Key Points
Question
Is serum neurofilament light level at the time of the first demyelinating event associated with long-term brain atrophy and disease progression?
Findings
In this cohort study of 308 patients with multiple sclerosis and clinically isolated syndrome in a clinical trial of intramuscular interferon β-1a, serum neurofilament light concentrations at baseline were associated with brain atrophy progression over 5 and 10 years.
Meaning
These findings suggest that serum neurofilament light measured at the time of clinically isolated syndrome or early multiple sclerosis may be useful as a biomarker associated with disease severity stratification in early multiple sclerosis.
This cohort study examines the association of neurofilament light levels with long-term brain atrophy and disease progression in multiple sclerosis.
Abstract
Importance
Data are needed on the potential long-term prognostic association of serum neurofilament light in multiple sclerosis (MS).
Objective
To evaluate serum neurofilament light as a biomarker associated with long-term disease outcomes in clinically isolated syndrome.
Design, Setting, and Participants
This post hoc cohort study used data from the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study, a 36-month, multicenter, placebo-controlled interferon β-1a randomized clinical trial conducted from April 1996 to March 2000, and its long-term (5- and 10-year) extension study from February 2001 to March 2009. Participants included individuals with a symptomatic initial demyelinating event and brain magnetic resonance imaging (MRI) lesions suggestive of MS. Data were analyzed from April 2017 through 2019.
Exposure
The variable of interest was naturally occurring serum neurofilament light concentration
Main Outcomes and Measures
Gadolinium-enhancing (Gd+) lesion number, T2 lesion volume, and brain parenchymal fraction, a measure of brain atrophy were measured at baseline and 5 and 10 years. Multivariate regression models evaluated whether age, sex, and baseline covariates, including serum neurofilament light, brain parenchymal fraction, Expanded Disability Status Scale, Gd+ lesion count, and T2 lesion volume, were associated with brain parenchymal fraction changes over 5 and 10 years.
Results
Among 308 included participants (mean [SD] age, 33.2 [7.6] years; 234 [76.0%] women), baseline serum neurofilament light concentrations were associated with Gd+ lesions (Spearman r = 0.41; P < .001) and T2 lesion volume (Spearman r = 0.42; P < .001). Among covariates for brain parenchymal fraction change, serum neurofilament light concentration had the greatest correlation with change in brain parenchymal fraction at 5 years (Spearman r = –0.38; P < .001) and was the only variable associated with brain parenchymal fraction at 10 years (Spearman r = –0.45; P < .001). Participants in the highest vs lowest baseline serum neurofilament light tertiles showed brain parenchymal fraction reduction at 5 years (−1.83% [95% CI, −1.49% to −2.18%] vs −0.95% [95% CI, −0.78% to −1.12%]; P < .001) and 10 years (−3.54% [95% CI, −2.90% to −4.17%] vs −1.90% [95% CI, −1.43% to −2.37%]; P < .001). At 5 years, 6 of 45 participants (13.3%) in the highest neurofilament tertile and 2 of 52 participants (3.8%) in the lowest neurofilament tertile achieved an Expanded Disability Status Scale score of 3.5 or greater.
Conclusions and Relevance
This cohort study found that higher baseline serum neurofilament light levels were associated with increased brain atrophy over 5 and 10 years. These findings suggest that serum neurofilament light could be a biomarker associated with disease severity stratification in early MS and may help to guide intervention.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disorder of the central nervous system with variable symptoms, disease course, prognosis, and response to treatment.1,2 A biomarker that could be used early in the disease course to estimate disease severity by identifying patients at risk of more aggressive disease would be useful in counseling patients and choosing disease-modifying treatments. Measures of brain atrophy and T2 hyperintense lesion burden based on magnetic resonance imaging (MRI), combined with clinical features at the time of diagnosis, have been used to gauge disease severity and inform treatment decisions.3,4,5 However, to our knowledge, standardized models based on these measures have not emerged, highlighting the need for additional sensitive, quantitative, cost-effective, and noninvasive biomarkers associated with prognosis. Despite efforts over the last decades to identify such biomarkers, there is no blood-based biomarker associated with prognosis currently available to inform clinical practice.
Neurofilament light chain, one of the intermediate filaments believed to play a key role in the growth and stability of axons, has been shown to increase in cerebrospinal fluid (CSF) and serum in response to neuroaxonal injury.6 Increased CSF and serum neurofilament light levels have been reported in several neurodegenerative disorders, including MS,7 Alzheimer disease,8 and amyotrophic lateral sclerosis,9 and in brain trauma.10 In MS, elevated levels of serum neurofilament light have been shown to correlate with disease activity11 and worse long-term clinical and paraclinical outcomes.7,11,12,13,14,15,16,17,18,19,20,21 Although these studies demonstrate utility for serum neurofilament light level as a potential biomarker associated with disease activity and shorter-term prognosis, information is scarce on the association of serum neurofilament light level at first MS presentation with long-term disease outcomes.
In this study, we evaluated serum neurofilament light level as a prognostic biomarker associated with 5- and 10-year clinical and radiological outcomes in a well-characterized cohort of patients presenting with clinically isolated syndrome. We used data from a study that initiated in 2000; therefore, the definition of clinically isolated syndrome is slightly different than the current definition, which describes it as an acute or subacute single episode of at least 24 hours duration that occurs in an individual not previously known to have MS, who has symptoms and objective findings that reflect an inflammatory demyelinating event from which there has either been recovery or no recovery.22 In this phase 3 study, clinically isolated syndrome was defined as a neurologic event consistent with demyelination of the optic nerve, spinal cord, brainstem, or cerebellum and the presence of 2 or more clinically silent lesions of the brain at least 3 mm in diameter.23
Methods
Study Design
This study used serum samples from the Controlled High-Risk Avonex (intramuscular [IM] interferon [IFN] β-1a) Multiple Sclerosis Prevention Study (CHAMPS)23 and clinical outcome data from the long-term follow-up study, Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurologic Surveillance (CHAMPIONS).24,25 The study protocols were approved by the institutional review board at each participating site, and the studies were conducted according to the International Council for Harmonization Guideline on Good Clinical Practice and the Declaration of Helsinki.26 Participants in CHAMPS and CHAMPIONS provided written consent to participate and provide serum samples for possible use in future MS research.23,24,25 This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
CHAMPS23 was a 36-month placebo-controlled phase 3 randomized clinical trial of IM IFN-β-1a in participants with early MS conducted from April 1996 until March 2000, with first data publication in September 2000.23 Details of the study methods have been published elsewhere.23 Participants with an initial demyelinating event and cranial MRI evidence of subclinical demyelination were randomly assigned to receive weekly IM injections of 30 mg IFN-β-1a or placebo.23 The primary end point was the development of clinically definite MS, which was defined as either the occurrence of a new symptomatic neurological event attributable to a different part of the central nervous system than the initial episode and in the absence of fever or infection lasting more than 48 hours, or an increase of at least 1.5 points in Expanded Disability Status Scale (EDSS) score. Patients who developed clinically definite MS were withdrawn from the study. MRI data collected from each participant included baseline number of gadolinium-enhancing (GD+) lesions and T2 lesion load and volume; these data were derived at a central MRI reading center using a standardized method. All participants in CHAMPS23 were eligible to participate in the long-term follow-up study, CHAMPIONS,24,25 regardless of outcome or treatment during or after CHAMPS.24,25 CHAMPIONS24,25 began enrolling in February 2001 and continued for 10 years; the first data publication was in 2006 (ie, 5-year data).25 At the start of the extension study, patients receiving placebo started IM IFN-β-1a treatment.24,25 Brain parenchymal fraction (BPF), a normalized measure of brain volume, was calculated from the brain MRIs as previously described27 and measured at entry into the trial (baseline) and at 6 months, 1 year, and 18 months, as well as years 5 and 10. Information about the overall study design and number of measurements for MRI and clinical and radiological measurements are summarized in the eTable in the Supplement.
Serum samples were collected as part of CHAMPS23 at baseline before beginning treatment. Samples were stored at –70 °C until serum neurofilament light concentrations were determined. Eligibility for inclusion in our analysis was availability of a baseline serum sample (eTable in the Supplement).
Serum Neurofilament Light Assay
A sensitive single molecule array assay (SIMOA NF-light Advantage Kit; Quanterix) was used to measure serum neurofilament light levels in sera from participants enrolled in CHAMPS.23 The assay was analytically validated for the fit-for-purpose serum neurofilament light evaluation. The measurements were performed in 1 round of experiments using 1 batch of reagents.
Statistical Analysis
Descriptive statistics were used to summarize the demographic characteristics of participants with MS. Baseline serum neurofilament light concentrations were correlated with baseline MRI parameters, including number of GD+ lesions and T2 lesion volume, using Spearman rank correlation. Baseline serum neurofilament light concentrations were also correlated with BPF change at 5 and 10 years using Spearman rank correlation. Changes in BPF were calculated using the 6-month measurement as baseline to avoid artifactually low changes due to pseudoatrophy. Participants were split into tertiles based on their baseline serum neurofilament light concentration; intertertile differences in GD+ lesion count, T2 lesion volume, and BPF were calculated using analysis of variance. The prognostic value associated with serum neurofilament light was explored by comparing long-term BPF change and disability progression among the tertiles.
Multivariate regression models were used to explore whether baseline serum neurofilament light levels, as well as other baseline factors, might be associated with 5- and 10-year BPF changes. The full regression models included age, sex, log(baseline serum neurofilament light), baseline BPF, baseline EDSS, baseline GD+ lesion count, and log(baseline T2 lesion volume). The natural log was used as the base for logged values. A generalized linear regression model was used. In CHAMPS,23 there was no difference between delayed and immediate initiation of IFN-β-1a and change in BPF at 5 years; therefore, treatment was not included as a covariate in the model. Missing data were not imputed, and some covariates or outcomes had missing values. Notably, in the regression model, most of the CHAMPS23 participants were included and contributed to the final analysis.
The association between cumulative probability of progression to clinically definite MS in 10 years and baseline serum neurofilament light concentration tertiles was tested using a Cox proportional hazards model; the covariates in this model included age, sex, baseline EDSS score, and log(T2 volume baseline).
All analyses were performed using R statistical software (R Project for Statistical Computing) and SAS version 9.4 (SAS Institute) on an HP-UX platform (Hewlett Packard). GraphPad Prism software version 7 (GraphPad Software) was used for graphical representation. P values were 2-sided and assessed at a threshold of .05. Data were analyzed from 2017 to 2019.
Results
Overview of Participants Data Set
The 308 included participants with serum neurofilament light measurements at baseline (eTable in the Supplement) had a mean (SD) age of 33.2 (7.6) years, and 234 (76.0%) were women (Table 1). The demographic characteristics of the study population were similar to those recruited into CHAMPS,23 and the values represent a cohort with mild to moderate disease activity, as would be expected from patients experiencing an initial demyelinating event.
Table 1. Demographic and Baseline Characteristics for the Original CHAMPS Participants and the Subset With Baseline Serum Neurofilament Light Samples.
| Characteristic | Mean (SD) | |
|---|---|---|
| Participants in CHAMPS (n = 383) | Participants with baseline serum neurofilament light data (n = 308) | |
| Age, y | 33.0 (7.5) | 33.2 (7.6) |
| Female, No. (%) | 289 (75) | 234 (76) |
| EDSS score, median (range) | 2 (0-6.5) | 2 (0-6.5) |
| Brain parenchymal fraction | ||
| Baseline | 0.855 (0.01) | 0.855 (0.01) |
| Month 6 | 0.858 (0.01) | 0.858 (0.01) |
| T2 lesion volume, mm3 | 3433.6 (3923.3) | 3513.0 (4030.6) |
| Gd+ lesions, No. | 0.61 (1.3) | 0.65 (1.4) |
| Serum neurofilament light, pg/mL | NA | 24.0 (45.4) |
Abbreviations: CHAMPS, Controlled High-Risk Avonex Multiple Sclerosis Prevention Study; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; NA, not available.
Serum Neurofilament Light Concentrations and Baseline MRI Disease Burden
Confirming earlier findings, serum neurofilament light concentrations at baseline were associated with baseline number of GD+ lesions (Spearman r = 0.41; P < .001) and T2 lesion volume (Spearman r = 0.42; P < .001). When participants were stratified into tertiles based on baseline serum neurofilament light concentration, there was a difference between the lowest and highest tertiles with respect to the mean (SD) number of GD+ lesions (0.20 [0.63] vs 1.35 [2.04]; P < .001) and T2 lesion volume (1903.98 [2215.21] vs 5062.99 [4280.87]; P < .001) (eFigure 1 in the Supplement). Baseline serum neurofilament light levels were not correlated with baseline or 6-month BPF measurements.
Serum Neurofilament Light Concentrations and Long-term Brain Atrophy
The covariates associated with BPF change over 5 years were baseline serum neurofilament light (β = −0.3586 [95% CI, –0.5614 to –0.1558]; P < .001; R2 = 0.08) and baseline T2 lesion volume (β = −0.1577 [95% CI, –0.2855 to –0.0299]; P = .02; R2 = 0.03) (Table 2). The total variance explained by these baseline variables was 17% for associating with BPF variance at 5 years, and baseline serum neurofilament light represented the greater proportion (8%). From the model, the magnitude of the association suggests that a 2.7-fold increase in serum neurofilament light baseline levels would have an expected mean (SD) BPF change of –0.3586 (95% CI, –0.5614 to –0.1558) over 5 years. The association between baseline serum neurofilament light level with 5-year percent BPF change is shown in Figure 1A (Spearman r = –0.38; P < .001). When separated into tertiles, participants with the highest baseline serum neurofilament light concentrations had greater reductions in BPF at 5 years compared with those with the lowest baseline serum neurofilament light concentrations (BPF change, –1.83% [95% CI, –1.49% to –2.18%] vs –0.95% [95% CI, –0.78% to –1.12%]; P < .001) (Figure 1B). Similar findings were observed when baseline serum neurofilament light levels were stratified by previously proposed thresholds of 8 pg/mL and 16 pg/mL (eFigure 2 in the Supplement).28
Table 2. Multivariate Regression Models to Identify Risk Factors Associated With Brain Parenchymal Fraction Change.
| Follow-up | β (95% CI) | P value | Covariate R2 |
|---|---|---|---|
| 5 y (n = 117) | NA | NA | 0.17 |
| Intercept | 0.8045 (−0.1591 to 1.7682) | .10 | NA |
| log(baseline serum neurofilament light level) | −0.3586 (–0.5614 to –0.1558) | <.001 | 0.08 |
| log(T2 volume at baseline) | −0.1577 (–0.2855 to –0.0299) | .02 | 0.03 |
| 10 y (n = 65) | NA | NA | 0.17 |
| Intercept | −0.4096 (–1.5336 to 0.7145) | .48 | NA |
| log(baseline serum neurofilament light level) | −0.7902 (–1.2046 to –0.3758) | <.001 | NA |
Abbreviation: NA, not available.
Figure 1. Association of Baseline Serum Neurofilament Light With Brain Atrophy at Years 5 and 10.
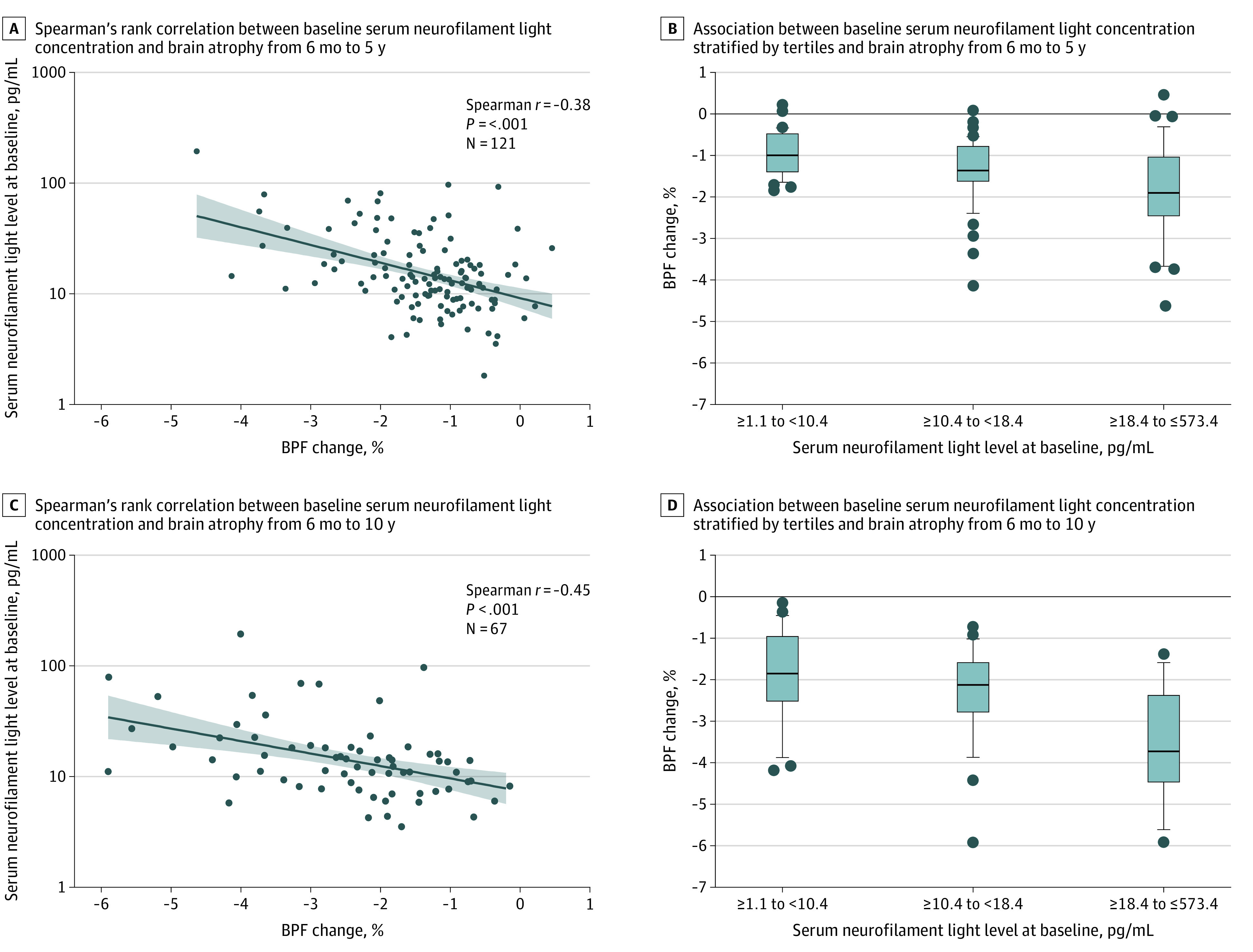
All brain parenchymal fraction (BPF) changes were calculated using the 6-month BPF measurement as baseline. A and C. Lines indicate linear fit to the observed data; shading, 95% CI for the estimated values from the linear fit; and dots, individual data points. B and D, lines indicate median; boxes, interquartile range; and dots, outliers.
When BPF change over 10 years was considered as an outcome, the only associated covariate was baseline serum neurofilament light level (β = −0.7902 [95% CI, –1.2046 to –0.3758]; P < .001) (Table 2). Owing to the smaller sample size at 10 years, extensive covariate adjustments in this analysis were not meaningful. Association between baseline serum neurofilament light concentration and BPF change over 10 years is shown in Figure 1C (Spearman r = –0.45; P < .001). The difference in 10-year BPF change between participants with the highest tertile serum neurofilament light levels and the lowest tertile serum neurofilament light levels was statistically significant (–3.54% [95% CI, –2.90% to –4.17%] vs –1.90% [95% CI, –1.43% to –2.37%]; P < .001) (Figure 1D). We found similar results when patients were grouped by baseline serum neurofilament light levels according to proposed thresholds of 8 pg/mL and 16 pg/mL (eFigure 2 in the Supplement). The nature of the initial qualifying event (ie, optic neuritis, spinal cord syndrome, or brainstem syndrome) was not associated with 5- or 10-year BPF change.
Serum Neurofilament Light Concentrations, Disease Activity, and Disability Progression
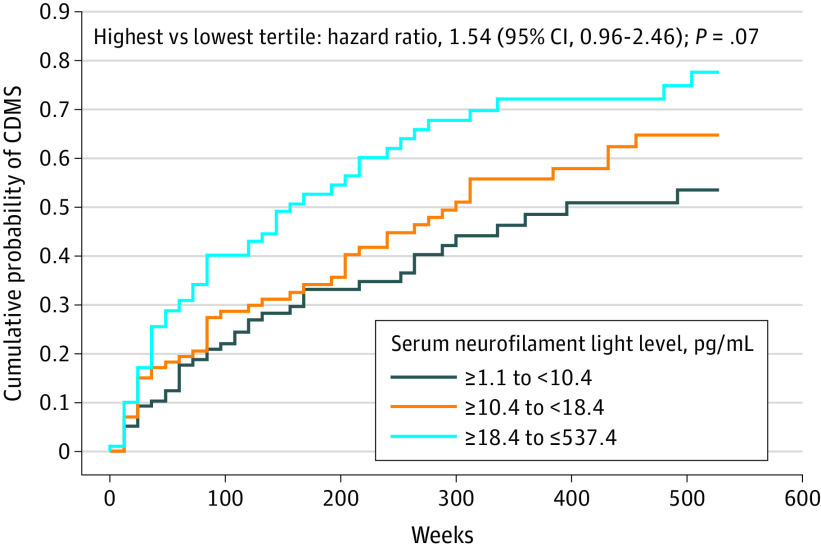
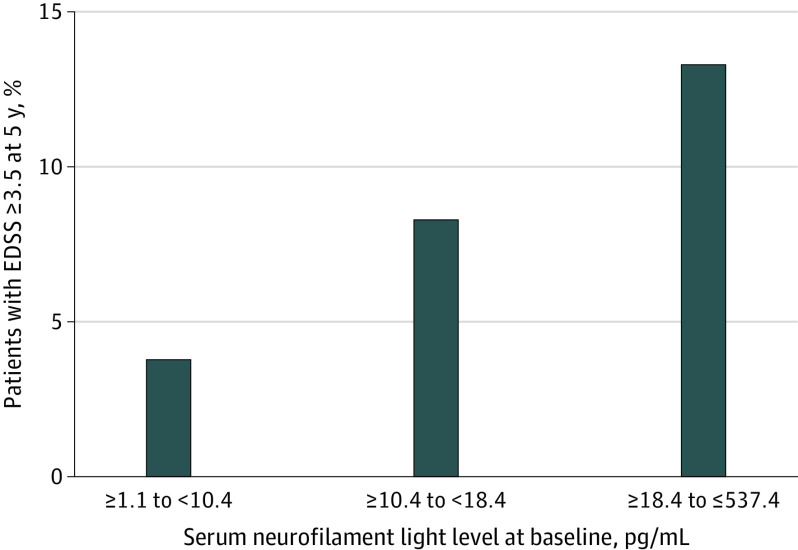
The association between baseline serum neurofilament light level and time to clinically definite MS was analyzed, adjusted for age, sex, baseline serum neurofilament light level (treated as a categorical variable based on tertile), baseline EDSS score, and T2 lesion volume. Compared with the lowest baseline serum neurofilament light tertile, there was no statistically significant difference in risk of clinically definite MS at 10 years among participants in the highest tertile (hazard ratio, 1.54 [95% CI, 0.96 to 2.46]; P = .07) (Figure 2). The estimated proportion of the study population with clinically definite MS at 10 years was 54% for the lowest tertile and 78% for the highest tertile of baseline serum neurofilament light concentration. Furthermore, at 5 years, 6 of 45 participants (13.3%) in the highest serum neurofilament light tertile and 2 of 52 participants (3.8%) in the lowest serum neurofilament light tertile reached an EDSS score of 3.5 or greater (Figure 3); however, the small numbers preclude statistical testing.
Figure 2. Association of Baseline Serum Neurofilament Light Concentration With Time to Clinically Definite Multiple Sclerosis (CDMS).
P value and hazard ratio based on stratified Cox proportional hazards model with adjustment for serum neurofilament light tertile at baseline, age, sex, Expanded Disability Status Scale score at baseline, and log(T2 volume at baseline).
Figure 3. Proportion of Patients Reaching Expanded Disability Status Scale (EDSS) Score 3.5 or Greater at 5 Years .
Data are shown for 157 patients who had both baseline serum neurofilament light level data and 5-year EDSS data.
Discussion
In this post hoc cohort study, we examined whether measurements of neurofilament light in serum samples from a large group of well-characterized patients with clinically isolated syndrome from CHAMPS23 were associated with long-term clinical and standardized MRI outcomes. Our findings support prior reports that showed cross-sectional correlations at baseline between serum neurofilament light concentration and GD+ lesion count or T2 lesion volume.7,11,29,30
Baseline serum neurofilament light levels at the time of the first demyelinating event were found to be associated with brain atrophy, as measured by BPF change over 5 and 10 years. Baseline T2 lesion volume was also associated with brain atrophy over 5 years. Therefore, whereas baseline T2 lesion volume is often associated with future inflammation,31 T2 lesions are nonspecific and may not reflect destructive pathogenesis. We speculate that the high serum neurofilament light levels reflect neuroaxonal damage associated with the inflammatory process, and that this explains the added value beyond T2 lesion volume alone.
Participants with low serum neurofilament light (ie, within lowest tertile, consistent with the serum neurofilament light levels observed in a healthy population32) at the time of the first demyelinating event had low rates of brain atrophy, with a mean BPF change of –0.95% (95% CI, –0.78% to –1.12%) over the next 5 years. In contrast, participants with high serum neurofilament light levels around the first demyelinating event had much greater brain atrophy over 5 years, with a mean BPF change of –1.83% (95% CI, –1.49% to –2.18%). Notably, in CHAMPS,23 all patients received high-dose methylprednisolone that suppressed baseline BPF.33 Indeed, 72% of the patients exhibited a mean increase in BPF of 0.6% from baseline to month 6. Therefore, we used the 6-month BPF measurement for the baseline when calculating 5- and 10-year changes.
Results from this study suggest that serum neurofilament light level may be an important component of a multidimensional measure to estimate prognosis and stratify risk of severe disease. This is important clinically because better risk stratification would facilitate the selection of patients for early aggressive therapy. High neurofilament light levels during the first demyelinating event indicate a large amount of neuroaxonal damage associated with the immune attack, and are hypothesized to be indicative of the strength of the immune attack, resilience of neuronal cells to that attack, and as a result, overall disease severity in patients.13 In light of this, serum neurofilament light level could represent a useful indicator associated with disease severity for those patients with MS presenting with low MRI-apparent disease activity. Other studies have investigated the value of serum neurofilament light concentration for estimating short-term disease activity. Those studies demonstrated that higher serum neurofilament light concentrations were associated with a greater risk of enhancing lesions,11,14 clinical disease activity or progression,14 and brain12,14 and spinal cord atrophy.12 A 2018 longitudinal study in a small cohort of patients with RRMS34 found that a cutoff of 14.2 ng/L serum neurofilament light concentration was associated with no evidence of disease activity at 1, 2, and 4 years, with high sensitivity but low specificity. Although they provide important information, those studies have been mainly based on relatively small cross-sectional and short-term studies, did not have serum neurofilament light measurements early in the disease course, or lacked quantitative MRI assessments. In contrast, our study included a relatively large number of well-characterized patients with standardized clinical and radiological assessments early in the disease and throughout the long-term follow-up.
Despite the growing body of evidence supporting the hypothesis that high serum neurofilament light concentrations are associated with worse short- and long-term prognoses in patients with MS, a number of factors need to be considered before serum neurofilament light measurements can be incorporated into clinical practice. First, the data should be reproduced in well-characterized real-world cohorts. Second, robust cutoff values for serum neurofilament light should be established for MS. In this study, we constructed baseline serum neurofilament light tertiles and the thresholds were approximately 10 pg/mL for the lower bound and 18 pg/mL for the upper bound; other studies have suggested thresholds of 8 pg/mL for low and 16 pg/mL for high.28,35 Third, although an association with age and increasing serum neurofilament light concentration has been identified in healthy cohorts,11,35 this association does not seem to be as clear in disease populations. Therefore, the need for an approach to correct for age or other covariates (eg, comorbidities) when considering serum neurofilament light levels in the MS population remains to be established. However, in this study, the population was relatively young; therefore, the association of age with serum neurofilament light levels is expected to have been small. It has been shown in a cohort of healthy individuals that serum neurofilament light levels remain relatively stable until age 40 or 45 years, at which time they begin to steadily increase.35 Finally, development of a robust, validated, and broadly implementable assay to enable integration of serum neurofilament light measurement into clinical practice is essential.
Limitations
This study has some limitations. Although our study sample was of relatively large size, this was a retrospective analysis. Additionally, not all MRI and clinical measures were available for all patients at all time points, especially during the long-term follow-up; this long follow-up period (10 years) could create the potential for ascertainment bias because more than half of the original patients were lost to follow-up. Furthermore, data were derived from patients recruited to a randomized clinical trial and therefore may not be representative of real-world populations. Another potential limitation of this study is the routine use of methylprednisolone prior to entry into CHAMPS23; however, published data showed no effect of methylprednisolone on CSF neurofilament light levels.36
Conclusions
This post hoc cohort study found that serum neurofilament light concentrations measured during the first demyelinating event were associated with contemporaneous radiological measures of disease activity and were associated with long-term (up to 10 years) brain atrophy. These observations lend further support to the view that serum neurofilament light represents a promising potential biomarker for disease severity stratification in early MS, which may help to guide treatment decisions. Repeating this analysis using samples from real-world studies and publicly available repositories would help to validate these findings. Importantly, it remains to be demonstrated that lowering serum neurofilament light levels results in reduced disease progression in later years. If that were the case, serum neurofilament light could be used as a prognostic marker and a treatment-response marker. Such a finding would require a prospective clinical study.
In a data set that included 10 years of follow-up, serum neurofilament light represents a promising biomarker associated with prognosis in early MS. If validated in prospective studies, it could potentially be used alongside clinical and radiological assessments for prognosticating disease in routine clinical practice, thereby facilitating the selection of appropriate treatment and potentially leading to better outcomes in patients with MS.
eFigure 1. Association of Baseline Serum Neurofilament Light Concentrations With Baseline Magnetic Resonance Imaging Measurements of Disease Burden
eFigure 2. Association of Baseline Serum Neurofilament Light Concentration With Brain Atrophy
eTable. Data Points Available for the CHAMPS and CHAMPIONS Studies
References
- 1.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi: 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eran A, García M, Malouf R, et al. MRI in predicting conversion to multiple sclerosis within 1 year. Brain Behav. 2018;8(9):e01042. doi: 10.1002/brb3.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808-817. doi: 10.1093/brain/awm329 [DOI] [PubMed] [Google Scholar]
- 5.Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol. 2013;12(7):669-676. doi: 10.1016/S1474-4422(13)70103-0 [DOI] [PubMed] [Google Scholar]
- 6.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 7.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550-1559. doi: 10.1177/1352458515623365 [DOI] [PubMed] [Google Scholar]
- 8.Zetterberg H, Skillbäck T, Mattsson N, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73(1):60-67. doi: 10.1001/jamaneurol.2015.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79(1):152-158. doi: 10.1002/ana.24552 [DOI] [PubMed] [Google Scholar]
- 10.Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788-1794. doi: 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 13.Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology. 2019;92(7):e733-e741. doi: 10.1212/WNL.0000000000006902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88(9):826-831. doi: 10.1212/WNL.0000000000003653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhan A, Jacobsen C, Myhr KM, Dalen I, Lode K, Farbu E. Neurofilaments and 10-year follow-up in multiple sclerosis. Mult Scler. 2018;24(10):1301-1307. doi: 10.1177/1352458518782005 [DOI] [PubMed] [Google Scholar]
- 16.Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2019;77(1):58-64. doi: 10.1001/jamaneurol.2019.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359-1366. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5(12):1478-1491. doi: 10.1002/acn3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Håkansson I, Tisell A, Cassel P, et al. Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol. 2017;24(5):703-712. doi: 10.1111/ene.13274 [DOI] [PubMed] [Google Scholar]
- 20.Jakimovski D, Kuhle J, Ramanathan M, et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol. 2019;6(9):1757-1770. doi: 10.1002/acn3.50872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellebjerg F, Royen L, Soelberg Sørensen P, Oturai AB, Jensen PEH. Prognostic value of cerebrospinal fluid neurofilament light chain and chitinase-3-like-1 in newly diagnosed patients with multiple sclerosis. Mult Scler. 2019;25(11):1444-1451. doi: 10.1177/1352458518794308 [DOI] [PubMed] [Google Scholar]
- 22.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 23.Jacobs LD, Beck RW, Simon JH, et al. ; CHAMPS Study Group . Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343(13):898-904. doi: 10.1056/NEJM200009283431301 [DOI] [PubMed] [Google Scholar]
- 24.Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O’Connor PW, Simon JH; Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance Investigators . Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol. 2012;69(2):183-190. doi: 10.1001/archneurol.2011.1426 [DOI] [PubMed] [Google Scholar]
- 25.Kinkel RP, Kollman C, O’Connor P, et al. ; CHAMPIONS Study Group . IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006;66(5):678-684. doi: 10.1212/01.wnl.0000200778.65597.ae [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Fisher E, Rudick RA, Cutter G, et al. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler. 2000;6(6):373-377. doi: 10.1177/135245850000600602 [DOI] [PubMed] [Google Scholar]
- 28.Calabresi PA, Arnold DL, Kinkel RP, et al. Serum neurofilament light (NfL): towards a blood test for prognosis and disease/treatment monitoring in multiple sclerosis patients. Neurology. 2018;90(15)(suppl):S24.003. [Google Scholar]
- 29.Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siller N, Kuhle J, Muthuraman M, et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler. 2019;25(5):678-686. doi: 10.1177/1352458518765666 [DOI] [PubMed] [Google Scholar]
- 31.Kaunzner UW, Gauthier SA. MRI in the assessment and monitoring of multiple sclerosis: an update on best practice. Ther Adv Neurol Disord. 2017;10(6):247-261. doi: 10.1177/1756285617708911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hviid CVB, Knudsen CS, Parkner T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest. 2020;1-5:1-5. doi: 10.1080/00365513.2020.1730434 [DOI] [PubMed] [Google Scholar]
- 33.Rao AB, Richert N, Howard T, et al. Methylprednisolone effect on brain volume and enhancing lesions in MS before and during IFNβ-1b. Neurology. 2002;59(5):688-694. doi: 10.1212/WNL.59.5.688 [DOI] [PubMed] [Google Scholar]
- 34.Håkansson I, Tisell A, Cassel P, et al. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflammation. 2018;15(1):209. doi: 10.1186/s12974-018-1249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabresi PA, Kuhle J, Arnold DL, et al. Serum neurofilament light (sNfL) for disease prognosis and treatment monitoring in multiple sclerosis patients:towards implementation into clinical care. Neurology. 2019;92(15 Suppl):S26.001. [Google Scholar]
- 36.Danielson M, Reinsfelt B, Westerlind A, Zetterberg H, Blennow K, Ricksten SE. Effects of methylprednisolone on blood-brain barrier and cerebral inflammation in cardiac surgery—a randomized trial. J Neuroinflammation. 2018;15(1):283. doi: 10.1186/s12974-018-1318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Association of Baseline Serum Neurofilament Light Concentrations With Baseline Magnetic Resonance Imaging Measurements of Disease Burden
eFigure 2. Association of Baseline Serum Neurofilament Light Concentration With Brain Atrophy
eTable. Data Points Available for the CHAMPS and CHAMPIONS Studies