Abstract
Background:
Computed tomography (CT) scans are being utilized to examine the influence of skeletal muscle and visceral adipose quantity and quality on health-related outcomes in clinical populations. However, little is known about the influence of contrast administration on these parameters.
Methods:
Pre-contrast, arterial, and 3 minute post contrast CT images of 45 clear cell renal cell carcinoma patients were downloaded from The Cancer Imaging Archive and retrospectively analyzed for visceral adipose cross-sectional area (CSA) and density, and muscle CSA and density at the 3rd lumbar vertebrae. Low muscle CSA index was defined as ≤38.9 cm2/m2 for women and ≤55.4 cm2/m2 for men. Low muscle density was defined as <41 Hounsfield units (HU) for body mass index (BMI) <24.9 kg/m2 and <33 HU for BMI ≥25.0 kg/m2.
Results:
In both the arterial and 3 minute phases, contrast administration decreased visceral adipose CSA (−20.9 and −20.9 cm2, p<0.001) and increased visceral adipose density (4.8 and 5.8 HU, p<0.001), relative to pre-contrast images. Muscle CSA index marginally increased in the arterial (0.6 cm2/m2, p=0.007) and 3 minute phase (0.8 cm2/m2, p<0.001). This likely represents clinically insignificant changes, as it does not alter the identification of low muscle CSA (44.4% vs 42.2%, p=1.00). Skeletal muscle density increased in the arterial (6.4 HU, p<0.001) and 3 minute phases (8.7 HU, p<0.001), which altered the identification of low muscle density (6.7% vs. 31.1%, p<0.001).
Conclusions:
Future analyses should consider the phase of contrast during CT imaging, as it may alter the interpretations of several parameters.
Keywords: body composition, computed tomography, sarcopenia, visceral adipose, muscle quality
Introduction
Quantifying body composition in clinical populations is of increasing importance, as emerging literature is demonstrating that lower than normal skeletal muscle quantity and quality (fat infiltration), and visceral obesity are associated with several deleterious outcomes, including increased rates of morbidity1,2 and mortality.3-7 However, accurately quantifying body composition in clinical settings is challenging, as many methodologies commonly utilized in research settings are inaccurate or impractical (high cost, limited accessibility) in these populations. Axial computed tomography (CT) scans, when performed as part of standard care, provide an opportunity to accurately and reliably quantify skeletal muscle and adipose tissues.8 The cross-sectional area (CSA) of skeletal muscle and adipose tissues from a single transverse image of the 3rd lumbar vertebra has strong associations with whole body muscle and adipose tissue mass,9,10 which has led to this landmark being consistently utilized for body composition analysis in a variety of clinical populations.
However, these scans are not performed specifically for body composition purposes, and therefore several CT-dependent settings vary between patients, which may influence the analysis and interpretation of body composition results.11 Recently, van Vugt et al. (2017) and Rollins et al. (2017), demonstrated that administration of a contrast medium, which is often utilized in abdominal CT scans and cancer staging, did not change skeletal muscle CSA, however, it significantly increased mean skeletal muscle attenuation (muscle quality) in the arterial and portal-venous phase compared to the pre-contrast phase; which challenged the capacity to identify poor muscle quality. Rollins et al. (2017) also investigated the effects of contrast on total fat mass, observing significant decreases in the arterial and portal-venous phase compared to the pre-contrast phase. However, only a single investigation has examined the influence of contrast medium on visceral adipose separately from total fat mass. Vehman et al. (1996) observed that contrast administration (phase of contrast not stated) decreased visceral adipose tissue CSA, however this was performed in only 7 patients using a manually selected region of interest around the visceral cavity.
The purpose of this investigation was to evaluate changes in the visceral adipose and skeletal muscle depots before and after administration of a contrast medium. Specifically, we aimed to: 1) determine if contrast administration alters the analysis of visceral adipose tissue CSA and density, and 2) confirm the observations of contrast administration on skeletal muscle CSA and density.
Methods
Patient Cohort
CT images from 45 clear cell renal cell carcinoma patients from The Cancer Imaging Archive (TCIA) KIRC-BP group were included in this retrospective analysis.15 The data published here are in whole based upon data generated by The Cancer Genome Atlas (TCGA) Research Network: http://cancergenome.nih.gov/ and TCIA.16 CT images of patients who had repeated contrast-enhanced CT scans performed (pre-contrast, arterial, and 3 minute phases or pre-contrast and 3 minute phases) were downloaded from TCIA website and analyzed for body composition across all phases (see Table S1 for list of patient ID’s). CT scans were acquired using 120 kVp across all phases of contrast; changes in tube voltage (i.e. 100 vs. 120 kVp) has been shown influence measures of body composition.17 Phase of contrast was obtained from radiologist notes embedded within the image, but scans were confirmed visually to denote if contrast was or was not present (Figure S1). All data collected in TCGA has been collected and utilized under strict human subject protection guidelines, and institutional review board approval.
Body Composition Analysis of CT Images
Single axial cross-sectional scans of the 3rd lumbar vertebrae were analyzed for skeletal muscle and visceral adipose tissue using SliceOmatic (version 5.0) image analysis software (TomoVision, Montreal, Canada). This software applies specific Hounsfield unit (HU) thresholds to precisely quantify skeletal muscle (−29 to 150 HU) and visceral adipose tissue (−150 to −50) pixels. CSA is calculated by summing the pixels of tissues identified as skeletal muscle or visceral adipose tissue and multiplying by the pixel surface area. Muscle CSA measures normalized to a patients height squared (muscle CSA (cm2)/height2 (m2)), known as muscle CSA index, were utilized for identification of low muscularity.18 Muscle and visceral adipose tissue density was calculated as the mean radiological tissue attenuation, measured in HU, for pixels identified as skeletal muscle and visceral adipose tissue. Lower mean attenuation indicates less dense tissues; which for skeletal muscle, specifies increased lipid content within the myocytes,19 and for visceral adipose tissue, indicates increased adipocyte size and lipid stores.20 Patients were identified as having low muscle CSA index using sex specific cut-points of ≤38.9 cm2/m2 for women and ≤55.4 cm2/m2 for men,18 and low muscle density using body mass index (BMI) specific cut-points of <41 HU for BMI <24.9 kg/m2 and <33 HU for BMI≥25.0 kg/m2.21
Initially, pre-contrast scans were landmarked and analyzed as described above, however subsequent landmarking and analysis of the arterial and 3 minute phase scans were performed with reference to the pre-contrast scans (visually ensuring identical bony landmark and tissue analysis), to reduce variation in analysis, allowing a more precise quantification of the effects of contrast administration (Figure S2).
To isolate the effects of the contrast medium on visceral adipose tissue, we performed a spot analysis and selected 3 separate locations, within the same image, that were visually distinct from lean tissues within the visceral cavity (Figure S3). This approach was taken to avoid the influence of the surrounding lean tissues (i.e. intestines), which have been previously shown to be altered by contrast administration and may confound the adipose analysis.14 The largest analysis aperture, found under the region growing function in SliceOmatic, was used in patients with larger visceral adipose tissue CSA (to ensure visceral adipose tissue is distinct from surrounding organs), defined as ≥130 cm2.22 Thirty four patients (visceral adipose CSA ≥130 cm2) were included in the spot analysis, with a mean CSA, standard deviation (SD), and range of 271.1 cm2, 73.1 cm2, and 139.0 – 433.0 cm2, respectively. Spot analysis was performed on the pre-contrast and 3 minute phase CT scans.
Statistical Analysis
Normality of continuous variables was assessed using quantile-quantile plots; normality was not violated and descriptive statistics are presented as mean ±SD or mean [95% confidence interval (CI)]. Differences in descriptive variables between those patients with and without arterial phase scans was analyzed using an independent t-test. The differences and agreement between pre-contrast and 3 minute phase scans for identification of low muscle CSA index and low muscle density were analyzed using McNemar’s test and Cohen’s kappa, respectively.
The effects of contrast on skeletal muscle and visceral adipose tissue depots across pre-contrast, arterial, and 3 minute phase scans was analyzed using repeated measures linear mixed model analysis. A one-way repeated measures ANOVA was not utilized due to 23 patients not having the arterial phase scan. Linear mixed model analysis was performed for muscle CSA index, muscle density, visceral adipose CSA, and visceral adipose density, with the phase of contrast (pre-contrast, arterial, and 3 minute phase) as a fixed variable and a covariance matrix of compound symmetry. Post hoc pairwise comparison for main effects of contrast was analyzed using Bonferroni’s test.
Bland-Altman plot analysis was performed comparing muscle CSA index, muscle density, visceral adipose CSA, and visceral adipose density across all contrast phases, limits of agreement (95% CI for difference between phases) were calculated and utilized for interpretation. To ensure the validity of the limits of agreement, two diagnostic checks were examined: 1) a correlation analysis was performed for the differences against averages to assess for proportional bias, and 2) homoscedasticity of the differences was assessed visually by examining the spread of the residuals of the regression analysis against the averages.24
A Pearson correlation analysis was performed between the change in muscle/visceral adipose density (3 minutes – pre-contrast) and muscle/visceral adipose CSA (average between 3 minute and pre-contrast), to examine if the size of a tissue depot influenced the magnitude of change in density following contrast administration. Correlation coefficients were interpreted as weak (0.30-0.50), moderate (0.50-0.70 and strong (0.70-1.00).25 All analysis was performed using SPSS (IBM, Chicago, IL, USA, version 24.0) and the level of significance was set at p≤0.05.
Results
Patient cohort
The analytic cohort consisted of 45 clear cell renal cell carcinoma patients, 82% of whom were male. Patients had an average BMI of 30.2 ±4.8, with wide distributions across the normal, overweight and obese categories (Table 1). No differences in age (p=0.868), weight (p=0.444), height (0.142), or BMI (p=0.949) were observed between those patients with (n=22) arterial phase CT scans available compared to those without (n=23) (data not shown).
Table 1.
Characteristics of patient cohort
| Variable Mean ±SD |
All (n=45) |
|---|---|
| Age, years | 58.6 ±12.7 |
| Weight, kg | 92.1 ±17.6 |
| Height, m | 1.75 ±0.09 |
| Sex, % male | 82.2 |
| BMI, kg/m2 | 30.2 ±4.8 |
| Underweight <18.5 kg/m2 |
0 |
| Normal 18.5 – 24.9 kg/m2 |
9 |
| Overweight 25.0 – 29.9 kg/m2 |
11 |
| Obese ≥30.0 kg/m2 |
25 |
BMI, body mass index; SD, standard deviation
Visceral Adipose Analysis
Mean visceral adipose CSA significantly decreased in the arterial (208.5 ±97.1 cm2, p<0.001) and 3 minute phases (208.6 ±96.4 cm2, p<0.001) compared with the pre-contrast phase (229.5 ±96.4 cm2), and there were no differences observed between the arterial and 3 minute phases (Figure 1). Mean visceral adipose density significantly increased from the pre-contrast phase (−96.4 ±5.1 HU) to the arterial (−91.6 ±5.3 HU, p<0.001) and 3 minute phases (−90.6 ±5.1 HU, p<0.001) (Figure 1). Mean visceral adipose density further increased from the arterial to 3 minute phase (−91.6 ±5.3 vs −90.6 ±5.1 HU, p=0.021). The changes in visceral adipose CSA and density from the pre-contrast phase to the arterial and 3 minute phases, represent 9.1 % and 5.0 – 6.0 %, respectively (Table 2).
Figure 1.
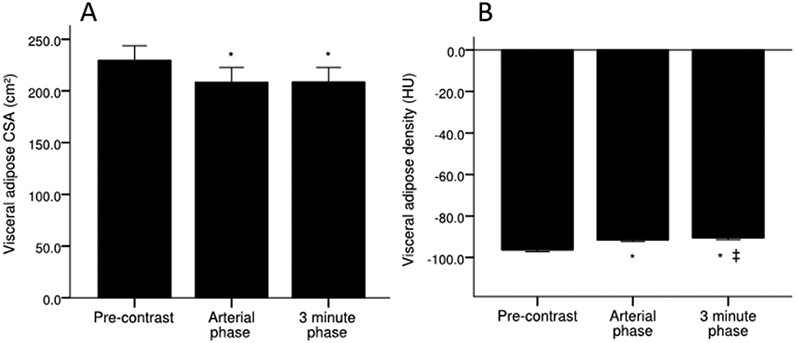
Visceral adipose A) CSA and B) density across pre-contrast, arterial, and 3 minute phase of contrast. Values are mean ±SEM. *Significantly different from pre-contrast. ǂSignificantly different from arterial phase. CSA, cross-sectional area; HU, Hounsfield unit; SEM, standard error of the mean.
Table 2.
Visceral adipose changes across all phases of contrast
| Variable [95% CI] |
Visceral adipose CSA | Visceral adipose density | ||||
|---|---|---|---|---|---|---|
| Mean difference, cm2 |
% change from pre- contrast |
p-value | Mean difference, HU |
% change from pre-contrast |
p-value | |
| Arterial phase – pre-contrast | −20.9 [−27.6 – − 14.3] | 9.1 % | <0.001 | 4.8 [4.0 – 5.6] | 5.0 % | <0.001 |
| 3 minute phase – pre-contrast | −20.9 [−25.8 – −15.9] | 9.1 % | <0.001 | 5.8 [5.1 – 6.4] | 6.0 % | <0.001 |
| 3 minute phase – arterial phase | 0.1 [−6.6 – 6.7] | - | 1.000 | 0.9 [0.1 – 1.8] | - | 0.021 |
CI, confidence interval; CSA, cross-sectional area; HU, Hounsfield unit.
No correlation was observed (r=0.17, p=0.277) between changes in visceral adipose density and visceral adipose CSA (Figure 2), indicating that the increased visceral adipose density is not associated with the size of the depot. Visceral adipose spot analysis, comparing the pre-contrast and 3 minute phase, demonstrated no changes in CSA (6.4 ±1.1 cm2 vs 6.6 ±1.2 cm2, p=0.329), despite increased in visceral adipose density (−103.8 ±4.0 HU vs −96.8 ±4.5 HU, p<0.001) (Table S2). Bland-Altman plot analysis demonstrated no proportional bias or heteroscedasticity for visceral adipose CSA and visceral adipose density across all contrast phase comparisons, with the exception of CSA for the arterial – pre-contrast phases, where proportional bias was present (Figure S4).
Figure 2.
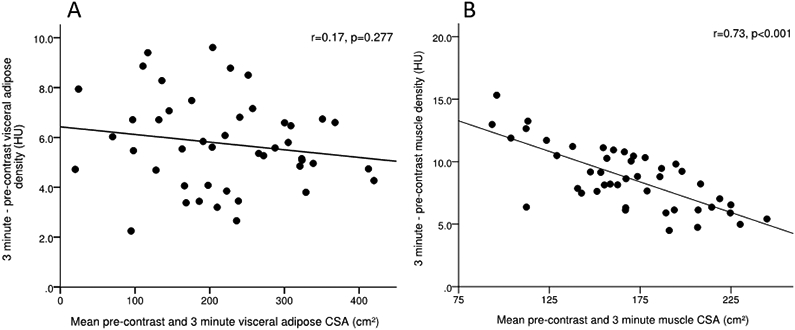
Correlation analysis between A) change in muscle density and muscle CSA and B) change in visceral adipose density and visceral adipose CSA from the 3 minute to pre-contrast phase. CSA, cross-sectional area; HU, Hounsfield unit.
Skeletal Muscle Analysis
Following contrast administration, mean muscle CSA index marginally increased in the arterial (54.9 ±9.6 cm2/m2, p=0.007) and 3 minute phase (55.0 ±9.6 cm2/m2, p<0.001), compared to the pre-contrast phase (54.3 ±9.6 cm2/m2), with no differences observed between the arterial and 3 minute phase (Figure 3). Importantly, these data represent <2% change in pre-contrast muscle CSA index (Table 3), which is likely to be clinically insignificant. Furthermore, there was no difference (44.4 % vs 42.2 %) and strong agreement (κ=0.956, p<0.001) for the identification of low muscle CSA index between the pre-contrast and 3 minute phases (Table 4).
Figure 3.
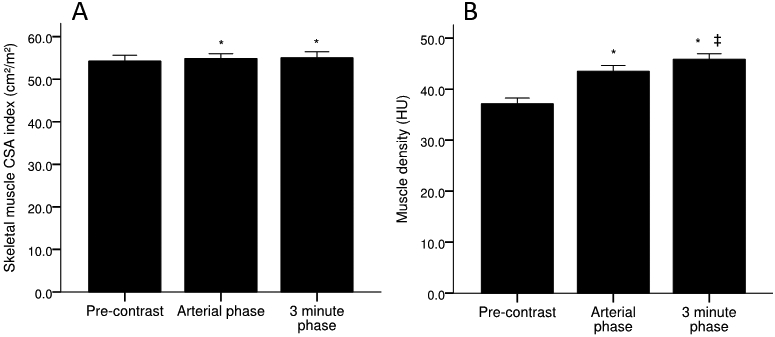
Skeletal muscle A) CSA index and B) density across pre-contrast, arterial, and 3 minute phase of contrast. Values are mean ±SEM. *Significantly different from pre-contrast. ǂSignificantly different from arterial phase. CSA, cross-sectional area; HU, Hounsfield unit; SEM, standard error of the mean.
Table 3.
Skeletal muscle changes across all phases of contrast
| Variable [95% CI] |
Muscle CSA index | Muscle density | ||||
|---|---|---|---|---|---|---|
| Mean difference, cm2/m2 |
% change from pre-contrast |
p-value | Mean difference, HU |
% change from pre-contrast |
p-value | |
| Arterial phase – pre-contrast | 0.6 [0.1 – 1.1] | 1.2 % | 0.007 | 6.4 [5.2 – 7.5] | 17.2 % | <0.001 |
| 3 minute phase – pre-contrast | 0.8 [0.4 – 1.2] | 1.4 % | <0.001 | 8.7 [7.9 – 9.6] | 23.4 % | <0.001 |
| 3 minute phase – arterial phase | 0.1 [−0.4 – 0.6] | - | 1.000 | 2.4 [1.2 – 3.5] | - | <0.001 |
CI, confidence interval; CSA, cross-sectional area; HU, Hounsfield unit.
Table 4.
Identification of low muscle CSA index and density between pre-contrast and 3 minute phase
| Pre-contrast | 3 minute phase |
p-value | |
|---|---|---|---|
| Muscle CSA index | |||
| Identified as low, n/N | 20/45 | 19/45 | 1.000 |
| Kappa Agreement | 0.956 | <0.001 | |
| Muscle density | |||
| Identified as low, n/N | 14/45 | 3/45 | 0.001 |
| Kappa Agreement | 0.273 | 0.008 | |
Compared to the pre-contrast phase (37.2 ±7.5 HU), mean skeletal muscle density significantly increased in the arterial (43.5 ±7.7 HU, p<0.001) and 3 minute phases (45.9 ±7.5 HU, p<0.001) (Figure 3). Unlike skeletal muscle CSA index, mean skeletal muscle density further increased in the 3 minute phase compared to the arterial phase (43.5 ±7.7 vs. 45.9 ±7.5 HU, p<0.001) (Figure 3). These alterations in muscle density likely represent a clinically significant change, as they represent 17.2 % and 23.5 % increases compared to pre-contrast values for the arterial and 3 minute phases, respectively (Table 3). The changes in muscle density from the pre-contrast to 3 minute phase resulted in significant differences in the identification of low muscle density (31.1 % vs 6.7 %, p<0.001), resulting in poor agreement between these phases (κ=0.256, p=0.008) (Table 4).
Correlation analysis of the change in muscle density from the 3 minute to pre-contrast phase and muscle CSA demonstrated a strong inverse (r=0.73, p<0.001) association (Figure 2); indicating that those individuals on the lower end of muscle CSA have larger increases in muscle attenuation following contrast administration. Bland-Altman plot analysis demonstrated no proportional bias or heteroscedasticity for muscle CSA index and muscle density across all contrast phase comparisons (Figure S5).
Discussion
In the present study, among clear cell renal cell carcinoma patients, we demonstrated that administration of a contrast medium alters CT-based measures of body composition by: 1) increasing visceral adipose attenuation and decreasing visceral adipose CSA, and 2) increasing skeletal muscle attenuation, without changing muscle CSA index. The artificial increase in skeletal muscle attenuation altered the identification of individuals with low skeletal muscle density, which may be more pronounced in those individuals with lower CSA; however, the identification of individuals with low skeletal muscle CSA index was not changed.
CT image analysis for body composition assessments provides a means to accurately and reliability quantify skeletal muscle and specific adipose tissue depots (i.e. visceral) in diverse clinical populations.26-28 These assessments may provide clinicians and researchers the opportunities to determine if the quantity or quality of body composition measures aid in determining cancer risk and prognosis3,6,7 or differentially modulate the response to a targeted intervention (i.e. increased protein intake in critically ill patients with low muscle CSA). In order to investigate these relationships using CT scans, a standardized approach is needed to ensure results are comparable across different studies; and determining if particular variables, such as administration of a contrast medium, alters our interpretation of the results is crucial.
Contrast medium has significant implications on visceral adiposity measures using CT analysis
In agreement with Vehman et al. (1996), this study demonstrated that visceral adipose CSA is decreased and visceral adipose attenuation is increased following contrast administration. These alterations in visceral adipose pose as a potential confounding factor if post-contrast scans are used for interpretation, as changes in visceral adipose tissue are associated with increased risk of cardiometabolic disease29,30 and earlier mortality in cachectic patients.3 For example, Di Sebastiano et al. (2013) observed in pancreatic cancer patients that the mean visceral adipose CSA loss from time of diagnosis to time of death was 52.8 ±55.0 cm2, which the mean change (−20.9 ±18.1 cm2) and Bland-Altman limits of agreement (±30.4 cm2) for the 3 minute – pre-contrast phase, would have substantial impact on the observed changes if the phase of contrast was not accounted for across longitudinal scans.
The quality of visceral adipose is also emerging as an important factor to consider in the risk of cardiometabolic disease.30,31 Several studies have observed that decreased visceral adipose attenuation, indicative of adipocyte hypertrophy and increased lipid stores, is associated with hypertension, insulin resistance, and metabolic syndrome in adults.2,32,33 If these findings were to be applied to clinical populations, in which the only available scans were post-contrast, the effects of the contrast medium would need to be taken into account, as here we observed artificial increases in visceral adipose density, which may falsely present as less lipid storage within the adipocytes.
Definitively determining the influence contrast medium on visceral adipose is challenging, as time-dependent movement of organs (i.e. intestines) within the viscera may also influence these measures (Figure S2). However, examination of the Bland-Altman plots of the 3 minute – pre-contrast and the 3 minute – arterial phase for visceral adipose CSA, provides two pieces of evidence that these changes are predominately due to contrast and not time. First, the 3 minute – pre-contrast Bland-Altman plot demonstrates that 91% of the patients present with a decreased visceral adipose CSA following contrast administration, resulting in a systematic difference in the mean CSA. One would expect that random movement of lean tissues within the viscera would lead to changes on an individual level, but not a systematic difference. Second, by examining the 3 minute – arterial phase, we observe exactly what we would expect, individual changes in CSA (limits of agreement = ± 18.0 cm2), but no systematic bias being present (mean difference = 0.7 cm2). These data suggest that although random time-dependent changes in the lean tissues within the viscera will effect visceral adipose CSA at the individual level, the administered contrast medium resulted in a substantial, systematic reduction in visceral adipose CSA.
After observing the 20.9 cm2 reduction in visceral adipose CSA post contrast administration, we initially hypothesized that this was due to visceral adipose pixels surrounding the lean tissues (i.e. intestines) increasing in attenuation above the upper HU threshold for visceral adipose (−50 HU). By performing the spot analysis (Figure S3), we observed that increases in attenuation also occurred in areas distinct from lean tissues, which may have occurred from the contrast medium entering adipose tissue depots or from contrast medium entering the microvasculature of the visceral adipose. However, no difference was observed in the CSA for the spot analysis between the pre-contrast and 3 minute phase, which may indicate that the loss in CSA is from the pixels surrounding the lean tissues, however, this is purely speculative.
Contrast enhanced CT images may confound assessment of skeletal muscle density
Our findings are in agreement with van Vugt et al. (2017) who found that contrast leads to a marginal, but statistically significant, increase in skeletal muscle CSA. However, these increases can be considered clinically insignificant because they don’t alter the identification of patients with low muscle CSA, in agreement with both van Vugt et al. (2017) and Rollins et al. (2017). In addition, the percent increase, compared with pre-contrast, is less than the intra-rater reliability coefficient of variation (<2%) generally observed in clinical populations.4,26 Therefore, if muscle CSA is the main outcome, it appears that post contrast phases, at least up to 3 minutes, can be utilized for accurate and valid quantification; despite these findings, further validation is certainly warranted.
In agreement with van Vugt et al. (2017) and Rollins et al. (2017), the present study demonstrated that skeletal muscle density is significantly increased following contrast administration, leading to poor identification of low muscle density. Interestingly, we observed that the change in muscle attenuation between the 3 minute and pre-contrast scans was inversely associated with muscle CSA, which may generate additional challenges in identifying low muscle density amongst individuals with low muscle CSA when a contrast medium has been administered. Taken together, these factors may preclude the use post-contrast phase scans for assessment of muscle density because the change in density is highly variable between patients, and would be difficult to accurately correct for this factor.
Limitations and conclusions
The most significant limitation of this retrospective analysis is that the type and dose of contrast may not have been standardized across all patients, however tube voltage (120 kVp), which has been shown to alter muscle density,17 was consistent across all scans. While the precise influence of different contrast agents and protocols is unknown, our results are in agreement with both van Vugt et al. (2017) and Rollins et al. (2017), which applied a highly standardised contrast administration approach. Moreover, due to our small, homogeneous sample size, which consisted of predominately male patients (82%), our results may have limited generalizability to other patient cohorts, which warrants further work, particularly if any sex differences are exist.
In conclusion, we demonstrated that skeletal muscle CSA increases post contrast administration, but to a clinically insignificant degree. However, skeletal muscle density, visceral adipose CSA, and visceral adipose density are significantly changed following contrast administration in both the arterial and 3 minute phase. This change in skeletal muscle density has significant impact on the identification of individuals with low density, particularly in those individuals with lower muscle CSA. Future analyses should consider if a contrast medium has been administered when interpreting CT based body composition analysis.
Supplementary Material
Clinical Relevancy Statement.
Computed tomography based measures of body composition are increasingly being utilized to examine nutritional status, identify patients at risk for complications, and improve prognosis and outcomes for several clinical populations. However, because these scans are often taken as part of routine clinical practice, several factors, such as the use of a contrast medium, are not standardized across all patients. Here, we demonstrate that administration of a contrast medium alters the analysis of visceral adipose cross-sectional area and visceral adipose and skeletal muscle attenuation. Future research should take into consideration the phase of contrast when performing computed tomography based analysis of body composition.
Acknowledgments
Financial disclosure: This work was supported by National Cancer Institute Cancer Center Core Grant (P30 CA008748), Chanel Endowment for Survivorship Research from Memorial Sloan Kettering Cancer Center, Early Researcher Award, and Canadian Foundation for Innovation.
List of Abbreviations
- ANOVA
Analysis of variance
- BMI
Body mass index
- CI
Confidence interval
- CSA
Cross-sectional area
- CT
Computed tomography
- HU
Hounsfield unit
- SD
Standard deviation
- SEM
Standard error of the mean
- TCGA
The Cancer Genome Atlas
- TCIA
The Cancer Imaging Archive
Footnotes
Conflict of Interest: None declared.
References
- 1.van Dijk DPJ, Bakens MJAM, Coolsen MME, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Sebastiano KM, Yang L, Zbuk K, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr. 2013;109(2):302–12. [DOI] [PubMed] [Google Scholar]
- 4.Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looijaard WGPM, Dekker IM, Stapel SN, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrieling A, Kampman E, Knijnenburg NC, et al. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2016. [DOI] [PubMed] [Google Scholar]
- 7.Psutka SP, Boorjian S a, Moynagh MR, et al. Decreased Skeletal Muscle Mass is Associated with an Increased Risk of Mortality after Radical Nephrectomy for Localized Renal Cell Cancer. J Urol. 2015;195(2):270–276. [DOI] [PubMed] [Google Scholar]
- 8.Paris M, Mourtzakis M. Assessment of skeletal muscle mass in critically ill patients. Curr Opin Clin Nutr Metab Care. 2016;19(2):125–130. [DOI] [PubMed] [Google Scholar]
- 9.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 10.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae KT. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology. 2010;256(1):32–61. [DOI] [PubMed] [Google Scholar]
- 12.van Vugt JLA, Coebergh van den Braak RRJ, Schippers HJW, et al. Contrast-enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Rollins KE, Javanmard-Emamghissi H, Awwad A, Macdonald IA, Fearon KCH, Lobo DN. Body composition measurement using computed tomography: Does the phase of the scan matter? Nutrition. 2017;41:37–44. [DOI] [PubMed] [Google Scholar]
- 14.Vehman T, Kairemo K, Taavitsainen M. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes. 1996;20:570–73. [PubMed] [Google Scholar]
- 15.Akin O, Elnajjar P, Heller M, et al. Radiology Data from the Cencer Genome Atlas Kidney Renal Clear Cell Carcinoma [TCGA-KIRC] collection. Cancer Imaging Arch. 2016. [Google Scholar]
- 16.Clark K, Vendt B, Smith K, et al. The cancer imaging archive (TCIA): Maintaining and operating a public information repository. J Digit Imaging. 2013;26(6):1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Werf A, Dekker IM, Meijerink MR, Wierdsma NJ, de van der Schueren MAE, Langius JAE. Skeletal muscle analyses: Agreement between non-contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin Physiol Funct Imaging. 2017. [DOI] [PubMed] [Google Scholar]
- 18.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. [DOI] [PubMed] [Google Scholar]
- 20.Côté JA, Nazare J-A, Nadeau M, et al. Computed tomography-measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte. 2016;5(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin L, Birdsell L, MacDonald N, et al. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J Clin Oncol. 2013;31(12):1539–1547. [DOI] [PubMed] [Google Scholar]
- 22.Hunter GR, Snyder SW, Kekes-Szabo T, Nicholson C, Berland L. Intra-abdominal adipose tissue values associated with risk of possessing elevated blood lipids and blood pressure. Obes Res. 1994;2(6):563–568. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. [DOI] [PubMed] [Google Scholar]
- 24.Ludbrook J. Confidence in Altman-Bland plots: A critical review of the method of differences. Clin Exp Pharmacol Physiol. 2010;37(2):143–149. [DOI] [PubMed] [Google Scholar]
- 25.Mukaka MM. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research Definitions of correlation and clarifications. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Paris MT, Mourtzakis M, Day A, et al. Validation of Bedside Ultrasound of Muscle Layer Thickness of the Quadriceps in the Critically Ill Patient (VALIDUM Study). J Parenter Enter Nutr. 2017;41(2):171–180. [DOI] [PubMed] [Google Scholar]
- 27.Tandon P, Ney M, Irwin I, et al. Severe Muscle Depletion in Patients on the Liver Transplant Wait List: Its Prevalence and Independent Prognostic Value. Liver Transplant. 2012;18:1209–1216. [DOI] [PubMed] [Google Scholar]
- 28.Pak K, Lee SH, Lee JG, Seok JW, Kim IJ. Comparison of visceral fat measures with cardiometabolic risk factors in healthy adults. PLoS One. 2016;11(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenquist KJ, Pedley A, Massaro JM, et al. Visceral and Subcutaneous Fat Quality is Associated with Cardiometabolic Risk. Int J Cardiovasc Imaging. 2013;6(7):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvey NJ, Pedley A, Rosenquist KJ, et al. Association of fat density with subclinical atherosclerosis. J Am Heart Assoc. 2014;3(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therkelsen KE, Pedley A, Rosenquist KJ, et al. Adipose tissue attenuation as a marker of adipose tissue quality: Associations with six-year changes in body weight. Obesity. 2016;24(2):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of Changes in Abdominal Fat Quantity and Quality With Incident Cardiovascular Disease Risk Factors. J Am Coll Cardiol. 2016;68(14):1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J, McKeown NM, Hwang S-J, Hoffman U, Jacques PF, Fox CS. Sugar-Sweetened Beverage Consumption is Associated With Change of Visceral Adipose Tissue Over 6 Years of Follow-Up. Circulation. 2016:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


