Abstract
Objective:
According to different reports, miR-9-5p either facilitates or suppresses the occurrence of tumors. BRAF is a serine/threonine kinase involved in the MAPK pathway and is a proto-oncogene promoting the progression of many tumors, especially melanoma. The present study aimed to reveal the mechanism of action of miR-9-5p and BRAF in choroidal melanoma (CM).
Methods:
RT-qPCR was used to detect the expression of miR-9-5p in CM cells after transfection with miR-9-5p mimics and inhibitor. EdU assay and Transwell assay, respectively, showed the proliferation, migration and invasion of CM cells after transfection with miR-9-5p mimics and inhibitor. A bioinformatics website was used for target prediction and the dual luciferase reporter assay was used to verify the interaction between miR-9-5p and BRAF. RT-qPCR and Western blot were performed to examine the expression of BRAF mRNA and protein, respectively. The BRAF protein was knocked down by siRNAs and then examined by Western blot. The effects of BRAF in CM cells were investigated by EdU assay and Transwell assay. Overexpressing BRAF and transfecting miR-9-5p mimics into choroidal melanoma cells confirmed the interaction between miR-9-5p and BRAF.
Results:
miR-9-5p could bind to the BRAF mRNA 3’UTR and inhibit the transcription and translation of BRAF, thereby suppressing the proliferation, migration and invasion of CM cell lines. Moreover, silencing BRAF inhibited the progression of CM cells.
Conclusions:
In conclusion, this study is the first to investigate the association among BRAF, miR-9-5p and the progression of CM cells. In addition, the interaction between BRAF and miR-9-5p was explored for the first time in CM. Thus, our study suggests that miR-9-5p, BRAF and their interaction may act as potential therapeutic targets for CM.
Keywords: choroidal melanoma, miR-9-5p, BRAF, proliferation, migration, invasion
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, with approximately 90% of UM occurring in the choroid.1 In addition to the skin, the eye is the second most common place, accounting for 5% of all melanomas.2 Choroidal melanoma (CM) is fatal in approximately 50% of patients,3 and while the mechanism of CM is not yet clear, numerous researchers infer that chromosomal aberrations, genetic mutations and activation of cell signaling pathways may initiate this tumor. CM often occurs at the age of 50-60 and is easily transferred through the blood circulation.4 It carries a risk of metastasizing to the liver, lung and skin.5 Many patients have liver metastases for many years prior to the appearance of clinically or radiologically significant large metastases.6 The clinical manifestations of CM are diverse, with many complications, a high degree of malignancy, easy invasion and metastasis and a poor prognosis, which seriously threaten the vision and health of patients.7 Early detection and treatment will contribute to improving the survival rate and prognosis of patients. Hence, thorough investigation of the pathogenesis and regulatory mechanism of CM will be helpful to discover the pivotal target molecules related to tumorigenesis and provide an effective marker for the treatment of CM.
Recently, research studies have focused on ncRNAs,8 which maintain cellular function through posttranscriptional regulation and lack protein-coding capacity. MicroRNAs (miRNAs), a type of ncRNA primarily binding to the 3’UTR of mRNA, influence cell proliferation, metastasis and differentiation.9,10 MiR-9-5p has been revealed to regulate the complicated biological process of many diseases.11-13 However, the regulatory modes and mechanisms of miR-9-5p in CM have not been reviewed and remain to be explored deeply.
V-raf murine sarcoma viral homolog B1 (BRAF) is a serine/threonine kinase involved in the MAPK (RAS/BRAF/MEK/ERK) pathway that acts as a proto-oncogene.14 Since 2002, scholars have successively shown that high frequency BRAF mutations exist in melanoma,15 and the most common mutation is the mutation from T to A at a single site in the exon 15 kinase domain. The missense mutation occurs when the thymine residue at nucleotide 1799 is replaced by the adenine residue, which is called the BRAF V600E mutation.16 This mutation has also been detected in thyroid carcinomas,17 colorectal cancer18 and many other tumors, activating the MAPK pathway and leading to tumorigenesis. In summary, accumulating evidence indicates that BRAF is a significant factor in the process of tumor development.
To further illuminate the impact of miR-9-5p on CM development, the CM cell lines MUM-2B and MUM-2C were used to conduct a series of in vitro experiments. We predicted the downstream gene of miR-9-5p by a bioinformatics website to further explore the mechanism of miR-9-5p in the progression of CM. The above prediction was verified by the dual luciferase reporter assay. We also constructed BRAF-overexpressing stable cells to verify the interaction between miR-9-5p and BRAF rigorously. Considering our results, we might better understand the pathogenesis and discover a novel strategy for the treatment of CM.
Materials and Methods
Cell Culture, Plasmids, Transfection and Stable Cell Lines Establishment
The human aggressive CM eye cell lines MUM-2B and MUM-2C were purchased from Shanghai Bioleaf Biotech (China). All cryopreserved cell lines were rapidly thawed in 60°C water and cultured in MEM (HyClone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) to replace the liquid cryopreservation medium. Then, the cells were incubated at 37°C in an incubator containing 5% CO2.
The small interfering RNAs (siRNAs) targeting BRAF, miR-9-5p mimics, miR-9-5p inhibitor, negative control (NC) and inhibitor negative control (inhibitor NC) were synthesized by GenePharma (Suzhou, China). The BRAF overexpression (BRAF-OE) plasmid and pEGFP-N1 empty vector (Vector) were purchased from GeneChem (Shanghai, China). The above RNAs and plasmids were transfected into MUM-2B and MUM-2C cells with LipofectamineTM 3000 according to the manufacturer’s guidelines (Life Technologies Corporation, USA). Target cell lines that overexpressed BRAF were selected with 600 μg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA) until stable cell lines were established. The above RNA sequences were as follows: si-BRAF-1, 5’-GCAGAUUACAG
UGGGACAATT-3’; si-BRAF-2, 5’-CCAUAUCAUUGAGACCAAATT-3’; miR-9-5p mimics, 5’-UCUUUGGUUAUCUAGCUGUAUGA-3’; miR-9-5p NC, 5’-UUCUCCGAA
CGUGUCACGUTT-3’; miR-9-5p inhibitor, 5’-UCAUACAGCUAGAUAACCAAAGA-3’; and miR-9-5p inhibitor NC, 5’-CAGUACUUUUGUGUAGUACAA-3’.
Quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from cells according to the instructions of the MiRcute miRNA Extraction and Separation Kit (Tiangen, Beijing). The concentration and purity of RNA were measured by a microspectrophotometer (BioDrop, UK). BRAF and miR-9-5p cDNA were synthesized with Prime Script RT Master Mix (Takara, Dalian) and Mir-XTM miRNA First-Strand Synthesis Kit (Takara, Dalian), respectively. The real-time PCR primer sequences are displayed in Table 1. To measure the relative levels of both miRNA and mRNA, a LightCycler 480 (Roche, Swiss) was used for PCR with a SYBR Premix Ex TaqTM kit (Takara, Dalian). Each sample was assayed 3 times. The experimental results were quantitatively analyzed by the 2-ΔΔCt method.
Table 1.
Real-Time PCR Primer Sequences.
| Name | Primer sequences (5’-3’) |
|---|---|
| miR-9-5p foward | CGCGGTCTTTGGTTATCTAGCTGTATGA |
| U6 foward | CGCTTCGGCAGCACATATAC |
| U6 reverse | TTCACGAATTTGCGTGTCAT |
| BRAF foward | AATACACCAGCAAGCTAGATGC |
| BRAF reverse | AATCAGTTCCGTTCCCCAGAG |
| β-actin foward | ACTTAGTTGCGTTACACCCTT |
| β-actin reverse | GTCACCTTCACCGTTCCA |
| miR, microRNA | |
Western Blot
CM cells were lysed with RIPA buffer (lysis buffer) containing protease inhibitor to extract total protein. The above procedure was performed on ice. The BCA Protein Assay Kit (Shanghai Beyotime Biotechnology, China) was used to measure the protein concentration according to the manufacturer’s instructions. The protein fractions were separated by 10% SDS-PAGE (Beijing Leagene Biotech, China) at 40 μg/well and 140 V. Then, the isolated protein was transferred onto PVDF membranes. After being blocked in 5% skim milk for 2 h at 37°C, the membranes were probed with primary rabbit anti-B-Raf (1:1,000; no. #14814, CST, USA) and rabbit anti-GAPDH (1:5,000; no. 5174s, CST, USA) antibodies overnight at 4°C. The membranes were washed with TBST 3 times for 5 min and incubated with the appropriate secondary antibody for 1 h at 37°C. After the membranes were washed 3 times with TBST, the specific proteins were detected by microchemistry 4.2 (Bio-Rad, CA, USA). GAPDH was used as an internal reference to standardize the expression of the target protein. The relative gene protein expression differences were calculated by ImageJ (National Institute of Health, Bethesda, MD, USA).
Double Luciferase Reporter Assay
Using the pMIR-REPORT Luciferase plasmid (Shanghai Heyuan Biotechnology, China) as the vector, the wild-type sequence of BRAF 3’UTR (WT), which could bind to miR-9-5p, and the mutant-type sequence of BRAF 3’UTR (MUT), which could not bind to miR-9-5p, were cloned into the vector. The Renilla luciferase-expressing plasmid pMIR-GLO was used as an internal reference. The experimental groups were divided into 4 groups: WT + NC, WT + miR-9-5p mimics, MUT + NC and MUT + miR-9-5p mimics. The plasmids and RNAs were cotransfected into 293 T cells with LipofectamineTM 3000 as the transfection reagent. Luciferase activity was detected by a Biotek multifunctional enzyme marker (America Biotek Biological Science and Technology, USA) using a Promega Double Luciferase Reporter Assay Kit (Promega, USA).
5-Ethynyl-2’–Deoxyuridine Assay
This assay was used to detect cell proliferation. After transfection with siRNAs or miR-9-5p (NC, mimics, inhibitor NC, inhibitor) for 48 h, cells were incubated with 5-ethynyl-2’–deoxyuridine assay (EdU) (Shanghai Beyotime Biotechnology, China) for 4 h. Then, 4% paraformaldehyde was used to fix cells for 15 min, and 0.5% Trion X-100 was used to permeabilize the cells for 10 min. Each time, the cells were washed with PBS 3 times. Cells were incubated in click reaction buffer for 1 h in the dark, and then cell nuclei were labeled with Hoechst 33342 for 15 min. Images were observed by a fluorescence microscope (Olympus Corporation, Japan).
Transwell Assay
After conventional transfection for 48 h, the cells were digested, centrifuged, suspended in serum-free medium and counted by a cell counter. A total of 600 µl culture medium containing 10% serum was added to the 24-well plate, and 200 µl of serum-free medium containing 20,000 cells or 40,000 cells was added to the chamber in the absence or presence of Matrigel. The cells were placed in an incubator at 37°C containing 5% CO2 for 24 h. The samples were stained with crystal violet for 10 min, washed with PBS, observed and imaged under a microscope, and the images were stored in tiff format. The numbers of migrating and invading cells were calculated by ImageJ.
Statistical Analysis
All of the above experiments were independently repeated 3 times, and the data are expressed as the mean ± SD. Statistical analyses were conducted using GraphPad Prism 7.0 (La Jolla, CA, USA) statistical software. The experimental data were analyzed by Student’s t-test. The difference in experimental results was statistically significant when P < 0.05.
Results
MiR-9-5p Suppressed the Proliferation of CM Cells
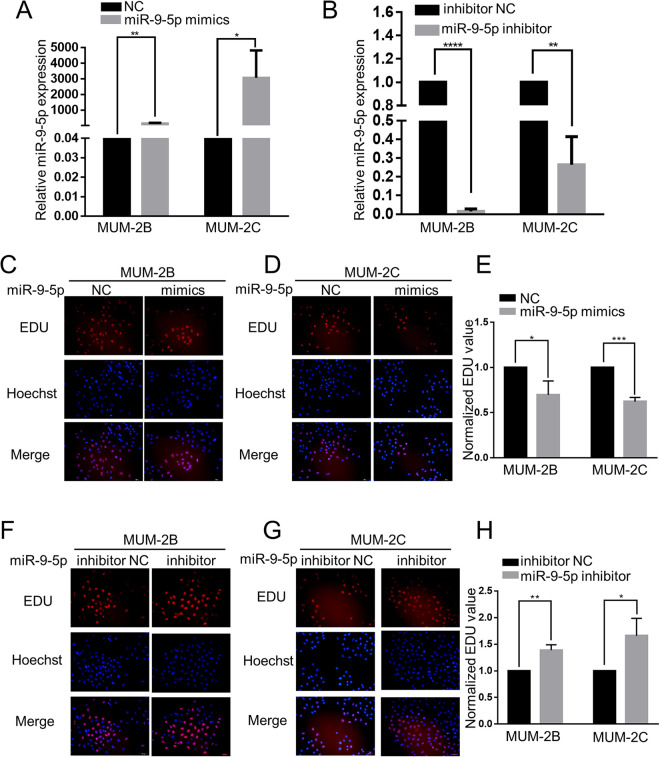
According to numerous studies, miR-9-5p is an important factor in different tumors. In this study, we explored the function of miR-9-5p in the progression of CM. RT-qPCR was used to detect the expression of miR-9-5p. In contrast to that in the NC group, miR-9-5p was obviously upregulated in the miR-9-5p mimic group (P < 0.05), while in contrast to the inhibitor NC group, miR-9-5p was obviously downregulated in the miR-9-5p inhibitor group (P < 0.05) (Figure 1A and B). EdU assays were carried out to examine the changes in MUM-2B and MUM-2C cells treated with miR-9-5p mimics and inhibitor. Compared to the NC group, miR-9-5p mimics significantly inhibited cell proliferation (P < 0.05) (Figure 1C-E). On the other hand, compared to the inhibitor NC group, the miR-9-5p inhibitor group had significantly enhanced cell proliferation (P < 0.05) (Figure 1F-H). These results confirm that miR-9-5p inhibits the proliferation of CM cells.
Figure 1.
MiR-9-5p suppressed the proliferation of choroidal melanoma cells. A and B, RT-qPCR was used to detect the expression of miR-9-5p. miR-9-5p was obviously upregulated in choroidal melanoma cells after transfection with miR-9-5p mimics, while miR-9-5p was obviously downregulated in choroidal melanoma cells after transfection with miR-9-5p inhibitor. ****P < 0.0001, **P < 0.01, *P < 0.05. C, D and E, EdU assays were used to examine the proliferation of MUM-2B and MUM-2C cells after transfection with miR-9-5p mimics. Compared to that in the NC group, the EdU value was clearly decreased. ***P < 0.001, *P < 0.05. F, G and H, EdU assays were used to examine the proliferation of MUM-2B and MUM-2C cells after transfection with miR-9-5p inhibitor. Compared to that in the inhibitor NC group, the EdU value was significantly increased. **P < 0.01,*P < 0.05. (NC, miR-9-5p negative control; inhibitor NC, miR-9-5p inhibitor negative control).
Effect of miR-9-5p on CM Cell Migration and Invasion
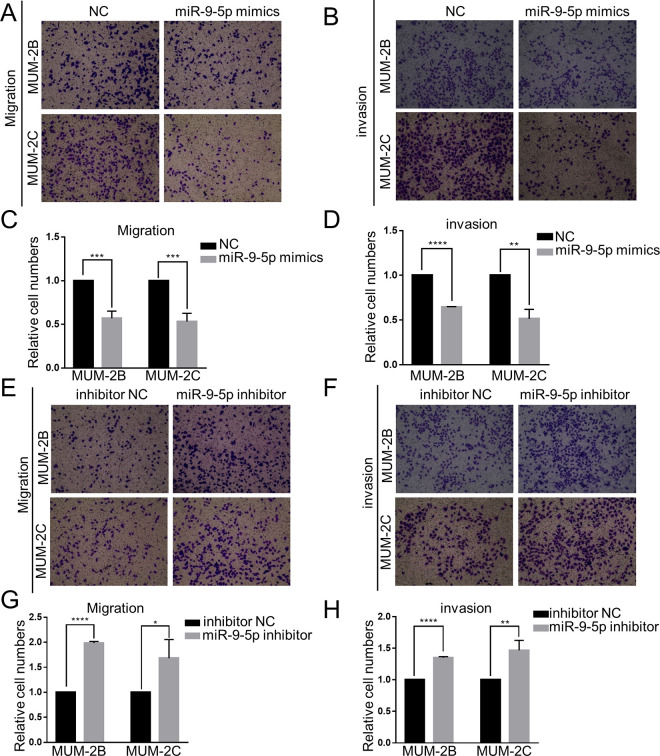
To further explore the effect of miR-9-5p in CM cells, Transwell experiments were conducted. The Transwell migration assay used chambers without Matrigel, while the invasion assay used chambers with Matrigel. The number of migrating and invading cells that exited the chambers was remarkably decreased in the miR-9-5p mimic group (P < 0.05) (Figure 2A-D). On the other hand, the number of migrating and invading cells that exited the chamber was remarkably increased in the miR-9-5p inhibitor group (P < 0.05) (Figure 2E-H). These results were identical in both MUM-2B and MUM-2C cells. This evidence strongly suggests that miR-9-5p inhibits the migration and invasion of CM cells.
Figure 2.
Effect of miR-9-5p on choroidal melanoma cell migration and invasion. A, B, C and D, Transwell migration and invasion assays were performed to detect the migration and invasion ability of choroidal melanoma cells after transfection with miR-9-5p mimics. Migration and invasion were notably inhibited in the miR-9-5p mimic group. ****P < 0.0001, ***P < 0.001, **P < 0.01. E, F, G and H, Transwell migration and invasion assays were performed to detect the migration and invasion ability of choroidal melanoma cells after transfection with miR-9-5p inhibitor. Migration and invasion were notably enhanced in the miR-9-5p inhibitor group. ****P < 0.0001, **P < 0.01, *P < 0.05. (NC, miR-9-5p negative control; inhibitor NC, miR-9-5p inhibitor negative control).
MiR-9-5p Regulates BRAF Transcription and Translation by Targeting a Specific Sequence in the BRAF mRNA 3’UTR
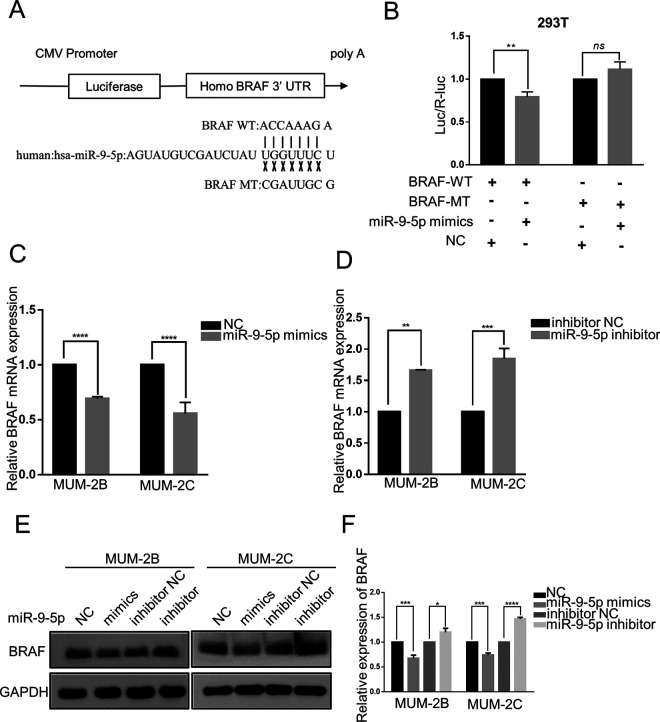
Recently, miRNAs were proven to act as important factors in the process of formation and progression of many tumors and regulate target gene expression by binding to the 3’UTR of the target gene mRNA. Through the TargetScan algorithm (http://www.targetscan.org/vert_71/), a conserved binding site for miR-9-5p within the BRAF mRNA 3’UTR was found. To further explore the relationship between miR-9-5p and BRAF, we constructed BRAF 3’UTR wild-type (WT) and mutant (MUT) luciferase reporter vectors (Figure 3A). They were cotransfected into 293 T cells with miR-9-5p mimics and miR-9-5p NC. Luciferase activity was obviously decreased in the miR-9-5p mimics + WT group compared with the other groups (P < 0.05) (Figure 3B). RT-qPCR was performed to examine BRAF mRNA expression. The expression of BRAF mRNA was downregulated when MUM-2B and MUM-2C cells were transfected with miR-9-5p mimics (P < 0.05) (Figure 3C), while the expression of BRAF mRNA was upregulated when MUM-2B and MUM-2C cells were transfected with miR-9-5p inhibitor (P < 0.05) (Figure 3D). Then, Western blotting was conducted to detect the expression of BRAF protein. Compared to that in the NC group, BRAF protein was downregulated in the miR-9-5p mimics group (P < 0.05), while compared to that in the inhibitor NC group, BRAF protein was upregulated in the miR-9-5p inhibitor group (P < 0.05) (Figure 3E and F). These experimental results showed that miR-9-5p negatively regulated BRAF transcription and translation by targeting the BRAF mRNA 3’UTR.
Figure 3.
miR-9-5p regulates BRAF transcription and translation by targeting the BRAF mRNA 3’UTR. A, The sequences of BRAF mRNA 3’UTR wild-type (WT) and mutant (MT) luciferase reporter vectors. B, After cotransfection with miR-9 mimics and WT (or MT) vector into HEK-293 T cells, luciferase activity was detected. miR-9-5p bound to wild-type BRAF sequences to reduce luciferase activity but could not inhibit mutant luciferase activity. **P < 0.01. C and D, miR-9-5p blocked the expression of BRAF mRNA. ****P < 0.0001, ***P < 0.001, **P < 0.01. E and F, miR-9-5p blocked the expression of BRAF protein. ****P < 0.0001, ***P < 0.001, *P < 0.05. (NC, miR-9-5p negative control; inhibitor NC, miR-9-5p inhibitor negative control; ns, P > 0.05).
Silencing BRAF Inhibited the Proliferation, Migration and Invasion of CM Cell Lines
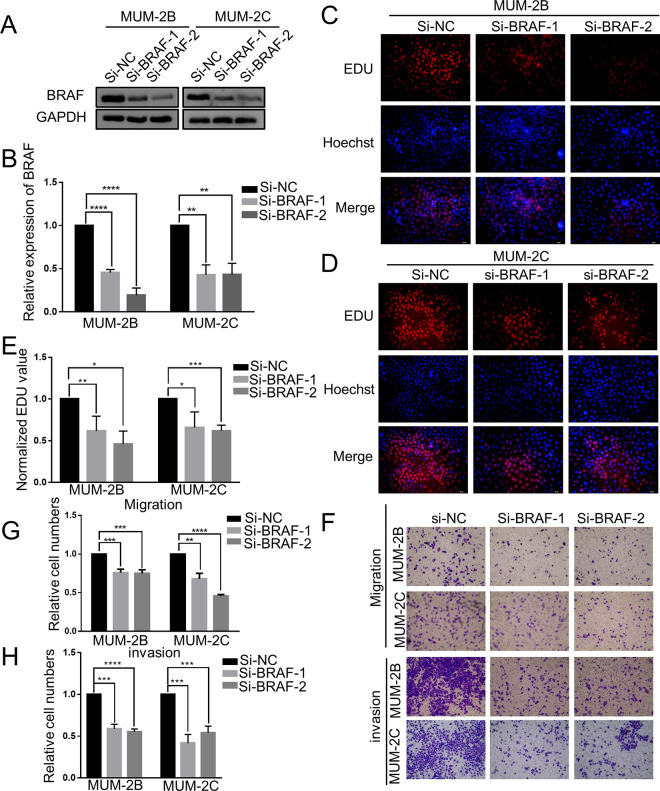
Based on previous experimental results, we continued to investigate the effect of BRAF on CM cells and conducted Western blot, EdU, Transwell migration and Transwell invasion assays. Western blot analysis showed that BRAF protein expression was notably downregulated after transfection with si-BRAF-1 and si-BRAF-2 (P < 0.05) (Figure 4A and B). Next, an EdU assay was performed to detect the effect of BRAF on the proliferation of MUM-2B and MUM-2C cell lines. Compared to the NC group, knockdown of BRAF with 2 siRNAs significantly suppressed the proliferation ability of MUM-2B (P < 0.05) (Figure 4C and E) and MUM-2C cells (P < 0.05) (Figure 4D and E). Then, Transwell assays were carried out to reveal the changes in the migration and invasion of MUM-2B and MUM-2C cells with BRAF knockdown. After the cells were treated with the 2 siRNAs in the Transwell assay, the numbers of migrating and invading cells decreased significantly (P < 0.05) (Figure 4F-H). The above results indicated the close relationship between low expression of BRAF protein and CM cell proliferation, migration and invasion.
Figure 4.
Silencing BRAF inhibited the proliferation, migration and invasion of choroidal melanoma cells. A and B, Western blot analysis revealed that the expression of BRAF protein was remarkably downregulated by transfecting si-BRAF-1 and si-BRAF-2. ****P < 0.0001, **P < 0.01. C, D and E, Compared to the NC group, silencing BRAF by 2 siRNAs inhibited cell proliferation. ****P < 0.0001, ***P < 0.001, **P < 0.01. F, G and H, After silencing BRAF by 2 siRNAs, the numbers of migrating and invading cells were noticeably decreased. ****P < 0.0001, ***P < 0.001. (si-NC, siRNA negative control).
The Effect of miR-9-5p on Decreasing BRAF Protein Levels Was Reversed in Stable Cells Overexpressing BRAF
On the basis of the above results, we overexpressed BRAF to further explore whether the above functional effects are due to direct regulation of BRAF. Western blot analysis demonstrated that stable cell lines were successfully constructed (Figure 5A). The effect of miR-9-5p mimics on BRAF protein expression was reversed by overexpressing BRAF in MUM-2B and MUM-2C cell lines (P < 0.05) (Figure 5B and C). We concluded that miR-9-5p decreases BRAF protein expression by directly targeting BRAF.
Figure 5.
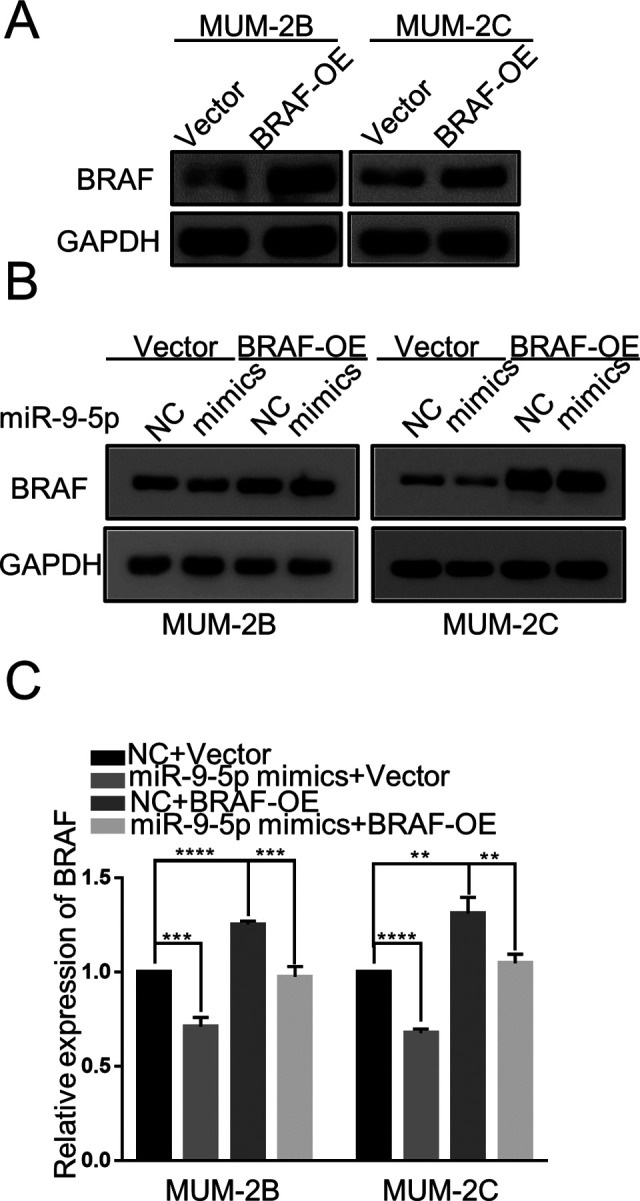
The effect of miR-9-5p on decreasing BRAF protein was reversed in stable cells expressing the BRAF-OE plasmid. A, Western blot analysis showed that the BRAF protein was overexpressed in the stable MUM-2B and MUM-2C cell lines transfected with the BRAF-OE plasmid. B and C, Compared with stable cells containing vector only, overexpression of BRAF restored the expression of BRAF protein in cells transfected with miR-9-5p mimics. ****P < 0.0001, ***P < 0.001, **P < 0.01. (NC, miR-9-5p negative control; BRAF-OE, BRAF overexpression).
The Effect of miR-9-5p on CM Cells Was Reversed in Stable Cells Overexpressing BRAF
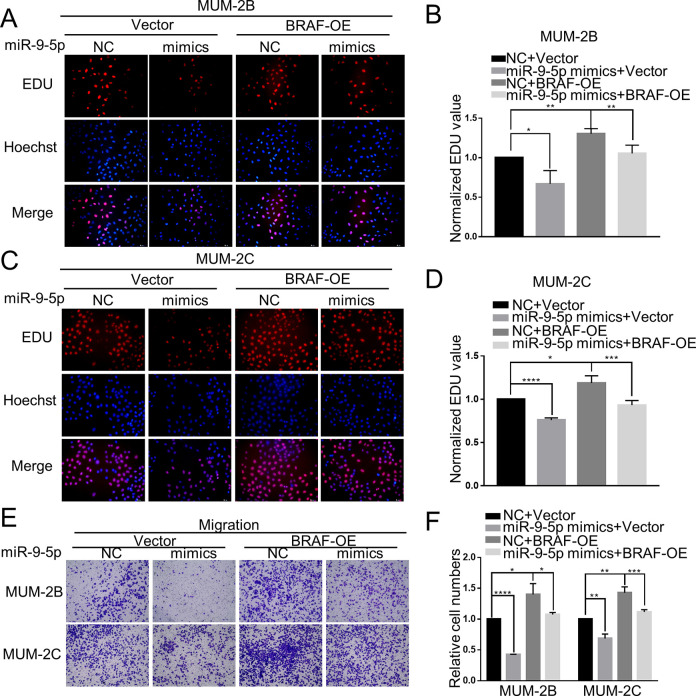
Although we found that miR-9-5p can regulate the proliferation and migration of CM cells, we wanted to confirm that the detected functional performance is achieved through direct targeting of BRAF. The inhibitory effect of miR-9-5p mimics on proliferation was also reduced in the stable MUM-2B (P < 0.05) and MUM-2C (P < 0.05) cell lines transfected with the BRAF overexpression plasmid (Figure 6A-D). The suppression of migration by miR-9-5p mimics was inverted in the stable MUM-2B (P < 0.05) and MUM-2C (P < 0.05) cell lines transfected with the BRAF overexpression plasmid (Figure 6E and F). Taking the above results into account, we confirmed that miR-9-5p inhibits the proliferation and migration of CM cells by targeting BRAF.
Figure 6.
The effect of miR-9-5p on CM cells was reversed in stable cells overexpressing BRAF. A, B, C and D, Inhibition of proliferation by miR-9-5p was enhanced in the stable MUM-2B and MUM-2C cell lines transfected with the BRAF overexpression plasmid. E and F, Suppression of migration by miR-9-5p was inverted in the stable MUM-2B and MUM-2C cell lines transfected with the BRAF-OE plasmid. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. (NC, miR-9-5p negative control, BRAF-OE, BRAF overexpression).
Discussion
Choroidal melanoma (CM), the most common primary intraocular malignant tumor in adults, has an annual incidence of approximately 20 per million. This tumor has a long latent and is prone to metastasis, with a mortality of approximately 50%.19 Patients with CM show a variety of clinical manifestations, many complications and a high degree of malignancy, which seriously threatens the vision and health of patients. The current treatment methods for CM include radiotherapy, percutaneous hyperthermia, removal and surgical resection of the tumor,20 but the therapeutic effects are not ideal. Therefore, we should deeply explore the molecular mechanism of occurrence and development of CM to form a better and more efficient method for the prevention and treatment of CM.
In recent years, microRNAs (miRNAs), which have approximately 21 nucleotides,21 have been studied extensively and have been demonstrated to play a regulatory role in various tumors. These molecules usually bind to the 3’UTR of mRNAs to regulate the expression of target genes and then affect the activation and inhibition of signaling pathways, which control the occurrence and development of tumors.22 Many studies have reported that miRNAs such as miR-155,23 miR-216a-5p,24 and miR-2125 negatively or positively regulate the biological function of CM by targeting specific genes. In addition, accumulating reports have also found that miRNAs play a key role in the proliferation, metabolism and apoptosis of UM 26; for example, miR-296-3p and FOXCUT act as tumor supressor of CM by jointly targeting MMP-2 and MMP-9.27 Another miRNA, miR-9-5p, has also been widely studied. Recent reports have indicated that miR-9-5p plays an important role in numerous tumors, such as gastric cancer,12,28 metastatic renal cell carcinoma29 and pancreatic cancer,30 regulating the proliferation, migration, invasion and other functions of tumor cells. MiR-9-5p inhibits the proliferation of glioblastoma cells by directly targeting FOXP2.31 In addition, miR-9-5p could inhibit the migration and invasion of colorectal cancer by targeting FOXP2 and then inhibit metastasis and the EMT process.32 Studies have shown that miR-9-5p has dual functions in different tumors. So far, the regulatory effect of miR-9-5p in CM has not been reported, so we hypothesized that miR-9-5p may play a key role in CM cell proliferation, migration and invasion.
In the present study, we discovered that miR-9-5p influenced the progression of CM cells. The EdU assay showed that miR-9-5p mimics could inhibit the proliferation of CM cells, while the miR-9-5p inhibitor could enhance the proliferation of CM cells. Transwell assays showed that miR-9-5p mimics could decrease the numbers of migrating and invading cells, while miR-9-5p inhibitor could increase the numbers of migrating and invading cells. Consequently, miR-9-5p may suppress the proliferation, migration and invasion of CM.
To further study the target gene of miR-9-5p in CM cells, we predicted that BRAF mRNA may be bound to miR-9-5p through a bioinformatics website. Then, we tested the targeted relationship between miR-9-5p and BRAF with a dual luciferase reporter assay. The results confirmed an interaction between miR-9-5p and BRAF, which was consistent with the previous prediction. Western blot and RT-qPCR showed that miR-9-5p mimics could downregulate the expression of BRAF, while miR-9-5p inhibitor could upregulate the expression of BRAF. Comprehensive analysis of the above evidence suggests that miR-9-5p is likely to exert a regulatory effect on CM cells by directly targeting BRAF.
It has been reported that BRAF, which is a proto-oncogene, is an important member of the MAPK signaling pathway. The most common mutation of BRAF is V600E, which is most frequently present in melanoma.33 In addition, it has been investigated in a variety of tumors and has been shown to be involved in the occurrence and progression of cancer, which provides a new perspective for the treatment of tumors. Colorectal cancer with the BRAF V600E mutation can be effectively treated by combining inhibitors of BRAF, MEK and EGFR proteins.18 Then, we explored the effect of BRAF on CM cells by transfecting siRNAs into cells and detected the proliferation, migration and invasion of cells. The results indicated that silencing BRAF inhibited the progression of CM cells.
To make the experiment more rigorous and further confirm the mechanisms of miR-9-5p targeting BRAF, we constructed stable cell lines with BRAF overexpression or empty vector that were transfected with miR-9-5p mimics. The results showed that BRAF overexpression reversed the results of the above experiments, such as BRAF protein expression and CM cell proliferation and migration. This further indicated that miR-9-5p may possess negative regulatory functions in CM cells by targeting BRAF.
In conclusion, our study proved that miR-9-5p may function as a tumor suppressor, inhibiting the proliferation, migration and invasion of CM cells by targeting BRAF. In addition, BRAF, as a downstream gene of miR-9-5p, promotes the proliferation, migration and invasion of CM cells. However, in our study, the main purpose of our experiment was to observe the function of choroidal melanoma cells, and the pathways and factors related to cell function will be further discussed in future investigations. Furthermore, due to the lack of clinical tissue samples, in subsequent experiments, we will focus on investigating the expression of miR-9-5p in choroidal melanoma tissues and its correlation with clinical characteristics.
Acknowledgments
The authors would like to thank the staff members of Department of ophthalmology, the First Hospital of China Medical University, for their valuable suggestions and assistance.
Abbreviations
- CM
choroidal melanoma
- UM
uveal melanoma
- EdU
5-ethynyl-2’–deoxyuridine
- BRAF
V-raf murine sarcoma viral homolog B1
- miRNA
microRNA
- 3’UTR
3’ -untranslated region
Authors’ Note: The study did not involve ethical review.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The current study was funded by the Natural Science Foundation of Liaoning Province (grant no. 20180551171).
ORCID iD: Hong Ning  https://orcid.org/0000-0003-4958-5268
https://orcid.org/0000-0003-4958-5268
References
- 1. Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond). 2017;31(2):241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amaro A, Gangemi R, Piaggio F, et al. The biology of uveal melanoma. Cancer Metastasis Rev. 2017;36(1):109–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damato B, Eleuteri A, Taktak AF, Coupland SE. Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res. 2011;30(5):285–295. [DOI] [PubMed] [Google Scholar]
- 4. Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46(1):151–166. [DOI] [PubMed] [Google Scholar]
- 5. Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014;25(3):177–185. [DOI] [PubMed] [Google Scholar]
- 6. Johnson DB, Daniels AB. Continued poor survival in metastatic uveal melanoma: implications for molecular prognostication, surveillance imaging, adjuvant therapy, and clinical trials. JAMA Ophthalmol. 2018;136(9):986–988. [DOI] [PubMed] [Google Scholar]
- 7. Frenkel S, Rosenne H, Briscoe D, et al. Long-term uveal melanoma survivors: measuring their quality of life. Acta Ophthalmol. 2018;96(4):e421–e426. [DOI] [PubMed] [Google Scholar]
- 8. Costa FF. Non-coding RNAs: meet thy masters. Bioessays. 2010;32(7):599–608. [DOI] [PubMed] [Google Scholar]
- 9. Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11(12):849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Amore S, Hardfeldt J, Cariello M, et al. Identification of miR-9-5p as direct regulator of ABCA1 and HDL-driven reverse cholesterol transport in circulating CD14+ cells of patients with metabolic syndrome. Cardiovasc Res. 2018;114(8):1154–1164. [DOI] [PubMed] [Google Scholar]
- 12. Fan Y, Shi Y, Lin Z, et al. miR-9-5p suppresses malignant biological behaviors of human gastric cancer cells by negative regulation of TNFAIP8L3. Dig Dis Sci. 2019;64(10):2823–2829. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Sun L, Jia K, Wang H, Wang X. miR-9-5p modulates the progression of Parkinson’s disease by targeting SIRT1. Neurosci Lett. 2019;701:226–233. [DOI] [PubMed] [Google Scholar]
- 14. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 15. Oikonomou E, Koustas E, Goulielmaki M, Pintzas A. BRAF vs RAS oncogenes: are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget. 2014;5(23):11752–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calipel A, Lefevre G, Pouponnot C, Mouriaux F, Eychene A, Mascarelli F. Mutation of B-Raf in human choroidal melanoma cells mediates cell proliferation and transformation through the MEK/ERK pathway. J Biol Chem. 2003;278(43):42409–42418. [DOI] [PubMed] [Google Scholar]
- 17. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janku F. Advances on the BRAF front in colorectal cancer. Cancer Discov. 2018;8(4):389–391. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Tang D, Wu T, Sun F. ELF1-mediated LUCAT1 promotes choroidal melanoma by modulating RBX1 expression. Cancer Med. 2020;9(6):2160–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamza HS, Elhusseiny AM. Choroidal melanoma resection. Middle East Afr J Ophthalmol. 2018;25(2):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. [DOI] [PubMed] [Google Scholar]
- 22. Anfossi S, Fu X, Nagvekar R, Calin GA. MicroRNAs, regulatory messengers inside and outside cancer cells. Adv Exp Med Biol. 2018;1056:87–108. [DOI] [PubMed] [Google Scholar]
- 23. Peng J, Liu H, Liu C. MiR-155 promotes uveal melanoma cell proliferation and invasion by regulating NDFIP1 expression. Technol Cancer Res Treat. 2017;16(6):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Huo Y, Wang D, et al. MiR-216a-5p/hexokinase 2 axis regulates uveal melanoma growth through modulation of Warburg effect. Biochem Biophys Res Commun. 2018;501(4):885–892. [DOI] [PubMed] [Google Scholar]
- 25. Wang YC, Yang X, Wei WB, Xu XL. Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein. Int J Ophthalmol. 2018;11(8):1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russo A, Caltabiano R, Longo A, et al. Increased levels of miRNA-146a in serum and histologic samples of patients with uveal melanoma. Front Pharmacol. 2016;7:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Hu Y, Cui J, Zhou Y, Chen L. Coordinated targeting of MMP-2/MMP-9 by miR-296-3p/FOXCUT exerts tumor-suppressing effects in choroidal malignant melanoma. Mol Cell Biochem. 2018;445(1-2):25–33. [DOI] [PubMed] [Google Scholar]
- 28. Zhang X, Qian Y, Li F, Bei S, Li M, Feng L. microRNA-9 selectively targets LMX1A to promote gastric cancer cell progression. Biochem Biophys Res Commun. 2018;505(2):405–412. [DOI] [PubMed] [Google Scholar]
- 29. Ralla B, Busch J, Florcken A, et al. miR-9-5p in nephrectomy specimens is a potential predictor of primary resistance to first-line treatment with tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma. Cancers (Basel). 2018;10(9):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Wang B, Ren H, Chen W. miR-9-5p inhibits pancreatic cancer cell proliferation, invasion and glutamine metabolism by targeting GOT1. Biochem Biophys Res Commun. 2019;509(1):241–248. [DOI] [PubMed] [Google Scholar]
- 31. Zhang H, Li Y, Tan Y, et al. MiR-9-5p inhibits glioblastoma cells proliferation through directly targeting FOXP2 (Forkhead Box P2). Front Oncol. 2019;9:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang WX, Yu HL, Liu X. MiR-9-5p suppresses cell metastasis and epithelial-mesenchymal transition through targeting FOXP2 and predicts prognosis of colorectal carcinoma. Eur Rev Med Pharmacol Sci. 2019;23(15):6467–6477. [DOI] [PubMed] [Google Scholar]
- 33. Wangari-Talbot J, Chen S. Genetics of melanoma. Front Genet. 2012;3:330. [DOI] [PMC free article] [PubMed] [Google Scholar]