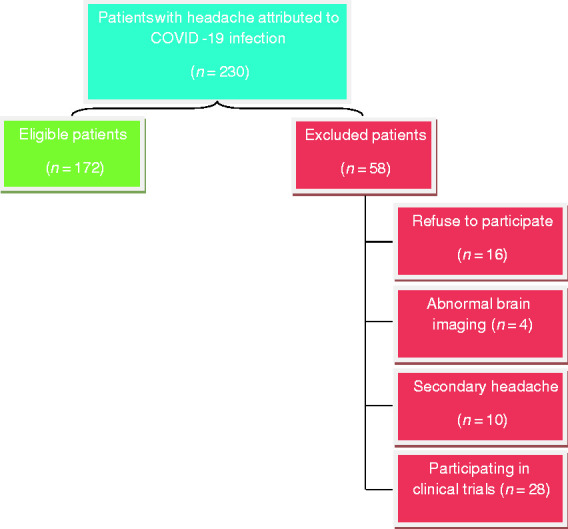
Flow diagram for the eligible and excluded patients.
Data collection
We collected data on patient demographics, body mass index (BMI) and comorbid conditions. In addition to baseline clinical data, an expert neurologist made a detailed descriptive analysis of COVID-19 related headache by face-to-face interviews. A stepwise approach was applied to see if there was pre-existing primary headache disorder according to the ICHD-3 criteria (15). COVID-19 related headache analysis data included site of headache, frequency, duration, character, intensity according to the visual analogue scale (VAS) (18), and response to analgesics. The onset of headache in temporal relation to other COVID-19 symptoms was recorded. The relation of headache to administration of steroids, periods of high fever and dehydration, if present, were also reported.
According to the 2015 Cochrane review (19), dehydration was defined as serum osmolality of more than 294 mOsm/kg in the context of water loss. Serum osmolality was calculated using the following formula: 2 Na (mEq/L) + (Urea [mg/dL])/2.8 + (Glucose [mg/dL])/18.
Laboratory data, including baseline serum C-reactive protein (CRP), D-dimer, ferritin and lymphocytic count done within 24 hours of hospital admission, were collected. The results of initial computerized tomography (CT) – chest were recorded.
Sampling
All patients with COVID-19 related headache who were admitted to the two centers during the recruitment period that fulfilled the eligibility criteria and were accepted to take part in the study were included in the study (172 patients).
Statistical analysis
The data were analyzed using IBM SPSS (Statistical Package of Social Science) Version 21. Normality distribution of the data was tested by using the Shapiro-Wilk test. Clinical characteristics and laboratory data of the patients, in addition to the characteristics of COVID-19 related headache, were presented by using the median and interquartile range (IQR) for non-normally distributed numerical data and by using frequency and percentage for qualitative data. The associations of intensity, frequency, and duration of headache with characteristics of the patient were assessed through χ2 tests for categorical variables and through Mann-Whitney and Kruskal-Wallis tests for continuous variables (with results non-normally distributed according to the Shapiro-Wilk test). Multivariate analysis was done with construction of a multiple linear regression model to identify predictors of frequency and intensity of COVID-19 related headache after being adjusted for their potential mutual confounding effect. We did not include in this model factors that showed collinearity; we analysed multicollinearity by using the variance inflation factor (VIF) and tolerance. We considered multicollinearity critical when VIF was >3 and tolerance less than 0.1. The magnitude of the dependence between the variables was explored using the adjusted R2 value. The results are presented as odds ratios (OR) and 95% confidence intervals (CIs). A p-value less than 0.05 was considered statistically significant. All tests were two-tailed.
Ethical considerations
The study proposal was revised and approved by the Neurology Department Ethical Committee, Cairo University. The aim and nature of the study were explained for each patient before inclusion. A policy of data confidentiality was firmly followed. Informed written consent was obtained from all participants before enrolment. The study design followed the requirements of the Revised Helsinki Declaration of biomedical ethics.
Results
Demographics and clinical characteristics of the study population
The study population included 172 patients who had headache attributed to COVID-19 infection, with median age of 33 and interquartile range (IQR) 27.3–42 years. The study included 64 males (37.2%) and 108 females (62.8%).Other clinical characteristics and laboratory findings of the study group are summarized in Table 1. The characteristics of COVID-19 related headache are outlined in Table 2.
Table 1.
Demographics, clinical characteristics and laboratory data of the study group.
| Patients (n = 172) | |
| Age, median (IQR) | 33 (27.3–42) |
| Gender, n (%) | |
| Males | 64 (37.2%) |
| Females | 108 (68.2%) |
| BMI, median (IQR) | 27.7 (25.4–31.6) |
| Smokers, n (%) | 15 (8.7%) |
| Medical comorbidities*, n (%) | 34 (19.8%) |
| Preexisting primary headache disorder, n (%) | |
| Migraine | 45 (26.2) |
| Tension-type headache | 46 (26.7) |
| None | 81 (47.1%) |
| Laboratory data, median (IQR) | |
| Lymphocyte count | 1400 (800–2447.5) |
| CRP (mg/L) | 12 (4.39–48) |
| D-dimer (µg/mL) | 0.36 (0.2–0.56) |
| Ferritin (ng/mL) | 130 (52.1–261.5) |
| Grading severity of COVID-19 infection, n (%) | |
| Mild | 116 (67.4%) |
| Moderate | 42 (24.4%) |
| Severe | 14 (8.1%) |
*Eighteen patients with controlled HTN (BP ≥ 140/90 or on antihypertensive medications), seven patients were diabetic, five patients had hypothyroidism, three patients had ischemic heart disease and one patient had epilepsy.
BMI: body mass index; CRP: C-reactive protein; IQR: interquartile range.
Table 2.
Characteristics of COVID-19 related headache.
| Headache characteristics | Patients (n = 172) |
|---|---|
| Headache onset, n (%) | |
| Before other COVID-19 symptoms | 49 (28.5%) |
| With other COVID-19 symptoms | 98 (57%) |
| After other COVID-19 symptoms | 25 (14.5%) |
| Site, n (%) | |
| Diffuse | 91 (52.9%) |
| Temporal | 31 (18%) |
| Frontal | 40 (23.3%) |
| Occipital | 10 (5.8%) |
| Character of pain, n (%) | |
| Throbbing | 28 (16.3%) |
| Pressing | 70 (40.7%) |
| Exploding | 45 (26.2%) |
| Dull | 29 (16.9%) |
| Frequency (attacks/week), median (IQR) | 7 (3–7) |
| Visual analogue scale, median (IQR) | 7 (5–8) |
| Duration in hours, median (IQR) | 6 (2–16 ) |
| Relation to high fever*, n (%) | |
| Increase with fever | 59 (49.2%) |
| Decrease with fever | 4 (3.3%) |
| No effect | 57 (47.5%) |
| Relation to steroids intake**, n (%) | |
| Increases with steroids | 9 (16.7%) |
| Decreased with steroids | 22 (40.7%) |
| No effect of steroids | 23 (42.6%) |
| Relation to dehydration***, n (%) | |
| Increased with dehydration | 30 (27%) |
| Decreased with dehydration | 12 (10.8%) |
| No effect of dehydration | 69 (62%) |
| Response to analgesics, n (%) | |
| Excellent | 56 (32.6%) |
| Moderate | 80 (46.5%) |
| Poor | 36 (20.9%) |
*Only 120 (69.7%) patients had fever.
**Only 54 (31.4%) of patients received steroids.
***Only 111 (64.5%) patients had dehydration.
IQR: interquartile range.
Characteristics of COVID-19 related headache in relation to pre-existing primary headache disorder
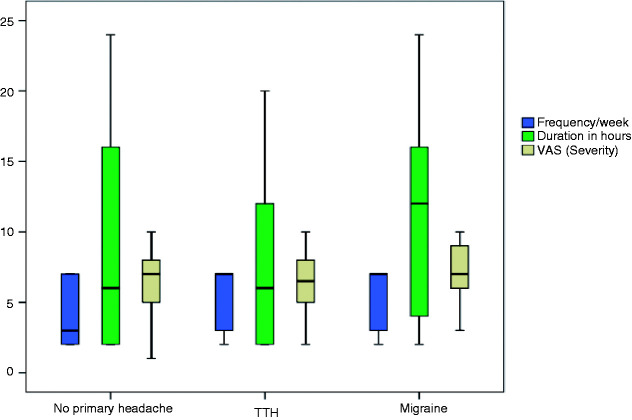
Patients having a pre-existing primary headache disorder had significantly more frequent COVID-19 related headache (p = 0.003) (Table 3). Frequency of COVID-19 related headache was significantly different between the three groups: Patients with migraine, patients with tension-type headache (TTH) and patients without pre-existing primary headaches (Figure 2). Post hoc pairwise comparisons of the Kruskal-Wallis test showed that such frequency was significantly higher in patients with migraine and TTH compared to patients without pre-existing primary headache, (p < 0.001 and p = 0.011), respectively.
Table 3.
Characteristics of COVID-19 related headache in relation to gender, smoking state, pre-existing primary headache disorder, fever, dehydration, steroids, comorbidities and COVID-19 severity.
| Frequency/week, median (IQR) | Duration (hours), median (IQR) | VAS, median (IQR) | |
|---|---|---|---|
| Gender | |||
| Males, n = 64 (37.2%) | 3 (2–7) | 16 (2–24) | 7 (0–10) |
| Females, n = 108 (62.8%) | 5 (2–7) | 12 (2–24) | 8 (0–10) |
| p-value | 0.56 | 0.54 | 0.51 |
| Smoking state | |||
| Smoker, n = 15 (8.7%) | 6 (2–7) | 16 (2–24) | 7 (4–10) |
| Non-smoker, n = 157 (91.2 %) | 4 (2–7) | 10 (2–24) | 5 (0–10) |
| p-value | 0.45 | 0.36 | 0.77 |
| Pre-existing primaryheadache* | |||
| With, n = 91 (52.9%) | 7 (3–7) | 12 (4–16) | 7 (5–8) |
| Without, n = 81 (47.1%) | 3 (2–7) | 6 (2–16) | 7 (5–8) |
| p-value | <0.001* | 0.55 | 0.34 |
| Fever* | |||
| With, n = 120 (69.8%) | 7 (3–7) | 11 (4–16) | 7 (6–8) |
| Without, n = 52 (30.2%) | 3 (2–7) | 6 (2–16) | 6 (4–8) |
| p-value | 0.003* | 0.292 | 0.012* |
| Dehydration* | |||
| With, n = 111 (64.5%) | 7 (3–7) | 6 (2–16) | 7 (5–8) |
| Without, n = 61 (35.5%) | 3 (2–7) | 6 (2–16) | 6 (5–8) |
| p-value | 0.029* | 0.506 | 0.201 |
| Steroids | |||
| Received, n = 54 (31.4%) | 7 (3–7) | 6 (2–13) | 7 (5–8) |
| Not received, n = 118 (68.6%) | 7 (3–7) | 6 (4–16) | 7 (5–8) |
| p-value | 0.965 | 0.295 | 0.924 |
| Comorbidities | |||
| With, n = 34 (19.8%) | 7 (7–7) | 12 (2–16) | 8 (6–10) |
| Without, n = 138 (80.2%) | 5 (2–7) | 6 (2–16) | 6.5 (5–8) |
| p-value | 0.006* | 0.235 | 0.003* |
| Grading severity of COVID-19 infection | |||
| Mild, n = 116 (67.4%) | 3 (2–7) | 6 (2–15) | 6 (5–8) |
| Moderate, n = 42 (24.4%) | 7 (3–7) | 12 (2–16) | 8 (6.75–9) |
| Severe, n = 14 (8.1%) | 7 (2.75–7) | 6 (2–16) | 7 (5–8.25) |
| p-value | 0.032* | 0.134 | 0.007* |
VAS: visual analogue scale.
Note: Bold p-values < 0.05 are considered significant.
*These groups were matched regarding age, sex, comorbidities and COVID-19 severity.
Figure 2.
Comparison between patients with migraine, tension type headache and patients without primary headache, regarding characteristics of COVID-19 related headache.
Characteristics of COVID-19 related headache in relation to other COVID-19 symptoms
Patients with fever had significantly higher frequency and intensity of COVID-19 related headache compared to patients without fever. Headache frequency was significantly higher in patients with dehydration in comparison to those without dehydration (Table 3).
Characteristics of COVID-19 related headache in relation to comorbidities, gender and smoking status of the patients
Patients with comorbidities had significantly higher frequency and intensity of COVID-19 related headache compared to patients without comorbidities (Table 3). Nevertheless, headache characteristics did not differ in terms of gender or smoking status.
Characteristics of COVID-19 related headache in relation to severity of COVID-19 infection
Each of frequency and intensity of COVID-19 related headache was significantly different between the three groups (Table 3). Post hoc pairwise comparisons of the Kruskal-Wallis test showed that frequency and intensity were significantly higher in patients with moderate COVID-19 than in those with mild COVID-19 infection (p = 0.031, p = 0.005), respectively.
Factors associated with frequency and intensity of COVID-19 related headache
A multivariate analysis was done including all variables that were believed to be relevant for frequency and intensity of COVID-19 headache, and these showed no collinearity with each other (VIF < 3).
Factors associated with high frequency of attacks of COVID-19 related headache were the presence of primary headache group disorders (p = 0.026), dehydration (p = 0.007) and comorbidities (p = 0.026), (adjusted R2, 0.28) (Table 4).
Table 4.
Factors related to frequency of COVID-19 related headache, multivariate analysis.
| Variables | B | Odds ratio | Sig. |
95.0% confidence interval for B |
Correlations |
Collinearity statistics |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Zero-order | Partial | Part | Tolerance | VIF | ||||
| Constant | 3.528 | 0.042 | 0.125 | 6.931 | ||||||
| Lymphocyte count | −0.005 | −0.016 | 0.892 | −0.001 | 0.000 | −0.037 | −0.019 | −0.014 | 0.807 | 1.239 |
| CRP (mg/L) | 0.003 | 0.062 | 0.650 | −0.010 | 0.016 | 0.314 | 0.063 | 0.048 | 0.607 | 1.647 |
| D-dimer | 0.220 | 0.041 | 0.741 | −1.109 | 1.548 | 0.142 | 0.046 | 0.035 | 0.742 | 1.348 |
| Ferritin (ng/mL) | −0.005 | −0.015 | 0.914 | 0.001 | 0.001 | 0.178 | −0.015 | −0.011 | 0.563 | 1.776 |
| Primary headache | 1.437 | 0.314 | 0.026* | 0.182 | 2.692 | 0.296 | 0.304 | 0.243 | 0.599 | 1.669 |
| Dehydration | 1.586 | 0.334 | 0.007* | 0.444 | 2.728 | 0.463 | 0.361 | 0.294 | 0.775 | 1.290 |
| Fever | 0.794 | 0.149 | 0.228 | −0.513 | 2.102 | 0.202 | 0.167 | 0.129 | 0.745 | 1.342 |
| Moderate COVID-19 | 0.231 | 0.045 | 0.721 | −1.057 | 1.519 | 0.150 | 0.050 | 0.038 | 0.711 | 1.406 |
| Severe COVID-19 | −0.666 | −0.095 | 0.528 | −2.768 | 1.436 | 0.051 | −0.088 | −0.067 | 0.497 | 2.011 |
| Age | −0.031 | −0.175 | 0.248 | −0.084 | 0.022 | 0.044 | −0.160 | −0.123 | 0.495 | 2.021 |
| Sex | −0.284 | −0.061 | 0.670 | −1.612 | 1.045 | 0.018 | −0.059 | −0.045 | 0.549 | 1.823 |
| Smoking | 0.686 | 0.072 | 0.554 | −1.626 | 2.999 | 0.090 | 0.082 | 0.063 | 0.769 | 1.301 |
| Comorbidities | 2.055 | 0.394 | 0.026* | 0.256 | 3.853 | 0.304 | 0.303 | 0.242 | 0.378 | 2.644 |
| Dependent variable: Frequency/week, adjusted R2 0.28. | ||||||||||
CRP: C-reactive protein; VIF: variance inflation factor.
Note: Bold p-values < 0.05 are considered significant.
Factors associated with high pain intensity of COVID-19 related headache were female gender (p = 0.006), dehydration (p = 0.024) and fever (p = 0.046), (adjusted R2, 0.3) (Table 5).
Table 5.
Factors related to intensity of COVID-19 related headache, multivariate analysis.
| Variables | B | Odds ratio | Sig |
95.0% confidence interval for B |
Correlations |
Collinearity statistics |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Zero-order | Partial | Part | Tolerance | VIF | ||||
| Constant | 0.429 | 0.803 | −3.002 | 3.860 | ||||||
| Lymphocyte count | 0.000 | 0.065 | 0.577 | 0.000 | 0.001 | −0.025 | 0.078 | 0.058 | 0.807 | 1.239 |
| CRP (mg/L) | 0.000 | −0.004 | 0.976 | −0.013 | 0.013 | 0.281 | 0.004 | −0.003 | 0.607 | 1.647 |
| D-dimer | 0.306 | 0.055 | 0.649 | −1.034 | 1.645 | 0.172 | 0.063 | .048 | 0.742 | 1.348 |
| Ferritin (ng/mL) | 0.001 | 0.206 | 0.144 | 0.000 | 0.002 | 0.221 | 0.202 | 0.154 | 0.563 | 1.776 |
| Primary headache | 0.097 | 0.021 | 0.878 | −1.168 | 1.363 | 0.124 | 0.021 | 0.016 | 0.599 | 1.669 |
| Dehydration | 1.332 | 0.274 | 0.024* | 0.180 | 2.483 | 0.292 | 0.306 | 0.241 | 0.775 | 1.290 |
| Fever | 1.346 | 0.247 | 0.046* | 0.027 | 2.664 | 0.339 | 0.273 | 0.213 | 0.745 | 1.342 |
| Moderate COVID-19 | 0.587 | 0.112 | 0.369 | −0.711 | 1.885 | 0.237 | 0.125 | 0.094 | 0.711 | 1.406 |
| Severe COVID-19 | 0.031 | 0.004 | 0.977 | −2.088 | 2.150 | 0.115 | 0.004 | 0.003 | 0.497 | 2.011 |
| Age | −0.010 | −0.057 | 0.700 | −0.064 | 0.043 | 0.141 | −0.054 | −0.040 | 0.495 | 2.021 |
| Sex | 1.897 | 0.399 | 0.006* | 0.558 | 3.236 | 0.252 | 0.367 | 0.296 | 0.549 | 1.823 |
| Smoking | 1.724 | 0.176 | 0.144 | −0.608 | 4.055 | 0.119 | 0.202 | 0.154 | 0.769 | 1.301 |
| Comorbidities | 1.119 | 0.209 | 0.221 | −0.694 | 2.932 | 0.377 | 0.169 | 0.129 | 0.378 | 2.644 |
| Dependent variable: VAS (intensity), adjusted R2 0.3. | ||||||||||
CRP: C-reactive protein; VAS: visual analog scale; VIF: variance inflation factor.
Note: Bold p-values < 0.05 are considered significant.
Discussion
Clinicians involved in the care of COVID-19 patients need to be cognizant of the diverse clinical characteristics of COVID-19 related headache. This is the first study that offers a detailed analysis of COVID-19 related headache in relation to history of pre-existing primary headache disorder, medical comorbidities, fever, dehydration, intake of steroids and laboratory biomarkers of SARS-COV-2 infection.
In general, the headaches that accompany systemic infections are typically nonspecific without any particular distinguishing or characteristic features (20). Although diffuse pain and moderate or severe intensity are listed in the ICHD-3 criteria of “Acute headache attributed to systemic viral infection”, they are not mandatory for diagnosis (15). The present study showed that the majority of our patients had a diffuse headache (52.9%), pressing in 40.7%, with median intensity of 7.
Pathogenesis of headaches attributed to systemic infection is not clearly settled. It has been speculated that microorganisms may activate inflammatory and nociceptive mediators that stimulate headache, such as nitric oxide, prostaglandins and cytokines (14,21). Likewise, COVID-19 infection is accompanied by release of a large amount of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α (22) that are involved in various pathological pain states (23). The response to steroids in 40.7% of our patients who received steroids may be indicative of immunological/inflammatory mechanisms for the generation of COVID-19 related headache. However, we did not find any statistically significant relationship between the use of steroids and frequency, duration or intensity of COVID-19 related headache.
There is another point of view: Fever, as a part of a systemic infection, may be a trigger for headache (14,19). The principal inflammatory mediators of SARS-COV-2 infection (IL1 and IL6) activate the hypothalamus and promote fever (24). In accordance with our results, COVID-19 patients with fever were more likely to develop more frequent COVID-19 related headache attacks, compared to patients without fever. Nevertheless, we should not rely on this principle alone, as some patients (30.2%) had headache in the absence of fever.
The present study showed that patients with pre-existing primary headache disorder had significantly more frequent attacks of COVID-19 related headache. Pre-existing primary headache disorder is a demodulatory pain process (25), where derangement of top-down pain modulatory pathways occurs with atypical release of nociceptive molecules (26). Such alterations can lead to sensitization of central and peripheral nociceptive pathways resulting in a decrease in the pain threshold and an increase in receptive fields (27,28). Specifically for migraine, cortical spreading depression (CSD) may add to hyperexcitability of the trigeminovascular neurons (29).
The current study showed that dehydration is a predictor of higher frequency and intensity of COVID-19 related headache attacks. It is well known that water deprivation triggers migraine and other types of headache due to intracranial dehydration and total plasma volume (30–32. Therefore, healthcare providers must be aware of fluid balance states in patients with COVID-19, especially for those with headache.
Medical comorbidities are considered some of the most evident predictors of COVID-19 severity (33). Although patients with and without comorbidities in our study were not matched regarding COVID-19 severity, we could not neglect the significant impact of comorbidities and both frequency and intensity of headache revealed by the regression model. Furthermore, medical comorbidities were indicated by multiple researchers as risk factors for headache (34–36).
To our knowledge, laboratory markers of COVID-19 infection were studied for the first time in relation to frequency and intensity of COVID-19 related headache. It is well known that higher CRP is related to decreased pain threshold (37) and is implicated in the inflammatory process of various types of headache (38). However, in our study, CRP was not found to be associated with either frequency or intensity of COVID-19 related headache
Lymphocytes are the primary sources of proinflammatory and anti-inflammatory cells that are strongly involved during the systemic inflammatory response (39). Karabulut et al. (40) found a significantly higher neutrophil/lymphocyte ratio during the migraine attacks, compared to the healthy subjects. However, in our study, lymphocytic count was not found to be associated with either frequency or intensity of COVID-19 related headache.
Although the process of iron metabolism is supposed to increase the frequency of headaches by decreasing the pain threshold via different mechanisms – such as nitric oxide and neurotransmitters (40) – our study failed to find such a relation. Likewise, Aamodt et al. (41) found no association between ferritin levels and the prevalence of headaches.
D-dimers, as fibrin degradation products, are commonly used markers of inflammation and increased coagulation activity (42). Although higher D-dimer levels were supposed to play a role in neurogenic inflammation in migraine (43), we did not find any relation with frequency or intensity of COVID-19 related headache.
The most important limitation of our study is the low number of patients with severe COVID-19 infection (n = 14) and patients who received steroids (n = 54). As a result, we could not properly judge the impact of COVID-19 severity and intake of steroids on the frequency, intensity and duration of COVID-19 related headache. Secondly, we did not compare between the impact of different medical comorbidities on frequency, intensity and duration of COVID-19 related headache because the included subgroups (HTN, DM, hypothyroidism, ischemic heart disease and epilepsy) did not have comparable numbers of patients. Future studies should adopt follow-up of patients who have recovered from SARS-COV-2 infection to assess the possibility of development of chronic post-infection headache. In addition, much concern should be directed towards clarifying the pathogenesis of COVID-19 related headache.
Conclusion
COVID-19 related headache is diffuse in the majority of patients (52.9%), pressing in 40.7%, with a median intensity of 7 (as assessed by VAS) and a median frequency of 7 days/week. Frequency of COVID-19 related headache was higher in patients with primary headache group disorders, dehydration and comorbidities. Meanwhile, high pain intensity was associated with female gender, fever, and dehydration.
Clinical implications
COVID-19 related headache is diffuse, pressing in the majority of patients, with moderate to severe intensity.
Patients with a history of pre-existing primary headache disorders, comorbidities, or dehydration had more frequent COVID-19 related headache attacks.
Fever, dehydration and female gender are predictors of higher intensity of COVID-19 related headache.
Footnotes
Author contributions: RM participated in study design, collection and interpretation of data and helped to draft the manuscript. MH participated in study design, collection and interpretation of data and helped to draft the manuscript. CR participated in study design, sequence alignment and helped to draft the manuscript. HA participated in collection of data and helped to draft the manuscript. AK participated in collection and interpretation of data and helped to draft the manuscript. HR participated in study design, analysis and interpretation of data and helped to draft the manuscript. AD participated in study design, collection and interpretation of data and helped to draft the manuscript. All authors read and approved the final manuscript.
Availability of data and materials: Authors report that the datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Written informed consent was obtained from each participant in this study and the study was approved by the local ethical committee of the Neurology Department, Cairo University.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Christine Ragaie https://orcid.org/0000-0002-5795-5217
References
- 1.Gorbalenya AE, Baker SC, Baric RS et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 2020; 92: 401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, E IA, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Intern J Infect Dis 2020; 91: 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burki TK. Coronavirus in China. Lancet Resp Med 2020; 8: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One 2020; 15: e0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med 2020; 172: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA 2020; 323: 1499–1500. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JR. COVID-19 and the nervous system. J Neurovirol 2020; 26: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borges do Nascimento IJ, Cacic N, Abdulazeem HM, et al. Novel coronavirus infection (COVID-19) in humans: A scoping review and meta-analysis. J Clin Med 2020; 9: 941. [DOI] [PMC free article] [PubMed]
- 13.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marinis M, Welch KM. Headache associated with non-cephalic infections: Classification and mechanisms. Cephalalgia 1992; 12: 197–201. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Clinical management of COVID-19: Interim guidance, 27 May 2020. World Health Organization, https://apps.who.int/iris/handle/10665/332196 (2020).
- 17.COVID-19 treatment guidelines panel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. National Institutes of Health, https://www.covid19treatmentguidelines.nih.gov/ (2020). [PubMed]
- 18.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983; 16: 87–101. [DOI] [PubMed] [Google Scholar]
- 19.Hooper L, Abdelhamid A, Attreed NJ, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev 2015; 2015: CD009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladstone J, Bigal ME. Headaches attributable to infectious diseases. Curr Pain Headache Rep 2010; 14: 299–308. [DOI] [PubMed] [Google Scholar]
- 21.Marchioni E, Tavazzi E, Bono G, et al. Headache attributed to infection: Observations on the IHS classification (ICHD-II). Cephalalgia 2006; 26: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 22.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020; 11: 14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blomqvist A, Engblom D. Neural mechanisms of inflammation-induced fever. Neuroscientist 2018; 24: 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speciali JG, Fleming NRP, Fortini I. Primary headaches: Dysfunctional pains. Revista Dor 2016; 17: 72–74. [Google Scholar]
- 26.Ji R-R, Nackley A, Huh Y, et al. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129: 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurtray A, Saito E. Does primary headache type influence secondary headache symptoms? J Neurosci Rural Pract 2014; 5: 111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schankin CJ, Straube A. Secondary headaches: Secondary or still primary? J Headache Pain 2012; 13: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar S, Johnson KW, Ossipov MH, et al. CGRP and the trigeminal system in migraine. Headache 2019; 59: 659–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blau JN, Kell CA, Sperling JM. Water-deprivation headache: A new headache with two variants. Headache 2004; 44: 79–83. [DOI] [PubMed] [Google Scholar]
- 31.Popkin BM, D’Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 2010; 68: 439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price A, Burls A. Increased water intake to reduce headache: Learning from a critical appraisal. J Eval Clin Pract 2015; 21: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 33.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol 2008; 7): 354–361. [DOI] [PubMed] [Google Scholar]
- 35.Hagen K, Stovner LJ, Vatten L, et al. Blood pressure and risk of headache: A prospective study of 22 685 adults in Norway. J Neurol Neurosurg Psychiatry 2002; 72: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aamodt AH, Stovner LJ, Midthjell K, et al. Headache prevalence related to diabetes mellitus. The Head-HUNT study. Eur J Neurol 2007; 14: 738–744. [DOI] [PubMed] [Google Scholar]
- 37.Schistad EI, Stubhaug A, Furberg A-S, et al. C-reactive protein and cold-pressor tolerance in the general population: The Tromsø Study. Pain 2017; 158: 1280–1288. [DOI] [PubMed] [Google Scholar]
- 38.Hagen K, Stovner LJ, Nilsen KB, et al. The impact of C-reactive protein levels on headache frequency in the HUNT study 2006–2008. BMC Neurol 2019; 19: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske lekarske listy 2001; 102: 5–14. [PubMed] [Google Scholar]
- 40.Karabulut KU, Egercioglu TU, Uyar M, et al. The change of neutrophils/lymphocytes ratio in migraine attacks: A case-controlled study. Ann Med Surg 2016; 10: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aamodt AH, Borch-Iohnsen B, Hagen K, et al. Headache prevalence related to haemoglobin and ferritin. The HUNT Study. Cephalalgia 2004; 24: 758–762. [DOI] [PubMed] [Google Scholar]
- 42.Tripodi A. D-dimer testing in laboratory practice. Clin Chem 2011; 57: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 43.Yucel Y, Tanriverdi H, Arıkanoglu A, et al. Increased fibrinogen, d-dimer and galectin-3 levels in patients with migraine. Neurol Sci 2014; 35: 545–549. [DOI] [PubMed] [Google Scholar]