Key Points
Question
What are the regional and nationwide endogenous endophthalmitis hospitalization trends in the United States from 2003 to 2016?
Findings
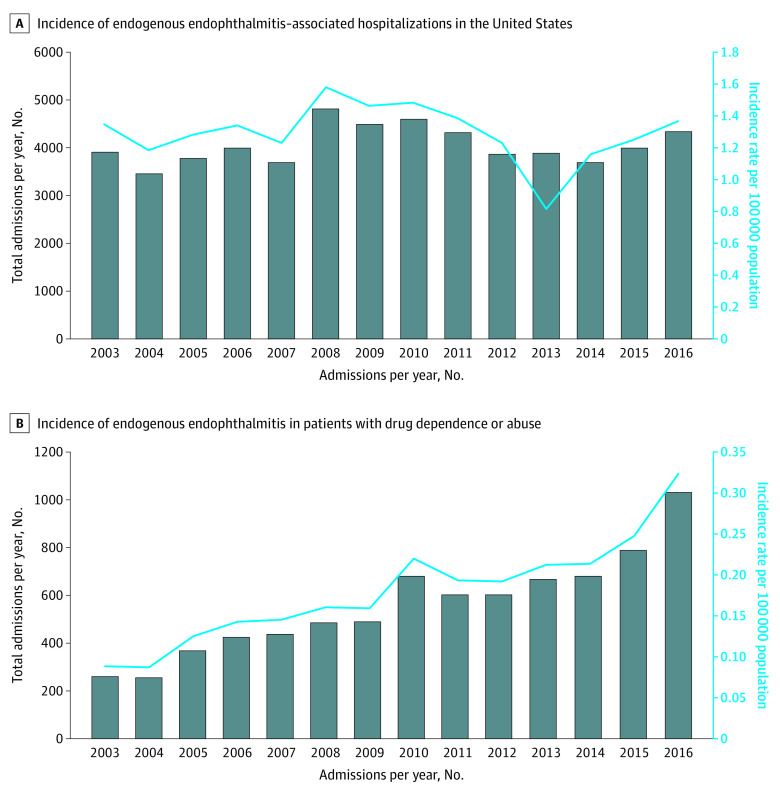
In this cross-sectional study, a 4-fold increase in hospital admissions for endogenous endophthalmitis, from 0.08 to 0.32 in 100 000 among patients with a history of drug dependence or use, was observed in the National Inpatient Sample from 2003 to 2016. The incidence of hospitalization for endogenous endophthalmitis during the same period remained stable, costing approximately $134 million.
Meaning
According to this study, patients with a history of drug use or dependence account for an increasing proportion of endogenous endophthalmitis cases in the United States.
Abstract
Importance
Complications arising from the nationwide opioid epidemic led to an increase in health care use. Few studies have investigated whether this is reflected in hospital admissions for endogenous endophthalmitis.
Objective
To report changing trends in epidemiology, risk factors, hospital course, and costs associated with drug use–related endogenous endophthalmitis hospitalizations in the United States from 2003 to 2016.
Design, Setting, and Participants
Nationwide, retrospective cross-sectional study using the National Inpatient Sample. A total of 56 839 patients admitted with a diagnosis of endogenous endophthalmitis were included. Data were analyzed between 2003 and 2016.
Exposures
Inpatient admission for endogenous endophthalmitis during the years 2003 to 2016.
Main Outcomes and Measures
The Nationwide Inpatient Sample was queried to identify all inpatient admissions with a diagnosis of endogenous endophthalmitis in the United States between the years 2003 and 2016. Analyses were performed to identify national and regional trends in incidence and prevalence of associated infectious and noninfectious comorbidities in patients with or without a history of drug dependence or use. Median and cumulative inflation-adjusted costs for admissions were calculated.
Results
Of all patients, 55.6% were White, 13.6% were Black, and 10.6% were Hispanic. There were an estimated 56 839 endogenous endophthalmitis–related hospitalizations; 13.7% of these patients (n = 7783) had a history of drug dependence or use. The drug-using population was significantly younger (49.6 vs 57.5 years; difference, 7.9; 95% CI, 6.93-8.88; P < .001) and more likely to be male (61.8% [n = 35 127] vs 49.0% [n = 21 712]; difference, 12.8%; 95% CI, 11.6%-14.0%; P < .001). The incidence of endogenous endophthalmitis associated with drug dependence or use increased from 0.08 per 100 000 in 2003 to 0.32 per 100 000 population in 2016 across all 4 US geographic regions.
Conclusions and Relevance
A 4-fold increase in drug use–related endogenous endophthalmitis hospitalizations was observed in the United States from 2003 to 2016, resulting in substantial health care use burden. These findings support the hypothesis that clinicians should maintain a high index of suspicion for endophthalmitis when evaluating patients with intraocular inflammation in the setting of drug dependence or use.
This study investigates changing trends in epidemiology, risk factors, hospital course, and costs associated with drug use–related endogenous endophthalmitis hospitalizations in the United States from 2003 to 2016.
Introduction
Endogenous endophthalmitis is a vision-threatening intraocular infection caused by hematogenous spread of pathogens to the retina and choroid, with eventual involvement of the vitreous.1 It is a rare condition and accounts for 2% to 15% of all cases of endophthalmitis.2,3,4 Despite advances in antibiotic therapy, the associated visual outcomes remain poor.2,3,5,6,7,8,9,10,11 Most patients have underlying risk factors for hematogenous infections, which in turn predispose to intraocular infection. Common systemic risk factors include diabetes, intravenous (IV) drug use, immunosuppression, and malignancy.2
Because the United States continues to be in the throes of an opioid crisis, IV drug use has evolved as an important risk factor for endogenous endophthalmitis, with studies reporting an increasing number of IV drug use–associated endophthalmitis cases.12,13,14 However, data presented in these studies are limited to a few institutions and geographic regions of the United States. There is an emerging need for physicians and public health professionals to recognize the nationwide burden of endogenous endophthalmitis among the drug-using US population, which will best prepare them for the ensuing rise in health care morbidity. This report represents a nationwide study of endogenous endophthalmitis–related hospitalizations in the United States during a 13-year study period (2003-2016). It aims to investigate national and regional trends in incidence of endophthalmitis-related hospitalizations, particularly among the drug-using population; comparison of infectious and noninfectious comorbidities in individuals with or without drug dependence or use; burden and economic costs associated with endogenous endophthalmitis–related inpatient admissions; and in-hospital mortality.
Methods
Data Source and Study Population
The Nationwide Inpatient Sample (NIS) was queried for the years 2003 to 2016 to identify all patients admitted with a diagnosis of endogenous endophthalmitis. The NIS is part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ) and is the largest all-payer inpatient database in the United States. Sampling strata used by the NIS is based on hospital characteristics (eg, urban or rural location, teaching status, and hospital bed size). The data can be weighted according to the NIS sampling frame to generate national estimates.15 Analyses discussed and results represent NIS-weighted national estimates. The sampling strategy of the NIS changed in 2012, prior to which the database was composed of a 20% stratified systematic sample of all US community hospitals, with all discharges retained from those hospitals. In 2012, in an effort to yield more precise estimates, the database was redesigned to include a 20% stratified sample of discharges from all hospitals in the HCUP. The NIS contains data for approximately 7 million hospital discharges per year from 44 participating states, representing 98% of the US population. Estimates were generated using online tools that are publicly available on the HCUP website. The institutional review board at the West Virginia University granted this study exempt status. All data are publicly available and do not include patient identifiers; therefore, patient consent was not obtained. This study was conducted in adherence to the Declaration of Helsinki, HCUP regulatory privacy protections, and US federal and state laws.
Patients with a diagnosis of endophthalmitis, associated systemic risk factors including drug dependence or use, and infectious etiologies were identified using relevant International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. All patients with postsurgical and posttraumatic etiologies likely representing exogenous endophthalmitis were excluded. Hospital charges were converted to costs using the cost-to-charge ratio. Median cost per hospitalization and total costs per year were calculated and adjusted for inflation using the Consumer Price Index for Hospital Services from the US Bureau of Labor Statistics.16
Statistical Analysis
Descriptive statistics were presented as frequencies with percentages for categorical variables, and mean with standard error of mean (SEM) for continuous variables. Comparisons between groups were made using the independent-samples t test and Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. All P values were nominal and 2-sided. P values of .05 or less were considered significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Demographics and Baseline Characteristics
Demographics and baseline characteristics are described in Table 1. From 2003 to 2016, there were an estimated 56 839 endogenous endophthalmitis–related hospitalizations in the United States; 13.7% of these patients (n = 7783) had a history of drug dependence or use. The mean (SD) age was 56.4 (0.25) years; this was lower in the drug-using population (49.6 years vs 57.5 years; difference 7.9; 95% CI, 6.93-8.88; P < .001), with most such cases presenting in the fifth decade of life. Fifty percent of the population (n = 28 420) were men, with a higher proportion of men in the drug-using population: 61.8% (n = 35 127) vs 49.0% (n = 27 851); difference, 12.8%; 95% CI, 11.6%-14.0%; P < .001. Most patients were White (55.6%; n = 31 602), followed by African American (13.6%; n = 7730) and Hispanic (10.6%; n = 6025). The highest frequency of admissions were from the South (38.8%; n = 22 054), followed by Midwest (22.3%; n = 34 729), West (20.2%; n = 11 481), and Northeast (18.7%; n = 10 629). Most patients were publicly insured through Medicare or Medicaid (69.4%; n = 39 446), followed by private insurance (20.9%; n = 11 879) and self-pay (5.2%; n = 2956). The drug-using population had a higher proportion of self-payers (13.5% [n = 7673] vs 3.9% [n = 2216]), and most frequently belonged to the lowest income quartile (37.4% [n = 21 258]; 0-25th median income quartile). Most admissions were in urban teaching hospitals (61.6%; n = 35 012) and hospitals with a large bed size (64.2%; n = 36 491).
Table 1. Sociodemographic and Baseline Characteristics.
| Demographic characteristic | Patients with or without history of drug dependence or use | |||
|---|---|---|---|---|
| No. (%) | Raw difference (95% CI) | |||
| All patients with endogenous endophthalmitis (n = 56 839) | History of drug dependence or use | |||
| No (n = 49 056) | Yes (n = 7783) | |||
| Age, mean (SEM), y | 56.4 (0.25) | 57.5 (0.28) | 49.6 (0.41) | 7.9 (6.93 to 8.88) |
| Median | 62 | 65 | 50 | |
| Age, y | ||||
| 0-19 | 7468 (13.2) | 7371 (15.0) | 97 (1.3) | NA |
| 20-29 | 2276 (4.0) | 1336 (2.7) | 939 (12.1) | NA |
| 30-39 | 3448 (6.1) | 2313 (4.7) | 1134 (14.6) | NA |
| 40-49 | 5290 (9.3) | 3690 (7.5) | 1600 (20.6) | NA |
| 50-59 | 7762 (13.7) | 5834 (11.9) | 1928 (24.8) | NA |
| 60-69 | 8573 (15.1) | 7400 (15.1) | 1173 (15.1) | NA |
| 70-79 | 9030 (15.9) | 8422 (17.2) | 608 (7.8) | NA |
| ≥80 | 12 934 (22.8) | 12 631 (25.8) | 303 (3.9) | NA |
| Male | 28 793 (50.7) | 23 989 (49.0) | 4805 (61.8) | −12.8 (−14.0 to −11.6) |
| Race/ethnicity | ||||
| White | 31 588 (55.6) | 26 790 (54.6) | 4798 (61.7) | −7.1 (−8.2 to −5.9) |
| Black | 7731 (13.6) | 6530 (13.3) | 1201 (15.4) | −2.1 (−3.0 to −1.3) |
| Hispanic | 6006 (10.6) | 5283 (10.8) | 723 (9.3) | 1.5 (0.8 to 2.2) |
| Others | 3429 (6.0) | 3152 (6.4) | 277 (3.6) | 2.9 (2.4 to 3.3) |
| Unknown | 8086 (14.2) | 7302 (14.9) | 784 (10.1) | 4.8 (4.1 to 5.6) |
| Primary expected payer | ||||
| Medicare and Medicaid | 39 453 (69.4) | 34 692 (70.7) | 4761 (61.2) | 9.6 (8.4 to 10.7) |
| Private insurance | 11 893 (20.9) | 10 540 (21.5) | 1354 (17.4) | 4.1 (3.2 to 5.0) |
| Self-pay | 2978 (5.2) | 1928 (3.9) | 1051 (13.5) | −9.6 (−10.4 to −8.8) |
| No charge | 341 (0.6) | 212 (0.4) | 129 (1.7) | −1.2 (−1.5 to −0.9) |
| Others | 2174 (3.8) | 1685 (3.4) | 489 (6.3) | −2.9 (−3.4 to −2.3) |
| Region | ||||
| Northeast | 10 641 (18.7) | 9189 (18.7) | 1452 (18.7) | 0.1 (−0.9 to 1.0) |
| Midwest | 12 662 (22.3) | 10 990 (22.4) | 1673 (21.5) | 0.9 (−0.1 to 1.9) |
| South | 22 057 (38.8) | 18 965 (38.7) | 3093 (39.7) | −1.1 (−2.3 to 0.1) |
| West | 11 478 (20.2) | 9913 (20.2) | 1565 (20.1) | 0.1 (−0.9 to 1.1) |
| Median income quartile | ||||
| 0-25th | 18 088 (31.8) | 15 181 (30.9) | 2907 (37.4) | −6.4 (−7.6 to −5.3) |
| 26th- 50th | 14 196 (25.0) | 12 328 (25.1) | 1868 (24.0) | 1.1 (0.1 to 2.2) |
| 51st -75th | 12 405 (21.8) | 10 762 (21.9) | 1644 (21.1) | 0.8 (−0.2 to 1.8) |
| 76th-100th | 10 579 (18.6) | 9583 (19.5) | 995 (12.8) | 6.8 (5.9 to 7.6) |
| Missing | 1572 (2.8) | 1203 (2.5) | 369 (4.7) | −2.3 (−2.8 to −1.8) |
| Disposition of patient | ||||
| Home or self-care | 30 152 (53.0) | 25 383 (51.7) | 4769 (61.3) | −9.5 (−10.7 to −8.4) |
| Transfer to short-term hospital | 4063 (7.1) | 3571 (7.3) | 492 (6.3) | 1.0 (0.4 to 1.5) |
| Skilled nursing or intermediate care facility | 13 608 (23.9) | 12 333 (25.1) | 1275 (16.4) | 8.8 (7.9 to 9.7) |
| Home health care | 7495 (13.2) | 6663 (13.6) | 832 (10.7) | 2.9 (2.1 to 3.6) |
| Against medical advice | 694 (1.2) | 341 (0.7) | 353 (4.5) | −3.8 (−4.3 to −3.4) |
| Others | 827 (1.5) | 766 (1.6) | 61 (0.8) | 0.8 (0.6 to 1.0) |
| Bed size of hospital | ||||
| Small | 7149 (12.6) | 6381 (13.0) | 768 (9.9) | 3.1 (2.4 to 3.9) |
| Medium | 12 891 (22.7) | 11 108 (22.6) | 1783 (22.9) | −0.3 (−1.3 to 0.7) |
| Large | 36 476 (64.2) | 31 294 (63.8) | 5182 (66.6) | −2.8 (−3.9 to −1.7) |
| Location and teaching status of hospital | ||||
| Rural | 5172 (9.1) | 4623 (9.4) | 549 (7.1) | 2.4 (1.8 to 3.0) |
| Urban nonteaching | 16 310 (28.7) | 14 417 (29.4) | 1893 (24.3) | 5.1 (4.0 to 6.1) |
| Urban teaching | 35 034 (61.6) | 29 743 (60.6) | 5291 (68.0) | −7.4 (−8.5 to −6.2) |
| Length of hospital stay, d | ||||
| Median | 5 | 5 | 5 | 1.42 |
| Mean (SEM) | 10.2 (0.16) | 10.4 (0.18) | 9.0 (0.31) | (0.72 to 2.13) |
Abbreviation: NA, not applicable.
National and Regional Trends in the Incidence of Endogenous Endophthalmitis–Related Hospitalizations
The total incidence of endogenous endophthalmitis–related hospitalizations in the United States was 1.3 per 100 000 population between 2003 and 2016. The incidence peaked in 2008 (1.6 per 100 000 population) and declined to a low in 2013 (0.8 per 100 000 population) before increasing again through 2016 (Figure 1A). The incidence of endogenous endophthalmitis–associated with drug dependence or use increased 4-fold from 0.08 per 100 000 in 2003 to the highest incidence in 2016 (0.32 per 100 000 population; Figure 1B). Although the regional incidence of endogenous endophthalmitis–related hospitalizations has remained stable (eFigure, A in the Supplement), incidence of drug use–associated endophthalmitis has increased in all 4 geographic regions over the study period (eFigure, B in the Supplement).
Figure 1. Incidence of Endogenous Endophthalmitis–Related Hospitalizations.
A, Incidence of endogenous endophthalmitis–related hospitalizations in the United States. B, Incidence of endogenous endophthalmitis in patients with drug dependence or abuse.
Prevalence of Comorbid Infectious Conditions
Approximately 17.7% of patients (n = 10 061) had a diagnosis of bacteremia (3.3%; n = 1876), fungemia (3.1%; n = 1762), or sepsis (11.3%; n = 6423). Prevalence of bacteremia (4.1% [n = 2330] vs 3.2% [n = 1819]; difference, 0.9%; 95% CI, 0.4%-1.3%; P < .001) and fungemia (4.6% [n = 2615] vs 2.9% [n = 1648]; difference, 1.7%; 95% CI, 1.3%-2.3%; P < .001) was higher in the drug-using population; however, the rates of sepsis were comparable. Urinary tract infection was the most common infectious comorbidity present in 13.4% of cases (n = 7616), followed by pneumonia (10.7%; n = 6082), skin and soft tissue infections (9.4%; n = 5343), Candida infection (6.6%; n = 3751) with disseminated candidiasis in 1.4% (n = 796), gastrointestinal infection (4.7%; n = 2671), orbital cellulitis and osteomyelitis (4.5%; n = 2558), infective endocarditis (3.7%; n = 2103), osteomyelitis (2.0%; n = 1137), intracranial and intraspinal abscess (0.9%; n = 512), meningitis (0.7%; n = 398), peritoneal and retroperitoneal infections (0.8%; n = 455), aspergillus infection (0.4%; n = 227), and hepatic abscess (0.3%; n = 171).
The incidence of Candida infection (9.7% vs 6.1%; difference, 3.6%; 95% CI, 2.9%-4.3%; P < .001), osteomyelitis (2.6% vs 2.0%; difference, 0.6%; 95% CI, 0.3%-1.1%; P < .001), infective endocarditis (5.8% vs 3.4%; difference, 2.4%; 95% CI, 1.9%-3.0%; P < .001), intracranial and intraspinal abscess (1.5% vs 0.8%; difference, 0.7%; 95% CI, 0.4%-1.0%; P < .001), meningitis (1.1% vs 0.7%; difference, 0.4%; 95% CI, 0.2%-0.7%; P < .001), skin and soft tissue infections (11.9% vs 9.0%; difference, 2.9%; 95% CI, 2.1%-3.7%; P < .001), and gastrointestinal infections (5.1% vs 4.6%; difference, 0.5%; 95% CI, 0.1%-1.0%; P < .001) was higher in the drug-using population, while the incidence of urinary tract infections (9.0% vs 14.1%; difference, 5.1%; 95% CI, 4.4%-5.8%; P < .001), septic arthritis (0.7% vs 1.2%; difference, 0.5%; 95% CI, 0.3%-0.7%; P < .001), and pneumonia (7.8% vs 11.1%; difference, 3.3%; 95% CI, 2.6%-3.9%; P < .001) was lower.
The prevalence of hepatitis C (11.3% vs 1.6%; difference, 9.7%; 95% CI, 9.0%-10.4%; P < .001), hepatitis B (1.1% vs 0.4%; difference, 0.7%; 95% CI, 0.4%-0.9%; P < .001), and HIV (3.5% vs 0.9%; difference, 2.6%; 95% CI, 2.3%-3.1%; P < .001) were higher in the drug-using population.
Prevalence of Systemic Comorbidities
The most common systemic risk factor associated with endogenous endophthalmitis was diabetes (27.6%; n = 15 688), followed by malignancy (19.2%; 10 913), chronic kidney disease (CKD; 14.2%; n = 8071), drug dependence or use (13.7%; n = 7787), chronic obstructive pulmonary disease (COPD)/asthma (10.1%; n = 5741), immunodeficiency diseases (2.0%; n = 1137), organ or tissue transplant (1.4%; n = 796), liver cirrhosis (1.9%; n = 1080), and neutropenia (1.2%; n = 682). A comparison of systemic risk factors in patients with or without a history of drug dependence or use is presented for infectious conditions in Table 2 and noninfectious conditions in Table 3. The prevalence of immunodeficiency diseases (4.3% vs 2.8%; P < .001), COPD/asthma (15.3% vs 9.7%; P < .001), and liver cirrhosis (3.3% vs 1.7%; P < .001) was higher in the drug-using population, while the incidence of diabetes (22.2% vs 28.5%; P < .001), CKD (9.0% vs 15.0%; P < .001), malignancy (15.4% vs 19.9%; P < .001), organ and tissue transplant (0.9% vs 1.5%; P < .001), and neutropenia (0.5% vs 1.3%; P < .001) was lower.
Table 2. Prevalence of Comorbid Infectious Conditions in Patients With Endogenous Endophthalmitis.
| Comorbid infectious conditions | Patients with or without history of drug dependence or use | ||||
|---|---|---|---|---|---|
| No. (%) | P value | Raw difference (95% CI) | |||
| All patients with endogenous endophthalmitis (n = 56 839) | History of drug dependence or use | ||||
| No (n = 49 056) | Yes (n = 7783) | ||||
| Fungemia | 1760 (3.1) | 1399 (2.9) | 361 (4.6) | <.001 | −1.8 (−2.3 to −1.3) |
| Candida infection | 3792 (6.7) | 3030 (6.2) | 762 (9.8) | <.001 | −3.6 (−4.3 to −2.9) |
| Disseminated candidiasis | 783 (1.4) | 641 (1.3) | 142 (1.8) | <.001 | −0.5 (−0.8 to −0.2) |
| Aspergillus infection | 252 (0.4) | 223 (0.5) | 29 (0.4) | .31 | 0.1 (−0.1 to 0.2) |
| Bacteremia | 1890 (3.3) | 1573 (3.2) | 317 (4.1) | <.001 | −0.9 (−1.3 to −0.4) |
| Sepsis | 6423 (11.3) | 5577 (11.4) | 846 (10.9) | .20 | 0.6 (−0.3 to 1.3) |
| Osteomyelitis | 1163 (2.0) | 957 (2) | 206 (2.6) | <.001 | −0.7 (−1.1 to −0.3) |
| Infective endocarditis | 2102 (3.7) | 1651 (3.4) | 451 (5.8) | <.001 | −2.4 (−3.0 to −1.9) |
| Hepatic abscess | 184 (0.3) | 174 (0.4) | 10 (0.1) | .001 | 0.2 (0.1 to 0.3) |
| Urinary tract infection | 7628 (13.4) | 6926 (14.1) | 702 (9) | <.001 | 5.1 (4.4 to 5.8) |
| Intracranial and intraspinal abscess | 509 (0.9) | 392 (0.8) | 117 (1.5) | <.001 | −0.7 (−1.0 to −0.4) |
| Meningitis | 422 (0.7) | 333 (0.7) | 89 (1.1) | <.001 | −0.5 (−0.7 to −0.2) |
| Skin/soft tissue infection | 5361 (9.4) | 4433 (9) | 928 (11.9) | <.001 | −2.9 (−3.7 to −2.1) |
| Septic arthritis | 640 (1.1) | 586 (1.2) | 54 (0.7) | <.001 | 0.6 (0.3 to 0.7) |
| Pneumonia | 6164 (10.8) | 5540 (11.3) | 624 (8) | <.001 | 3.3 (2.6 to 3.9) |
| Gastrointestinal infection | 2677 (4.7) | 2280 (4.6) | 397 (5.1) | .08 | −0.5 (−1.0 to 0.1) |
| Peritoneal and retroperitoneal infections | 442 (0.8) | 369 (0.8) | 73 (0.9) | .08 | −0.2 (−0.4 to 0) |
| Orbital cellulitis and osteomyelitis | 2566 (4.5) | 2226 (4.5) | 340 (4.4) | .50 | 0.2 (−0.3 to 0.7) |
| Hepatitis | |||||
| Type B | 282 (0.5) | 198 (0.4) | 84 (1.1) | <.001 | −0.7 (−0.9 to −0.4) |
| Type C | 1669 (2.9) | 790 (1.6) | 879 (11.3) | <.001 | −9.7 (−10.4 to −9.0) |
| HIV infection | 750 (1.3) | 460 (0.9) | 290 (3.7) | <.001 | −2.8 (−3.2 to −2.4) |
Table 3. Prevalence of Comorbid Noninfectious Conditions in Patients With Endogenous Endophthalmitis.
| Comorbid noninfectious conditions | Patients with or without history of drug dependence or use | Raw difference (95% CI) | |||
|---|---|---|---|---|---|
| No. (%) | P value | ||||
| All patients with endogenous endophthalmitis (n = 56 839) | History of drug dependence or use | ||||
| No (n = 49 056) | Yes (n = 7783) | ||||
| Diabetes mellitus | 15 690 (27.6) | 13 958 (28.5) | 1732 (22.3) | <.001 | 6.2 (5.2 to 7.2) |
| Chronic kidney disease | 8058 (14.2) | 7354 (15.0) | 704 (9.0) | <.001 | 6.0 (5.2 to 6.7) |
| Malignancy | 10 938 (19.2) | 9740 (19.9) | 1198 (15.4) | <.001 | 4.5 (3.6 to 5.3) |
| Organ or tissue transplant | 1152 (2.0) | 1039 (2.1) | 113 (1.5) | <.001 | 0.7 (0.4 to 1.0) |
| Neutropenia | 691 (1.2) | 653 (1.3) | 38 (0.5) | <.001 | 0.8 (0.7 to 1.0) |
| Immunodeficiency diseases | 1695 (3.0) | 1361 (2.8) | 334 (4.3) | <.001 | −1.5 (−2.0 to −1.0) |
| Liver cirrhosis | 1097 (1.9) | 844 (1.7) | 253 (3.3) | <.001 | −1.5 (−1.9 to −1.1) |
| COPD/asthma | 5760 (10.1) | 4569 (9.3) | 1191 (15.3) | <.001 | −6.0 (−6.8 to −5.2) |
| Drug dependence or use | 7783 (13.7) | 0 | 7783 (100) | NA | NA |
| Mortality | 1903 (3.4) | 1766 (3.6) | 137 (1.8) | <.001 | 1.8 (1.5 to 2.2) |
Abbreviation: NA, not applicable.
Resource Use and In-Hospital Mortality
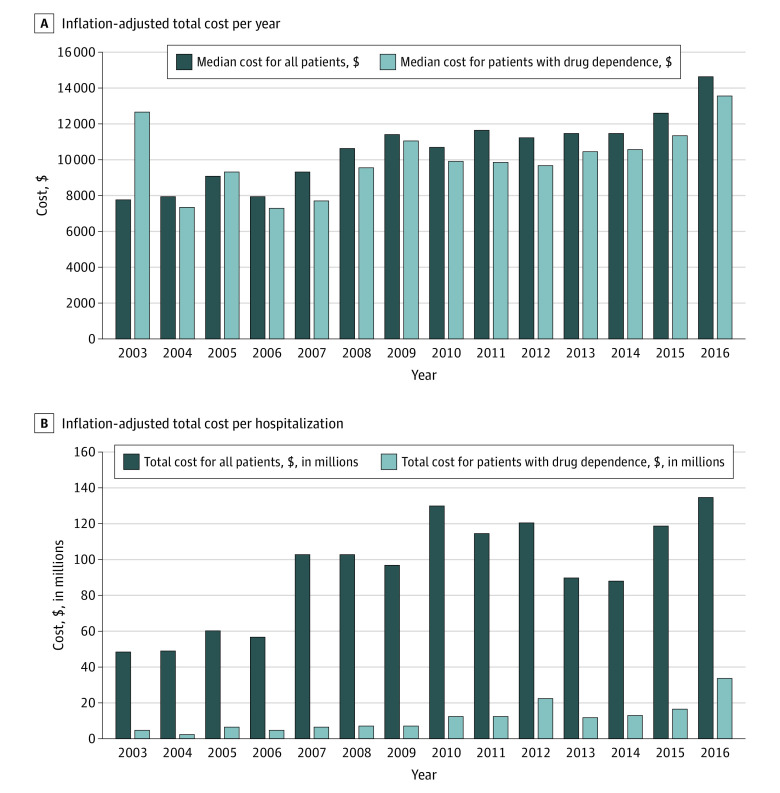
The median length of hospital stay was 5 days. In 2016, median inflation adjusted cost per endogenous endophthalmitis hospitalization was $14 593. Cumulative inflation-adjusted cost of all such hospitalizations totaled approximately $134 million. For the drug-using population, the median inflation-adjusted cost per hospitalization was $13 560, and the cumulative inflation-adjusted cost for all such hospitalizations was $33.6 million. Median cost per admission and the cumulative cost per year are summarized in Figure 2A and B.
Figure 2. Economic Burden Associated with Endogenous Endophthalmitis Hospitalizations in the United States.
A, Inflation-adjusted total cost per year. B, Inflation-adjusted median cost per hospitalization.
The overall mortality for endogenous endophthalmitis hospitalizations was 3.4%. The mortality was lower for patients with a history of drug dependence or use (1.8% vs 3.6%; P < .001).
Discussion
Drug dependence or use, in particular opioid use, remains a significant public health problem in the United States, affecting 19.7 million people, resulting in 70 200 deaths in 2017; indicating a 2-fold increase over the past decade, and costs the United States economy $740 billion annually.17,18,19 Studies have reported a rise in the incidence of IV drug use associated endophthalmitis in the United States,12,13,14 which closely mirrors the opioid epidemic. In this study, we investigated the national and regional trends of endogenous endophthalmitis hospitalizations across the United States; in particular the drug-using population. We also compared the prevalence of infectious and noninfectious comorbidities, economic burden, and in-patient mortality for patients with or without a history of drug dependence or use.
Our study reports the incidence of endogenous endophthalmitis–related hospitalizations to be 1.3 per 100 000 population in the United States. Regional analysis across all 4 geographic regions (Northeast, West, Midwest, and South) revealed stable incidence between 2003 and 2016 (eFigure, A in the Supplement). Approximately 13.7% of endophthalmitis hospitalizations in the United States were associated with a history of drug dependence or use. In a 2019 case series by Modjtahedi et al,12 47% of eyes with endogenous endophthalmitis had a history of drug dependence or use. In the Australian population, 30% to 38% of patients with endophthalmitis had a history of intravenous drug use,5 while lower rates have been reported in the Chinese (5%),20 Korean (<1%),11 and Danish (8%) populations.21
Patients with drug use–related endophthalmitis were younger (49.6 vs 57.5 years; difference, 7.9; 95% CI, 6.93-8.88; P < .001) than those without a history of drug dependence of use. This has implications because disease burden at a younger age may incur high lifetime health care spending, increased health care services or resource use, and loss of work productivity. In addition, subgroup analysis revealed that most of these cases belonged to the lowest income quartile, making them less equipped to deal with the ensuing visual disability.
The incidence of drug use–related endogenous endophthalmitis hospitalizations was 0.18 per 100 000 over a 14-year study period. The incidence showed an increasing trend over the years, with more than a 4-fold increase between 2003 (0.08 per 100 000) and 2016 (0.32 per 100 000 population). Regional analysis showed a marked increase in incidence across all 4 geographic regions. These findings corroborate prior studies reporting an exponential rise in IV drug use–related endophthalmitis cases in the northeast,12,13,14 which mirrors the regional trend in heroin-associated mortality and hospitalization.13
The most common infectious comorbidities were urinary tract infections, sepsis, and pneumonia, with higher rates in the non–drug-using population. This is similar to what has been previously reported.5,22 Patients with drug-use–related endophthalmitis had a higher rate of hepatitis B, hepatitis C, HIV infection, fungemia, bacteremia, Candida infection, osteomyelitis, infective endocarditis, meningitis, intracranial and intraspinal abscess, and skin and soft tissue infections (Table 2). The mortality rate in the drug-using population was 1.8% compared with 3.6% in the non–drug-using population. This can be explained by the fact that the non–drug-using population was significantly older with greater comorbidities, which likely attributes to the higher mortality rate. The most frequently reported systemic comorbidities in our study were diabetes, chronic kidney disease, and malignancy, with higher rates in the non–drug-using population. Patients with drug dependence or use had significantly higher rate of COPD/asthma, liver cirrhosis, and immunodeficiency diseases (Table 3).
Cumulative inflation-adjusted cost for drug-use–related endogenous endophthalmitis hospitalizations was $163 million over the study period. In addition, indirect costs resulting from disability, loss of work, decreased quality of life, and premature mortality can be substantial, posing a significant burden on the US health care system.
Strengths and Limitations
The strengths of this study include its large sample size and nationwide estimates, representative of the entire US population. To our knowledge, this is the first study that provides nationwide estimates on the incidence of drug-use–related endophthalmitis hospitalization in the United States over a 14-year study period.
This study has a number of limitations, including the potential for misdiagnosis based on ICD codes; it is particularly relevant because there was a switch from ICD-9 to ICD-10 in 2015; however, the large sample size may mitigate or render negligible the risk of such misclassifications. The NIS does not include information on the microbiologic profile of endophthalmitis cases, and this information, although clinically relevant in treating patients with endogenous endophthalmitis, was not included in our study. This study does not report the nationwide incidence of endogenous endophthalmitis because patients treated in an emergency department or outpatient setting are not included in the NIS database. Determining the incidence would require analysis of claims data from all payers in the United States, which is beyond the scope of this study, and our findings may not be generalizable to the overall drug-using population. Reluctance on the part of inpatients to divulge opioid use or dependence may have resulted in underestimates of the incidence.
The sampling strategy was changed in 2012 from a sample of discharges from 20% of hospitals to a 20% sample of discharges from all hospitals. The current sampling strategy provides more precise nationwide estimates than the strategy prior to 2012 by reducing sampling errors.15 Because the study data are only through 2016, one cannot determine with certainty how this might apply to considerations in 2020.
Conclusions
In summary, although these data support the hypothesis that the incidence of endogenous endophthalmitis–related hospitalizations has remained stable, the data also suggest there has been a 4-fold increase in drug use–related endogenous endophthalmitis hospitalizations, from 0.8 cases per 100 000 in 2003 to 3.2 cases per 100 000 in 2016. While these findings do not aid in the management of this condition, they do support the hypothesis that clinicians should maintain a high index of suspicion for endophthalmitis when evaluating patients with intraocular inflammation in the setting of drug dependence or use.
eFigure. Regional Comparison of Incidence Rate of Endogenous Endophthalmitis
References
- 1.Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986;31(2):81-101. doi: 10.1016/0039-6257(86)90076-7 [DOI] [PubMed] [Google Scholar]
- 2.Connell PP, O’Neill EC, Fabinyi D, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond). 2011;25(1):66-72. doi: 10.1038/eye.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: report of a ten-year retrospective study. Ophthalmology. 1994;101(5):832-838. doi: 10.1016/S0161-6420(13)31255-X [DOI] [PubMed] [Google Scholar]
- 4.Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001;12(6):464-470. doi: 10.1097/00055735-200112000-00012 [DOI] [PubMed] [Google Scholar]
- 5.Gounder PA, Hille DM, Khoo YJ, Phagura RS, Chen FK. Endogenous endophthalmitis in Western Australia: a sixteen-year retrospective study. Retina. 2020;40(5):908-918. doi: 10.1097/IAE.0000000000002512 [DOI] [PubMed] [Google Scholar]
- 6.Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137(4):725-731. [DOI] [PubMed] [Google Scholar]
- 7.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore). 2003;82(2):97-105. doi: 10.1097/00005792-200303000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Essman TF, Flynn HW Jr, Smiddy WE, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28(3):185-194. [PubMed] [Google Scholar]
- 9.Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014;59(6):627-635. doi: 10.1016/j.survophthal.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Patel SN, Rescigno RJ, Zarbin MA, Langer P, Bhagat N. Endogenous endophthalmitis associated with intravenous drug abuse. Retina. 2014;34(7):1460-1465. doi: 10.1097/IAE.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 11.Lim HW, Shin JW, Cho HY, et al. Endogenous endophthalmitis in the Korean population: a six-year retrospective study. Retina. 2014;34(3):592-602. doi: 10.1097/IAE.0b013e3182a2e705 [DOI] [PubMed] [Google Scholar]
- 12.Modjtahedi BS, Finn AP, Barb SM, et al. Characteristics and outcomes of endogenous endophthalmitis: eight-year experience at a tertiary care center. Ophthalmol Retina. 2019;3(1):61-72. doi: 10.1016/j.oret.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 13.Tirpack AR, Duker JS, Baumal CR. An outbreak of endogenous fungal endophthalmitis among intravenous drug abusers in New England. JAMA Ophthalmol. 2017;135(6):534-540. doi: 10.1001/jamaophthalmol.2017.0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modjtahedi BS, Finn AP, Papakostas TD, Durand M, Husain D, Eliott D. Intravenous drug use-associated endophthalmitis. Ophthalmol Retina. 2017;1(3):192-199. doi: 10.1016/j.oret.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 15.HCUP Methods series. Accessed October 1, 2020. https://hcup-us.ahrq.gov/reports/methods/2014-04.pdf
- 16.US Bureau of Labor Statistics Consumer price index for all urban consumers: hospital services. Accessed October 1, 2020. https://www.bls.gov/news.release/cpi.t02.htm
- 17.National Institute on Drug Abuse. Trends and statistics. Accessed September 28, 2020. https://www.drugabuse.gov/related-topics/trends-statistics.
- 18.2017. NSDUH Annual National Report. Accessed October 1, 2020. https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report
- 19.US Centers for Disease Control and Prevention CDC WONDER. Published 2017. Accessed September 28, 2020. https://wonder.cdc.gov/
- 20.Zhang H, Liu Z. Endogenous endophthalmitis: a 10-year review of culture-positive cases in northern China. Ocul Immunol Inflamm. 2010;18(2):133-138. doi: 10.3109/09273940903494717 [DOI] [PubMed] [Google Scholar]
- 21.Bjerrum SS, la Cour M. 59 eyes with endogenous endophthalmitis- causes, outcomes and mortality in a Danish population between 2000 and 2016. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):2023-2027. doi: 10.1007/s00417-017-3760-4 [DOI] [PubMed] [Google Scholar]
- 22.Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol. 2015;159(3):498-504. doi: 10.1016/j.ajo.2014.11.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Regional Comparison of Incidence Rate of Endogenous Endophthalmitis