Abstract
Background:
Microfracture (MFx) is the most common procedure for treating chondral lesions in the knee; however, initial improvements decline after 2 years. Autologous matrix-induced chondrogenesis (AMIC) may overcome this shortcoming by combining MFx with collagen scaffolds. However, the outcomes of AMIC and MFx in the knee have not been compared.
Purpose:
To compare the clinical and radiological outcomes of AMIC and MFx over a minimum 2-year follow-up.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic search of the MEDLINE, Embase, and Cochrane Library databases identified studies of patients who underwent AMIC or MFx and that reported validated clinical outcome measure and/or radiological evaluation findings at a follow-up of ≥2 years. There were 2 reviewers who performed study selection, a risk of bias assessment, and data extraction.
Results:
Overall, 29 studies were included in this systematic review. The mean improvement on the Lysholm score, Tegner activity scale, and visual analog scale for pain did not differ significantly between the 2 procedures. The mean improvement on the International Knee Documentation Committee (IKDC) subjective score was significantly greater in the AMIC (45.9 [95% CI, 36.2-55.5]) than in the MFx (27.2 [95% CI, 23.3-31.1]) group (P < .001). In addition, the mean magnetic resonance observation of cartilage repair tissue score was significantly higher in the AMIC (69.3 [95% CI, 55.1-83.5]) versus MFx (41.0 [95% CI, 27.3-54.7]) group (P = .005), and the mean adequate defect filling rate on magnetic resonance imaging scans was significantly better in the AMIC (77.3% [95% CI, 66.7%-87.9%]) versus MFx (47.9% [95% CI, 29.2%-66.6%]) group (P = .008) (odds ratio, 1.58 [95% CI, 1.07-2.33]).
Conclusion:
No significant differences in clinical outcomes, except for the IKDC subjective score, were found between the AMIC and MFx groups. Greater improvement in IKDC subjective scores and magnetic resonance imaging findings were seen in patients treated with AMIC compared with MFx at a minimum 2-year follow-up.
Keywords: autologous matrix-induced chondrogenesis, cartilage; meta-analysis, microfracture, scaffold, systematic review
The treatment of articular chondral lesions in the knee remains a challenge to orthopaedic surgeons, given the limited healing potential of cartilage tissue.11,30,35,41,42 If untreated, full-thickness chondral lesions may develop and potentially lead to pain, recurrent effusion, decreased activity, and progression of osteoarthritis in the long term.12,26 Chondral lesions accompanying such symptoms usually necessitate surgical treatment. Several options are currently available to repair articular chondral lesions including a marrow stimulation method, autologous chondrocyte implantation, and osteochondral autograft and allograft transplantation.6,10,63,69
Microfracture (MFx), a marrow stimulation technique, is considered the first-line treatment for chondral lesions because it is simple, cost-effective, minimally invasive, and a single-stage procedure, which is in contrast to other cartilage repair techniques.2,47,57 MFx recruits mesenchymal stem cells (MSCs) and growth factors in chondral defects by penetrating the subchondral plate.33,63 The resulting blood clot enriched with MSCs and growth factors, a so-called superclot, is capable of stimulating and differentiating into fibrocartilage for cartilage repair.33,63 Although MFx has demonstrated good short-term outcomes, potential concerns remain that the superclot may not be mechanically stable to sustain the tangential forces of the knee and the MSCs may widely diffuse into the joint rather than remain contained at the defect site.21,24,47,61
Autologous matrix-induced chondrogenesis (AMIC) was proposed to resolve these concerns by applying a collagen matrix at the microfractured chondral defect site.8,22,26 The collagen matrix enhances mechanical stability and confines the superclot to the defect site to provide a proper stimulus for chondrogenic differentiation and cartilage regeneration.22,26,39 Although AMIC is becoming a well-established treatment option with satisfactory clinical results compared with those of MFx,1,66 systematic evidence with respect to clinical efficacy comparing AMIC and MFx for cartilage repair in the knee is lacking. To address the lack of systematic information regarding the clinical efficacy of AMIC and MFx, a systematic review and meta-analysis of clinical studies was performed to determine the clinical efficacy of cartilage repair in the knee using both techniques. We compared the clinical and radiological outcomes of AMIC versus MFx at a minimum 2-year follow-up because short-term outcomes after different cartilage repair procedures have been acceptable in most patients.28,47,61
Methods
Literature Search
This systematic review and meta-analysis was performed according to the recommendations of Cochrane review methods. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020145264). A systematic literature search was performed in the PubMed (MEDLINE), Embase, and Cochrane Library databases up to June 2019 with no restriction on language or year of publication based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.48 The search terms used in the title, abstract, medical subject headings, and keywords fields included ([(“knee” OR “knees” OR “knee joint” OR “knee joints”) AND (“cartilage” OR “cartilages” OR “chondral”) AND (“defect” OR “defects” OR “lesion” OR “lesions” OR “damage” OR “damages”) AND (“chondroplasty” OR “chondrogenesis” OR “repair” OR “regeneration”)] AND [(“microfracture” OR “drilling”) OR (“autologous matrix-induced chondrogenesis” OR “AMIC” OR “type I/III collagen scaffold”)]) AND (“outcomes” OR “outcome” OR “scores” OR “score” OR “results” OR “result”). Manual searches were also performed for articles that could have been missed by the electronic search. Two investigators (J.-H.K. and J.-W.H.) independently screened the abstracts and titles of studies initially, and then full articles were reviewed when studies met the inclusion criteria.
Study Selection
Studies meeting the following criteria were identified: (1) patients who underwent AMIC or MFx for a cartilage defect in the knee joint, (2) clinical studies evaluating cartilage repair or clinical outcomes, (3) a full report of parameters including means ± SDs and sample numbers, and (4) follow-up of ≥2 years. Studies not clearly reporting parameters, biomechanical and cadaveric studies, technical notes, letters to the editor, expert opinions, review articles, meta-analyses, scientific conference abstracts, and case reports were excluded. Studies of cohorts with all patients undergoing high tibial osteotomy with AMIC or MFx were also excluded. Studies with a >10-year follow-up were excluded because clinical results of AMIC have been reported only since 2010.7,8,26
Data Extraction
Two investigators (J.-H.K. and J.-W.H.) independently extracted data from each article using a predefined data extraction form. Any disagreements between the 2 reviewers were resolved through a discussion. The data extracted were study design, number of knees, sex, age, body mass index, mean follow-up period, defect characteristics (mean size, grade, and location), details of the surgical technique (bone marrow stimulation method, membrane material, membrane fixation method, and approach), postoperative rehabilitation protocol, clinical outcome, and radiological outcome (magnetic resonance imaging [MRI] scoring system, mean score, and details of MRI findings of defect filling). Only data from outcome parameters with proven validity and reliability were selected because of methodological heterogeneity for the clinical outcome evaluations in the included studies. For clinical outcomes, the International Knee Documentation Committee (IKDC) subjective score, Lysholm score, Tegner activity scale score, and visual analog scale (VAS) for pain score were aggregated from pooled studies; the Knee injury and Osteoarthritis Outcome Score was excluded because of a lack of studies for the analysis. For radiological outcomes, the magnetic resonance observation of cartilage repair tissue (MOCART) score and details of defect filling after cartilage repair on MRI scans were aggregated from pooled studies.
Assessment of Methodological Quality
The same 2 investigators independently assessed the methodological quality of each study using the modified Coleman Methodology Score (CMS).15,22 Each study was scored for each of the 10 criteria from 2 parts of the grading system for a maximum score of 100. Any discrepancies in scores between the 2 reviewers were resolved through a discussion.
Statistical Analysis
The main outcomes of the meta-analysis were the mean difference in clinical outcomes and radiological outcome of defect filling after the cartilage repair procedures of AMIC versus MFx. Continuous variables, including the IKDC subjective score, Lysholm score, Tegner activity scale score, VAS for pain score, and MOCART score, were reported as means and 95% CIs. Binary outcomes including the adequate defect filling rate on MRI scans were reported as odds ratios (ORs) and 95% CIs. Heterogeneity was determined by estimating the proportion of between-study inconsistencies because of actual differences between studies, rather than differences due to random error or chance, using the I 2 statistic in which 25% was considered low heterogeneity; 50%, moderate heterogeneity; and 75%, high heterogeneity. Random-effects meta-analysis was performed to pool the outcomes across the included studies. A random-effects model using the restricted maximum likelihood method was applied, as this model has been known to allow greater generalization of conclusions for variable patient populations and different surgical procedures.29,52 Forest plots were used to show the outcomes, pooled estimate of effect, and overall summary effect of each study and were constructed using OpenMeta[Analyst] (Brown University; http://www.cebm.brown.edu/openmeta). Additional analyses were performed using Comprehensive Meta-Analysis software (Biostat) and R statistical software Version 3.4.0 (R Foundation for Statistical Computing). The standardized mean difference and standardized variance were calculated from the weighted estimates, standard errors, and sample size of each group by using the logit method.65,68 Summary ORs and 95% CIs were calculated based on the standardized mean difference and standardized variance. Statistical significance was set at P < .05.
Results
Identification of Studies
Overall, 600 articles were identified. Details regarding study identification as well as inclusion and exclusion criteria are shown in Figure 1. An electronic search yielded 222 studies in PubMed (MEDLINE), 290 in Embase, and 88 in the Cochrane Library. An additional 3 studies were identified via a manual search. After removing 345 duplicate studies, 258 studies remained. After screening the titles and abstracts and reading the full text, 229 studies were excluded. Ultimately, 29 studies were included in this systematic review.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for the identification and selection of studies included in this meta-analysis. AMIC, autologous matrix-induced chondrogenesis.
Study Characteristics and Methodological Quality Assessment
Of the 29 identified studies, only 2 studies directly compared the results of AMIC with MFx. Overall, 13 studies involving 360 knees evaluated the results after AMIC, and 18 studies involving 606 knees evaluated the results after MFx. The demographic data, study design, follow-up period, preoperative cartilage defect details, and quality score (modified CMS) of each included study are presented in Appendix Table A1. Although 14 studies were level 4, the mean modified CMS of the included studies was 72.9 ± 7.0 of 100 (95% CI, 70.4-75.4), regarded as fair to good quality. The mean modified CMS was 71.3 ± 8.0 (95% CI, 66.8-75.7) in the AMIC group and 74.3 ± 6.0 (95% CI, 71.3-77.3) in the MFx group, with a difference that was not statistically significant (P = .226). Details of the specific surgical technique, such as the bone marrow stimulation method, membrane material, membrane fixation method, and methodological approach, for the AMIC procedure are summarized in Table 1. Surgical indications and rehabilitation protocols for the AMIC group are presented in Table 2. Four parameters of clinical outcomes were compared between the 2 surgical procedures. MRI scores and adequate defect filling on MRI scans of the 2 surgical procedures are compared in Table 3.
Table 1.
Surgical Techniques of the AMIC Procedurea
| Author (Year) | Bone Marrow Stimulation Method | Membrane Material | Membrane Fixation Method | Approach |
|---|---|---|---|---|
| Anders1 (2013) | ||||
| Glued |
Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| Sutured | Chondropick awl (Steadman method) |
Chondro-Gideb | Suture (PDS 5-0) | Mini-arthrotomy |
| Dhollander18 (2011) | 1.2-mm K-wire drilling | Chondro-Gideb with PRP gel insertion under membrane | Suture (Vicryl 6-0) | Mini-arthrotomy |
| Dhollander19 (2012) | Chondropick awl (Steadman method) |
Chondrotissuec with immersing 3 mL autologous serum for 10 min | Transosseous bioresorbable pin (SmartNaild) | Mini-arthrotomy |
| Gille25 (2010) | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| Gille24 (2013) | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| de Girolamo16 (2019) | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| Kusano39 (2012) | Chondropick awl (Steadman method) |
Chondro-Gideb | Suture with fibrin glue injection under matrix | Mini-arthrotomy |
| Schiavone Panni56 (2018) | Chondropick awl (Steadman method) or 1.1-mm K-wire drilling |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| Pascarella50 (2010) | 2.0-mm K-wire drilling | Chondro-Gideb with immersing 1-2 mL bone marrow aspiration | Fibrin glue | Mini-arthrotomy |
| Sadlik51 (2017) | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Dry arthroscopic surgery |
| Schagemann55 (2018) | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy and dry arthroscopic surgery |
| Siclari58 (2014) | 1.8-mm K-wire drilling | Chondrotissuec with immersing 3 mL autologous PRP for 5-10 min | Transosseous bioresorbable pin (SmartNaild) or fibrin-like autologous PRP glue | Arthroscopic surgery |
| Volz66 (2017) | ||||
| Glued | Chondropick awl (Steadman method) |
Chondro-Gideb | Fibrin glue | Mini-arthrotomy |
| Sutured | Chondropick awl (Steadman method) |
Chondro-Gideb | Suture (PDS 5-0) | Mini-arthrotomy |
aAMIC, autologous matrix-induced chondrogenesis; PDS, polydioxanone suture; PRP, platelet-rich plasma.
bGeistlich Pharma AG.
cBioTissue AG.
dConMed Linvatec.
Table 2.
Surgical Indications and Rehabilitation Protocols of AMIC Groupa
| Author (Year) | Indication | Rehabilitation Protocol |
|---|---|---|
| Anders1 (2013) | Age >18-<50 y, defect size 2-10 cm2 | For condylar lesions: PWB with crutches until 6 wk, FWB
after 8 wk, 0°-60° of ROM until POD 10, and 0°-90° of ROM
until 6 wk For patellar lesions: PWB with crutches until 6 wk, FWB after 8 wk, 0°-30° of ROM until POD 10, and 0°-90° of ROM until 6 wk For all lesions: swimming after 3-6 wk, cycling and jogging after 7 wk, and return to contact sports after 18 mo |
| Dhollander18 (2011) | Age >18-<50 y |
NWB until 2 wk, FWB after 10 wk, 0°-15° of ROM until POD 2, full ROM after 8 wk, and return to low-impact sports after 12 mo |
| Dhollander19 (2012) | Age >16-<40 y | NWB until 2 wk, FWB after 10 wk, 0°-90° of ROM until 4 wk, full ROM after 8 wk, and return to low-impact sports after 12 mo |
| Gille25 (2010) | Defect size >1 cm2 | NWB until 6 wk, immobilization with knee extension until POD 7, and CPM exercise for 6 wk |
| Gille24 (2013) | Defect size >1 cm2 | NWB until 6 wk, immobilization with knee extension until POD 7, and CPM exercise for 6 wk |
| de Girolamo16 (2019) | Age >18-<55 y, defect size 2-8 cm2 | For condylar lesions: NWB with crutches until 3 wk, FWB
after 6 wk, and immediate full ROM For patellar lesions: progressive restoration of full ROM and FWB from early PODs |
| Kusano39 (2012) | Adult but <50 y, defect size >2 cm2 | CPM exercise at POD 10, 0°-60° of ROM until 4 wk, full ROM after 6 wk, PWB with crutches until 6 wk, FWB after 6 wk, and return to sports after 1 y |
| Schiavone Panni56 (2018) | Defect size >2 cm2 | For condylar lesions: PWB at POD 1, FWB after 4 wk, 0°-90°
of ROM at POD 1, and full ROM after 4 wk For patellofemoral lesions: PWB until POD 30 and 0°-60° of ROM until POD 30 For all lesions: heavy work after 3 mo and return to sports after 6 mo |
| Pascarella50 (2010) | Age >18-<50 y | NR |
| Sadlik51 (2017) | Age >18-<55 y | NWB with knee extension until 1 wk, PWB with crutches until 2 wk, FWB with knee extension until 4 wk, FWB with knee flexion after 6 wk, and FWB without crutches after 8 wk |
| Schagemann55 (2018) | Outerbridge grade III or IV | For condylar lesions: NWB with crutches until 8 wk, FWB
after 8 wk, and 0°-70° of ROM until 8 wk For patellar lesions: PWB with crutches until 2 wk, FWB after 2 wk, brace at 0°-20° until 8 wk, and CPM at 0°-50° immediately postoperatively |
| Siclari58 (2014) | Age >25-<65 y | NWB until 2 wk, PWB with crutches until 3 wk, FWB after 6 wk, swimming and cycling after 4 wk, and normal activities of daily life after 6 wk |
| Volz66 (2017) | Age >18-<50 y, defect size 2-10 cm2 | For condylar lesions: PWB with crutches until 6 wk, FWB
after 8 wk, 0°-60° of ROM until POD 10, and 0°-90° of ROM
until 6 wk For patellar lesions: PWB with crutches until 6 wk, FWB after 8 wk, 0°-30° of ROM until POD 10, and 0°-90° of ROM until 6 wk For all lesions: swimming after 3-6 wk, cycling and jogging after 7 wk, and return to contact sports after 18 mo |
aAMIC, autologous matrix-induced chondrogenesis; CPM, continuous passive motion; FWB, full weightbearing; NR, not reported; NWB, nonweightbearing; POD, postoperative day; PWB, partial weightbearing; ROM, range of motion.
Table 3.
Overall Radiological Outcomesa
| Author (Year) | MRI Scoring Systemb | MRI Findings Regarding Defect Filling |
|---|---|---|
| AMIC group | ||
| Anders1 (2013) | ||
| Glued | Surgeon-specific | >Two-thirds in 8/13, one-third to two-thirds in 1/13, <one-third in 3/13, and no defect filling in 1/13 |
| Sutured | Surgeon-specific | >Two-thirds in 5/8, one-third to two-thirds in 2/8, <one-third in 1/8, and no defect filling in 0/8 |
| Dhollander18 (2011) | MOCART (53.0 [47-59]) |
Complete in 0/5, hypertrophy in 2/5, incomplete >50% in 3/5, incomplete <50% in 0/5, and subchondral bone exposure in 0/5 |
| Dhollander19 (2012) | MOCART (67 [50-83]) |
Complete in 1/5, hypertrophy in 2/5, incomplete >50% in 2/5, incomplete <50% in 0/5, and subchondral bone exposure in 0/5 |
| Gille25 (2010) | MOCART | Complete to >50% in 10/15 |
| Gille24 (2013) | NR | NR |
| de Girolamo16 (2019) | MOCART | >Two-thirds in 1/2 and one-third to two-thirds in 1/2 |
| Kusano39 (2012) | MOCART | Complete in 3/16, hypertrophy in 3/16, incomplete >50% in 4/16, incomplete <50% in 4/16, and subchondral bone exposure in 2/16 |
| Schiavone Panni56 (2018) | MOCART (68.6) | Complete in 14/21, hypertrophy in 0/21, incomplete >50% in 5/21, incomplete <50% in 2/21, and subchondral bone exposure in 0/21 |
| Pascarella50 (2010) | Surgeon-specific | Significant enhancement of defect filling, cartilage shape, and subchondral edema in 53% |
| Sadlik51 (2017) | MOCART (58.3 [30-85]) |
NR |
| Schagemann55 (2018) | NR | NR |
| Siclari58 (2014) | MOCART (99) | Complete in 20/21, hypertrophy in 0/21, incomplete >50% in 1/21, incomplete <50% in 0/21, and subchondral bone exposure in 0/21 |
| Volz66 (2017) | ||
| Glued | Surgeon-specific | >Two-thirds in 10/15, one-third to two-thirds in 1/15, <one-third in 3/15, and no defect filling in 1/15 |
| Sutured | Surgeon-specific | >Two-thirds in 8/14, one-third to two-thirds in 1/14, <one-third in 2/14, and no defect filling in 3/14 |
| MFx group | ||
| Anders1 (2013) | Surgeon-specific | >Two-thirds in 3/4, one-third to two-thirds in 1/4, <one-third in 0/4, and no defect filling in 0/4 |
| Asik3 (2008) | NR | NR |
| Basad5 (2010) | NR | NR |
| Chung13 (2014) | Surgeon-specific | >Two-thirds in 2/12, one-third to two-thirds in 4/12, and <one-third in 6/12 |
| Domayer20 (2008) | MOCART | 100% in 7/24, 75%-100% in 9/24, 50%-75% in 3/24, 25%-50% in 4/24, and 0%-25% in 1/24 |
| Gobbi27 (2016) | NR | NR |
| Von Keudell67 (2012) | MOCART (19.6) | Complete in 1/13, hypertrophy in 0/13, incomplete >50% in 2/13, incomplete <50% in 0/13, and subchondral bone exposure in 10/13 |
| Koh34 (2016) | MOCART (51.8 ± 19.7) | Complete in 4/40, hypertrophy in 12/40, incomplete >50% in 11/40, incomplete <50% in 7/40, and subchondral bone exposure in 6/40 |
| Krych38 (2012) | NR | NR |
| Lee40 (2013) | NR | NR |
| Lim44 (2012) | Surgeon-specific | Outerbridge grade I in 4/25, grade II in 16/25, grade III in 3/25, and grade IV in 2/25 |
| Marquass46 (2012) | MOCART (39.4 ± 16.1) |
NR |
| Ossendorff49 (2019) | MOCART (54.1 ± 12.8) |
NR |
| Saris53 (2014) | Surgeon-specific | Complete to >50% in 53/69 |
| Sofu59 (2017) | MOCART | Complete in 4/24, hypertrophy in 0/24, incomplete >50% in 12/24, incomplete <50% in 8/24, and subchondral bone exposure in 0/24 |
| Solheim62 (2010) | NR | NR |
| Ulstein64 (2014) | NR | NR |
| Volz66 (2017) | Surgeon-specific | >Two-thirds in 2/6, one-third to two-thirds in 2/6, <one-third in 2/6, and no defect filling in 0/6 |
aAMIC, autologous matrix-induced chondrogenesis; MFx, microfracture; MOCART, magnetic resonance observation of cartilage repair tissue; MRI, magnetic resonance imaging; NR, not reported.
bValues are shown as mean, mean (range), or mean ± SD.
Clinical Outcomes
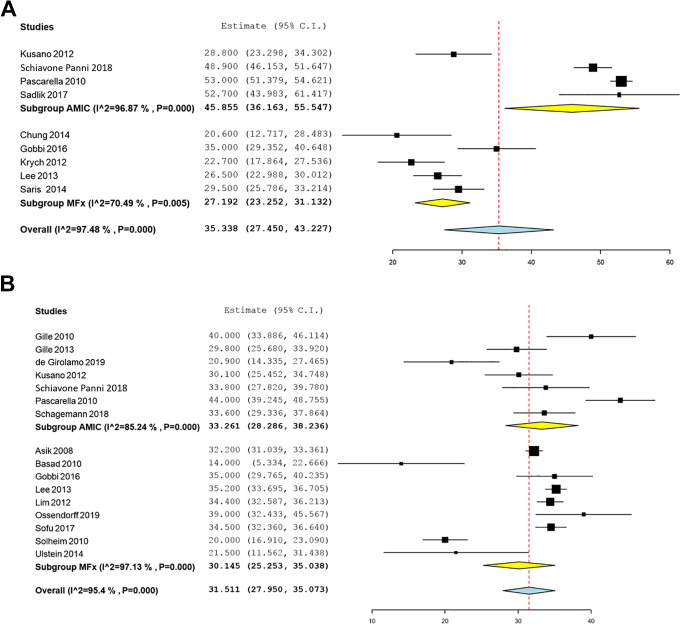
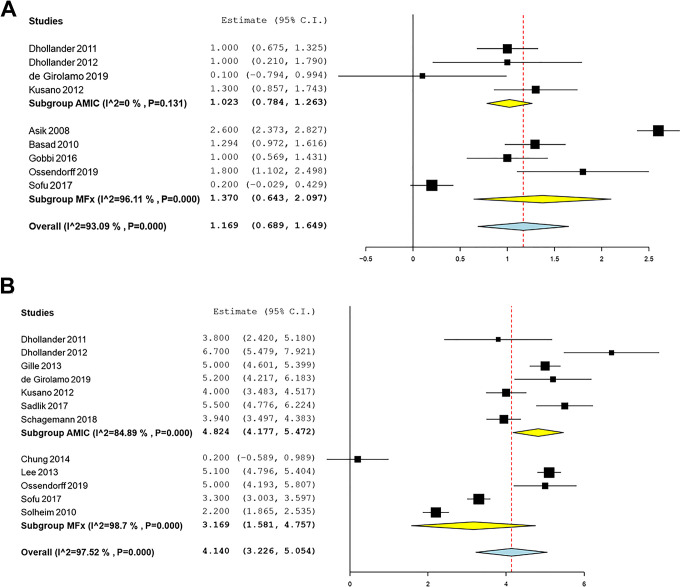
There were 4 AMIC studies and 5 MFx studies that reported changes in the IKDC subjective score from the preoperative to postoperative periods. Significant mean improvements in the IKDC subjective score were identified and were significantly in favor of AMIC: 45.9 (95% CI, 36.2-55.5) for AMIC and 27.2 (95% CI, 23.3-31.1) for MFx (P < .001) (Figure 2). The mean improvements on the Lysholm score and the Tegner activity scale were not significantly different (P = .38 and P = .37, respectively). Likewise, the mean reductions in the VAS for pain score were not significantly different for AMIC and MFx (P = .06) (Figure 3).
Figure 2.
Forest plots of the included studies showing changes in the (A) International Knee Documentation Committee score and (B) Lysholm score before and after cartilage repair using autologous matrix-induced chondrogenesis (AMIC) and microfracture (MFx). Squares represent the mean change in outcomes, with the size of the square being proportional to the sample size.
Figure 3.
Forest plots of the included studies showing changes in the (A) Tegner score and (B) visual analog scale for pain score before and after cartilage repair using autologous matrix-induced chondrogenesis (AMIC) and microfracture (MFx). Squares represent the mean change in outcomes, with the size of the square being proportional to the sample size.
Radiological Outcomes
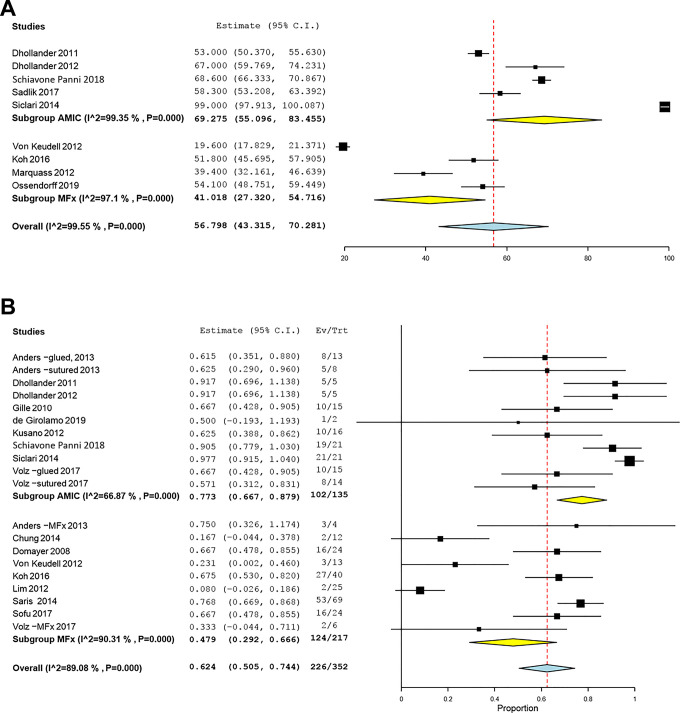
There were 5 AMIC studies and 4 MFx studies that reported statistically significant differences in pooled MOCART scores based on postoperative MRI findings, which were significantly in favor of AMIC: 69.3 (95% CI, 55.1-83.5) for AMIC and 41.0 (95% CI, 27.3-54.7) for MFx (P = .005) (Figure 4). Also, 9 AMIC studies and 9 MFx studies reported statistically significant differences in mean defect filling rates after cartilage repair on postoperative MRI scans: 77.3% (95% CI, 66.7%-87.9%) for AMIC and 47.9% (95% CI, 29.2%-66.7%) for MFx (P = .008) (Figure 4). The summary OR was 1.58 (95% CI, 1.07-2.33), which was significantly in favor of AMIC.
Figure 4.
Forest plots of the included studies showing changes in the (A) magnetic resonance observation of cartilage repair tissue score and (B) adequate defect filling rate on magnetic resonance imaging scan before and after cartilage repair using autologous matrix-induced chondrogenesis (AMIC) and microfracture (MFx). Squares represent the mean change in outcomes, with the size of the square being proportional to the sample size. Ev/Trt, observed number of events in the treatment group.
Discussion
The most important findings of this systematic review and meta-analysis indicated that clinical outcomes were comparable between the AMIC and MFx techniques after a minimum 2-year follow-up in terms of the Lysholm score, Tegner activity scale, and VAS for pain score. However, the IKDC subjective scores of the AMIC group were better than those of the MFx group. Furthermore, radiological outcomes as represented by the MOCART score and acceptable defect filling rates on MRI scans after AMIC were superior to those after MFx.
Despite the potential advantages of AMIC, the results of this study revealed that clinical outcomes, except for the IKDC subjective score, demonstrated comparable results over a 2-year follow-up between the AMIC and MFx procedures. Several potential explanations are proposed for the similar clinical outcomes. First, MFx is a crucial surgical step in the AMIC procedure, and thus, the clinical success of MFx may have depended on several prognostic factors, such as patient age, sex, body mass index, defect size, defect location, and depth of subchondral bone perforation.3,22,33,37,39 The heterogeneity of those factors might have resulted in confounding of the outcomes. Second, clinical outcomes could not fully represent the exact results of AMIC or MFx despite the use of well-established patient-reported scoring systems in this study.24,33,47 Other factors, such as inflammation, increased vascular penetration, nerve growth, complexity of the knee injury, and patient history, may have negatively affected the surgical outcome.14,17,33 Third, a lack of high-quality studies comparing the 2 techniques may have been a possible reason for the lack of statistical power to definitely define the superiority of clinical outcomes. Fourth, the follow-up period might not have been long enough to assess differences in outcomes between the 2 techniques, although we excluded studies with a short-term follow-up. However, the IKDC subjective score for AMIC, in contrast to other scores, was significantly superior to that of MFx in this study. Differences in the IKDC score over the 2-year follow-up are meaningful because the IKDC subjective score has been validated as an excellent tool for the assessment of cartilage repair surgery.32,33 Furthermore, a few studies have demonstrated that the IKDC score is strongly correlated with MRI parameters after cartilage repair.17,36,37,47
MRI has been considered a standard imaging tool for structural evaluation after cartilage repair.17,45,47 de Windt et al,17 in a systematic review and meta-analysis, found a correlation between MRI parameters and clinical outcomes after cartilage repair and reported that the MOCART score and defect filling rate were reliable predictors of clinical outcomes, although strong evidence supporting defect filling as a reliable parameter on MRI scans is lacking. Herein, the radiological outcomes of AMIC, in terms of the MOCART score and adequate defect filling rate on MRI scans, were significantly better than those of MFx over a 2-year follow-up. Our findings suggest that displacement of an initially fragile superclot from the MFx site may represent a potential explanation for inferior MOCART scores and lack of defect filling resulting from MFx compared with AMIC, as also described previously in experimental studies.31,47 Furthermore, insufficient concentrations of MSCs required to promote cartilage restoration may be another reason for inferior MRI results observed for MFx.2,4 The proposed benefits of AMIC theoretically derive from the enhanced concentration of MSCs available in the superclot and its stability during the healing process.2,4,22,43 These encouraging results and the advantages of a single-stage AMIC procedure are attractive considerations for knee surgeons when deciding on the first-line treatment for articular cartilage repair, as MFx is currently being challenged as a first-line treatment option, given the questionable long-term durability of the repair tissue.43,47,54,60,61
The current study had several limitations. First, the variable follow-up period of patients might represent a potential bias because the healing and maturation process of the repair tissue might have differed between the 2 groups,23 although we limited the follow-up duration to 2 to 10 years for the included studies. Second, because the outcomes of AMIC and MFx were examined only in 2 comparative studies, this meta-analysis was based on observational studies (level 4 evidence), which inevitably subjects this study to the limitations of a retrospective design including variability in sample sizes, patient characteristics, surgical techniques, chondral defect information, and cartilage repair and imaging techniques that may act as confounding factors. However, the advancement of meta-analyses has enhanced the performance of single-arm studies. Furthermore, we anticipate that the results of this meta-analysis could contribute to the establishment of level 1 or 2 evidence. A third potential limitation was the potential risk of bias caused by heterogeneity of the chondral defect location. The majority of the included studies evaluated in this systematic review failed to differentiate between femoral condylar and patellofemoral lesions. Although it is still unclear whether results vary depending on the location, some studies have found important differences,9,37 whereas other studies have not found any significant effects due to location.63 Thus, further studies are needed to specifically assess outcomes based on the defect location after cartilage repair.
Conclusion
The results of the present systematic review and meta-analysis indicate that clinical outcomes, with the exception of the IKDC subjective score, did not differ significantly among patients who underwent cartilage repair using the AMIC or MFx techniques and were assessed at a follow-up of ≥2 years. Improvement on the IKDC subjective score was greater in the AMIC group than in the MFx group. Furthermore, the MOCART score and adequate defect filling rate on MRI scans were improved after AMIC compared with MFx.
APPENDIX
TABLE A1.
Study Detailsa
| Author (Year) | Study Type | No. of Knees (M/F) | Mean Age, y | Mean Follow-up, mo | Mean Size of Chondral Lesion, cm2 | ICRS or Outerbridge Grade | Location of Chondral Lesion | Mean Modified CMS |
|---|---|---|---|---|---|---|---|---|
| Main Findings | ||||||||
| AMIC group | 360 | 36.1 | 38.3 | 3.5 | 71.3 | |||
| Anders1 (2013) | ||||||||
| Glued |
RCT (vs MFx and sutured AMIC) | 13 (10/3) | 39.0 | 24 | 3.8 | III (n = 5), IV (n = 8) | NR | 72 |
| Clinical outcome scores (ICRS and Cincinnati) showed significant improvement, irrespective of the technique used. MRI scans showed satisfactory and homogeneous defect filling. | ||||||||
| Sutured | RCT (vs MFx and glued AMIC) | 8 (7/1) | 35.0 | 24 | 3.8 | III (n = 3), IV (n = 5) | NR | 70 |
| Clinical outcome scores (ICRS and Cincinnati) showed significant improvement, irrespective of the technique used. MRI scans showed satisfactory and homogeneous defect filling. | ||||||||
| Dhollander18 (2011) | Case series | 5 (3/2) | 37.0 | 24 | 2.0 | III or IV | PU | 64 |
| AMIC was combined with PRP gel. Clinical outcome scores (KOOS and VAS for pain) showed gradual improvement, but improvement was not confirmed on MRI scans. | ||||||||
| Dhollander19 (2012) | Case series | 5 (4/1) | 36.0 | 24 | 2.3 | III or IV | MFC (n = 2), LFC (n = 2), PU (n = 1) | 64 |
| Clinical outcome scores (KOOS and VAS for pain) showed significant improvement. MRI scans showed adequate defect filling in 60% of cases. | ||||||||
| Gille25 (2010) | Case series | 32 (16/11) | 37.0 | 37 | 4.2 | IV | MFC (n = 7), LFC (n = 3), TG (n = 2), PU (n = 9), multiple (n = 6) | 78 |
| Clinical outcome scores (Meyer, Tegner, Lysholm, ICRS, and Cincinnati) showed significant improvement. MRI scans showed moderate to complete defect filling in most cases. | ||||||||
| Gille24 (2013) | Case series | 57 (38/19) | 37.3 | 24 | 3.4 | III (n = 20), IV (n = 37) | MFC (n = 32), LFC (n = 6), TG (n = 4), PU (n = 15) | 55 |
| Clinical outcome scores (Lysholm and VAS for pain) showed significant improvement. Most patients were highly satisfied. | ||||||||
| de Girolamo16 (2019) | RCT (vs BMAC) | 12 (7/5) | 30.0 | 24 | 3.8 | III or IV | MFC (n = 7), LFC (n = 3), PFJ (n = 2) | 75 |
| AMIC and BMAC were effective treatment methods for focal chondral lesions with beneficial effects on pain, functional scores, and MRI results. | ||||||||
| Kusano39 (2012) | Case series | 40 (23/17) | 35.6 | 28.8 | 3.9 | III or IV | FC (n = 20), PU (n = 20) | 71 |
| Clinical outcome scores (IKDC, Lysholm, Tegner, and VAS for pain) showed significant improvement. MRI scans showed generally incomplete tissue filling. | ||||||||
| Schiavone Panni56 (2018) | Case series | 21 (NR) | NR | 84 | 4.3 | III or IV | MFC (n = 11), LFC (n = 3), TG (n = 6), PU (n = 1) | 81 |
| Clinical outcome scores (IKDC and Lysholm) showed significant improvement, with 66.6% of patients showing good-quality repair tissue on MRI scans. Also, 76.2% of patients were satisfied or extremely satisfied. | ||||||||
| Pascarella50 (2010) | Case series | 19 (12/7) | 26 | 24 | 3.6 | III (n = 12), IV (n = 7) | MFC (n = 12), LFC (n = 5), TG (n = 2) | 64 |
| Clinical outcome scores (IKDC and Lysholm) showed significant improvement. MRI scans showed a significant reduction of the defect area in 53% of patients. | ||||||||
| Sadlik51 (2017) | Case series | 12 (7/5) | 36 | 38 | 2.5 | III (n = 7), IV (n = 5) | PU | 77 |
| Dry arthroscopic AMIC of patellar lesions was performed using a specific retraction system. Clinical outcome scores (IKDC, KOOS, and VAS for pain) and MRI scan showed significant improvement. | ||||||||
| Schagemann55 (2018) | Case series | 50 (30/20) | 35.5 | 24 | 3.3 | III or IV | MFC (n = 23), LFC (n = 8), TG (n = 3), PU (n = 15), TP (n = 1) | 62 |
| Mini-open AMIC was equivalent to the arthroscopic procedure. AMIC led to significant improvement of VAS for pain, KOOS, and Lysholm scores for up to 2 years compared with those before surgery. | ||||||||
| Siclari58 (2014) | Case series | 52 (20/32) | 44.0 | 60 | 3.0 | III (n = 16), IV (n = 36) | MFC (n = 12), MTP (n = 31), LTP (n = 9) | 74 |
| AMIC was combined with absorbable polymer-based implants immersed with autologous PRP. Clinical outcome scores (KOOS) showed significant improvement. MRI scans showed complete defect filling in 95% of patients. | ||||||||
| Volz66 (2017) | ||||||||
| Glued |
RCT (vs MFx and sutured AMIC) | 17 (15/2) | 39.0 | 60 | 3.9 | III or IV | NR | 80 |
| Significantly better clinical outcome scores (modified Cincinnati) were observed in the AMIC group, and MRI scans showed better defect filling in the AMIC group rather than the MFx group. | ||||||||
| Sutured | RCT (vs MFx and glued AMIC) | 17 (12/5) | 34.0 | 60 | 3.8 | III or IV | NR | 82 |
| Significantly better clinical outcome scores (modified Cincinnati) were observed in the AMIC group, and MRI scans showed better defect filling in the AMIC group rather than the MFx group. | ||||||||
| MFx group | 606 | 35.7 | 52.8 | 3.3 | 74.3 | |||
| Anders1 (2013) | RCT (vs sutured AMIC and glued AMIC) | 6 (4/2) | 41.0 | 24 | 3.8 | III (n = 1), IV (n = 5) | NR | 70 |
| Clinical outcome scores (ICRS and Cincinnati) showed significant improvement, irrespective of the technique used. MRI scans showed satisfactory and homogeneous defect filling. | ||||||||
| Asik3 (2008) | Case series | 90 (43/47) | 34.5 | 68 | <2 (n = 68), ≥2 (n = 22) | IV | MFC (n = NR), LFC (n = NR) | 76 |
| MFx was quite effective with regard to the improvement of daily activities, with a favorable effect on pain relief and better functional results at midterm follow-up. | ||||||||
| Basad5 (2010) | Case control study (vs MACI) | 20 (17/3) | 34.0 | 24 | 4-10 | NR | FC (n = 15), PFJ (n = 5) | 72 |
| MACI was superior to MFx in the treatment of larger (4 cm2) symptomatic articular defects over 2 y. | ||||||||
| Chung13 (2014) | PCS (vs MFx + biomembrane) | 12 (2/10) | 44.3 | 24 | 1.5 | III or IV | MFC (n = 6), LFC (n = 2), TG (n = 2), PU (n = 2) | 68 |
| Compared with conventional MFx, a biomembrane cover after MFx yielded superior outcomes in terms of the degree of cartilage repair during 2 y of follow-up. | ||||||||
| Domayer20 (2008) | Case series | 24 (17/7) | 41.0 | 29 | 2.0 | NR | MFC (n = 19), LFC (n = 5) | 70 |
| T2 mapping was sensitive to assess repair tissue function and provided information in addition to morphological MRI scans in the monitoring of MFx. | ||||||||
| Gobbi27 (2016) | PCS | 25 (16/9) | 42.7 | 60 | 4.5 | IV | MFC (n = 15), LFC (n = 11), PU (n = 3) | 67 |
| An HA-based scaffold with activated BMAC provided better clinical outcomes and more durable cartilage repair at medium-term follow-up compared with those with MFx. | ||||||||
| Von Keudell67 (2012) | Case series | 15 (9/6) | 45.0 | 48 | 1.9 | III or IV | MFC (n = 10), LFC (n = 5) | 62 |
| In 80% of patients, the cartilage defect size increased after MFx. Those with leg varus malalignment were more prone to an increase in defect size. | ||||||||
| Koh34 (2016) | PCS (vs adipose-derived MSCs with MFx) | 40 (16/24) | 39.1 | 27.4 | 4.6 | III or IV | NR | 74 |
| KOOS Pain and Symptom subscores were lower in the MFx alone group, but there were no differences in daily activity, sports, or quality of life subscores in both groups. In single cartilage defects that were ≥3 cm2, similar structural repair tissue was observed in both groups. | ||||||||
| Krych38 (2012) | RCT (vs OATS mosaicplasty) | 48 (32/16) | 32.5 | 60 | 2.6 | III or IV | MFC (n = 27), LFC (n = 16), TG (n = 5) | 78 |
| Clinical outcome scores (SF-36 and IKDC) showed significant improvement in both groups. There was no difference in clinical outcome scores for both groups. | ||||||||
| Lee40 (2013) | RCT (vs MFx + PRP) | 25 (15/10) | 46.0 | 28.0 | <4.0 | III or IV | FC | 79 |
| There were significant improvements in clinical results between the preoperative evaluation and 2 y postoperatively in both groups. At 2 y postoperatively, clinical results were significantly better in the MFx + PRP group than in the MFx alone group. | ||||||||
| Lim44 (2012) | Case control study (vs OATS and ACI) | 30 (17/12) | 32.9 | 80.4 | 2.8 | III or IV | MFC (n = 23), LFC (n = 7) | 82 |
| All 3 procedures showed improvement in functional scores (Lysholm, Tegner, and HSS). There were no differences in functional scores and postoperative MRI results among the groups. | ||||||||
| Marquass46 (2012) | Case control study (vs OATS) | 19 (NR) | 42.6 | 62.9 | 1.7 | IV | MFC | 67 |
| OATS had an unaltered significance in treating full-thickness cartilage defects and led to satisfying midterm results. | ||||||||
| Ossendorff49 (2019) | Case control study (vs ACI) | 22 (12/10) | 40.5 | 120 | 2.4 | III or IV | MFC (n = 12), LFC (n = 1), TG (n = 4), PU (n = 5) | 74 |
| The final Lysholm and functional pain scores were significantly higher in the MFx group than the ACI group. MRI scans showed similar results between the 2 groups. | ||||||||
| Saris53 (2014) | RCT (vs MACI) | 72 (48/24) | 32.9 | 24 | 4.7 | III (n = 15), IV (n = 57) | MFC (n = 53), LFC (n = 15), TG (n = 4) | 83 |
| Clinical outcome scores (KOOS) were significantly higher in the MACI group than the MFx group. Similar safety and defect filling results were observed in both groups. | ||||||||
| Sofu59 (2017) | Retrospective cohort study (vs MFx + HA-based cell-free scaffold) | 24 (7/17) | 43.0 | 25.7 | 3.6 | III or IV | MFC (n = 19), LFC (n = 5) | 81 |
| Cartilage regeneration surgery using an HA-based cell-free scaffold in combination with MFx for focal osteochondral lesions of the knee revealed promising clinical outcomes at 24-mo follow-up. | ||||||||
| Solheim62 (2010) | Case series | 110 (64/46) | 38.0 | 60 | 4.0 | IV | MFC (n = 62), LFC (n = 9), LTP (n = 11), TG (n = 18), PU (n = 10) | 78 |
| Clinical outcome scores (Lysholm and VAS for pain) showed significant improvement but were better in single defects rather than multiple defects. | ||||||||
| Ulstein64 (2014) | RCT (vs OATS) | 11 (6/5) | 31.7 | 117.6 | 2.6 | III or IV | MFC (n = 10), LFC (n = 1) | 76 |
| At long-term follow-up, there were no significant differences between MFx and OATS in clinical outcomes, muscle strength, or radiological outcomes. | ||||||||
| Volz66 (2017) | RCT (vs sutured AMIC and glued AMIC) | 13 (10/3) | 40.0 | 60 | 2.9 | III or IV | NR | 80 |
| Significantly better clinical outcome scores (modified Cincinnati) were observed in the AMIC group, and MRI scans showed better defect filling in the AMIC group rather than the MFx group. | ||||||||
aACI, autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; BMAC, bone marrow aspirate concentrate; CMS, Coleman Methodology Score; F, female; FC, femoral condyle; HA, hyaluronic acid; HSS, Hospital for Special Surgery; ICRS, International Cartilage Repair Society; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; LFC, lateral femoral condyle; LTP, lateral tibial plateau; M, male; MACI, matrix-induced autologous chondrocyte implantation; MFC, medial femoral condyle; MFx, microfracture; MRI, magnetic resonance imaging; MSC, mesenchymal stem cell; MTP, medial tibial plateau; NR, not reported; OATS, osteochondral autograft transfer system; PCS, prospective comparative study; PFJ, patellofemoral joint; PRP, platelet-rich plasma; PU, patellar undersurface; RCT, randomized controlled trial; SF-36, 36-Item Short Form Health Survey; TG, trochlear groove; TP, tibial plateau; VAS, visual analog scale.
Footnotes
Final revision submitted March 7, 2020; accepted April 10, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC(R)) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arshi A, Fabricant PD, Go DE, Williams RJ, McAllister DR, Jones KJ. Can biologic augmentation improve clinical outcomes following microfracture for symptomatic cartilage defects of the knee? A systematic review. Cartilage. 2018;9(2):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214–1220. [DOI] [PubMed] [Google Scholar]
- 4. Bark S, Piontek T, Behrens P, Mkalaluh S, Varoga D, Gille J. Enhanced microfracture techniques in cartilage knee surgery: fact or fiction? World J Orthop. 2014;5(4):444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. [DOI] [PubMed] [Google Scholar]
- 6. Bedi A, Feeley BT, Williams RJ., III Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009. [DOI] [PubMed] [Google Scholar]
- 7. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): a one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76(2):260–263. [PubMed] [Google Scholar]
- 8. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1(1):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 10. Brown D, Shirzad K, Lavigne SA, Crawford DC. Osseous integration after fresh osteochondral allograft transplantation to the distal femur: a prospective evaluation using computed tomography. Cartilage. 2011;2(4):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. [DOI] [PubMed] [Google Scholar]
- 12. Chubinskaya S, Haudenschild D, Gasser S, Stannard J, Krettek C, Borrelli J., Jr Articular cartilage injury and potential remedies. J Orthop Trauma. 2015;29(suppl 12):S47–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung JY, Lee DH, Kim TH, Kwack KS, Yoon KH, Min BH. Cartilage extra-cellular matrix biomembrane for the enhancement of microfractured defects. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1249–1259. [DOI] [PubMed] [Google Scholar]
- 14. Clancy CM, Eisenberg JM. Outcomes research: measuring the end results of health care. Science. 1998;282(5387):245–246. [DOI] [PubMed] [Google Scholar]
- 15. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. [DOI] [PubMed] [Google Scholar]
- 16. de Girolamo L, Schonhuber H, Vigano M, et al. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med. 2019;8(3):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695–1702. [DOI] [PubMed] [Google Scholar]
- 18. Dhollander AA, De Neve F, Almqvist KF, et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):536–542. [DOI] [PubMed] [Google Scholar]
- 19. Dhollander AA, Verdonk PC, Lambrecht S, et al. The combination of microfracture and a cell-free polymer-based implant immersed with autologous serum for cartilage defect coverage. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1773–1780. [DOI] [PubMed] [Google Scholar]
- 20. Domayer SE, Kutscha-Lissberg F, Welsch G, et al. T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome. Preliminary results. Osteoarthritis Cartilage. 2008;16(8):903–908. [DOI] [PubMed] [Google Scholar]
- 21. Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26(17):3617–3629. [DOI] [PubMed] [Google Scholar]
- 22. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1):222–231. [DOI] [PubMed] [Google Scholar]
- 23. Gao L, Orth P, Cucchiarini M, Madry H. Effects of solid acellular type-I/III collagen biomaterials on in vitro and in vivo chondrogenesis of mesenchymal stem cells. Expert Rev Med Devices. 2017;14(9):717–732. [DOI] [PubMed] [Google Scholar]
- 24. Gille J, Behrens P, Volpi P, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456–1464. [DOI] [PubMed] [Google Scholar]
- 26. Gille J, Wimmer J, Schuseil E, Gellissen J, Wallstabe S, Behrens P. Mid-term results of autologous matrix induced chondrogenesis (AMIC) for treatment of focal cartilage defects in the knee. Cartilage. 2010;1(2):57S. [DOI] [PubMed] [Google Scholar]
- 27. Gobbi A, Whyte GP. One-stage cartilage repair using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: five-year follow-up. Am J Sports Med. 2016;44(11):2846–2854. [DOI] [PubMed] [Google Scholar]
- 28. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579–1588. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinzpeter J, Zamorano A, Barahona M, Campos P. Treatment of osteochondritis dissecans of the knee with autologous iliac bone graft and hyaluronic acid scaffold. Knee Surg Relat Res. 2019;31(2):143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoemann CD, Hurtig M, Rossomacha E, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671–2686. [DOI] [PubMed] [Google Scholar]
- 32. Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):107–114. [DOI] [PubMed] [Google Scholar]
- 33. Jones KJ, Kelley BV, Arshi A, McAllister DR, Fabricant PD. Comparative effectiveness of cartilage repair with respect to the minimal clinically important difference. Am J Sports Med. 2019;47(13):3284–3293. [DOI] [PubMed] [Google Scholar]
- 34. Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109. [DOI] [PubMed] [Google Scholar]
- 35. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. 2018;46(4):995–999. [DOI] [PubMed] [Google Scholar]
- 36. Kreuz PC, Steinwachs M, Erggelet C, et al. Importance of sports in cartilage regeneration after autologous chondrocyte implantation: a prospective study with a 3-year follow-up. Am J Sports Med. 2007;35(8):1261–1268. [DOI] [PubMed] [Google Scholar]
- 37. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119–1125. [DOI] [PubMed] [Google Scholar]
- 38. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., III Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971–978. [DOI] [PubMed] [Google Scholar]
- 39. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109–2115. [DOI] [PubMed] [Google Scholar]
- 40. Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23(5):581–587. [DOI] [PubMed] [Google Scholar]
- 41. Lee HW, Choi KH, Kim JY, et al. Proteomic classification and identification of proteins related to tissue healing of platelet-rich plasma. Clin Orthop Surg. 2020;12(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee OS, Lee ES, Lee YS. Disparity between preoperative target correction amount and postoperative correction amount in open wedge high tibial osteotomy. Knee Surg Relat Res. 2019;31(2):126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee YH, Suzer F, Thermann H. Autologous matrix-induced chondrogenesis in the knee: a review. Cartilage. 2014;5(3):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470(8):2261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310–319. [DOI] [PubMed] [Google Scholar]
- 46. Marquass B, Mahn T, Engel T, et al. Clinical and radiological mid-term results after autologous osteochondral transplantation under consideration of quality of life. Article in German. Z Orthop Unfall. 2012;150(4):360–367. [DOI] [PubMed] [Google Scholar]
- 47. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 48. Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ossendorff R, Franke K, Erdle B, Uhl M, Südkamp NP, Salzmann GM. Clinical and radiographical ten years long-term outcome of microfracture vs. autologous chondrocyte implantation: a matched-pair analysis. Int Orthop. 2019;43(3):553–559. [DOI] [PubMed] [Google Scholar]
- 50. Pascarella A, Ciatti R, Pascarella F, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):509–513. [DOI] [PubMed] [Google Scholar]
- 51. Sadlik B, Puszkarz M, Kosmalska L, Wiewiorski M. All-arthroscopic autologous matrix-induced chondrogenesis-aided repair of a patellar cartilage defect using dry arthroscopy and a retraction system. J Knee Surg. 2017;30(9):925–929. [DOI] [PubMed] [Google Scholar]
- 52. Sangnawakij P, Bohning D, Niwitpong SA, Adams S, Stanton M, Holling H. Meta-analysis without study-specific variance information: heterogeneity case. Stat Methods Med Res. 2019;28(1):196–210. [DOI] [PubMed] [Google Scholar]
- 53. Saris D, Price A, Widuchowski W, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42(6):1384–1394. [DOI] [PubMed] [Google Scholar]
- 54. Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10S–19S. [DOI] [PubMed] [Google Scholar]
- 55. Schagemann J, Behrens P, Paech A, et al. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch Orthop Trauma Surg. 2018;138(6):819–825. [DOI] [PubMed] [Google Scholar]
- 56. Schiavone Panni A, Del Regno C, Mazzitelli G, D’Apolito R, Corona K, Vasso M. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1130–1136. [DOI] [PubMed] [Google Scholar]
- 57. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siclari A, Mascaro G, Kaps C, Boux E. A 5-year follow-up after cartilage repair in the knee using a platelet-rich plasma-immersed polymer-based implant. Open Orthop J. 2014;8:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sofu H, Kockara N, Oner A, Camurcu Y, Issin A, Sahin V. Results of hyaluronic acid-based cell-free scaffold application in combination with microfracture for the treatment of osteochondral lesions of the knee: 2-year comparative study. Arthroscopy. 2017;33(1):209–216. [DOI] [PubMed] [Google Scholar]
- 60. Solheim E, Hegna J, Inderhaug E. Long-term survival after microfracture and mosaicplasty for knee articular cartilage repair: a comparative study between two treatments cohorts. Cartilage. 2020;11(1):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826–831. [DOI] [PubMed] [Google Scholar]
- 62. Solheim E, Oyen J, Hegna J, Austgulen OK, Harlem T, Strand T. Microfracture treatment of single or multiple articular cartilage defects of the knee: a 5-year median follow-up of 110 patients. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):504–508. [DOI] [PubMed] [Google Scholar]
- 63. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. [DOI] [PubMed] [Google Scholar]
- 64. Ulstein S, Aroen A, Rotterud JH, Loken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 66. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797–804. [DOI] [PubMed] [Google Scholar]
- 67. Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: a long-term follow-up study. Eur J Radiol. 2012;81(7):1618–1624. [DOI] [PubMed] [Google Scholar]
- 68. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1–15. [Google Scholar]
- 69. Welch T, Mandelbaum B, Tom M. Autologous chondrocyte implantation: past, present, and future. Sports Med Arthrosc Rev. 2016;24(2):85–91. [DOI] [PubMed] [Google Scholar]