Abstract
Coronavirus disease 2019 (COVID‐19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). COVID‐19 mainly causes damage to the lung, as well as other organs and systems such as the hearts, the immune system and so on. Although the pathogenesis of COVID‐19 has been fully elucidated, there is no specific therapy for the disease at present, and most treatments are limited to supportive care. Stem cell therapy may be a potential treatment for refractory and unmanageable pulmonary illnesses, which has shown some promising results in preclinical studies. In this review, we systematically summarize the pathogenic progression and potential mechanisms underlying stem cell therapy in COVID‐19, and registered COVID‐19 clinical trials. Of all the stem cell therapies touted for COVID‐19 treatment, mesenchymal stem cells (MSCs) or MSC‐like derivatives have been the most promising in preclinical studies and clinical trials so far. MSCs have been suggested to ameliorate the cytokine release syndrome (CRS) and protect alveolar epithelial cells by secreting many kinds of factors, demonstrating safety and possible efficacy in COVID‐19 patients with acute respiratory distress syndrome (ARDS). However, considering the consistency and uniformity of stem cell quality cannot be quantified nor guaranteed at this point, more work remains to be done in the future.
Keywords: clinical trials, COVID‐19, mesenchymal stem cells, stem cell therapy
The potential mechanisms of MSCs therapy for COVID‐19. MSCs have great therapeutic potential in immunomodulation and tissue repair through secretion of soluble paracrine protein factors and exosomes. MSCs can regulate the functions of a variety of immune cells, secrete several cytokines, promote tissue repair and regeneration, and may play important therapeutic roles in patients with COVID‐19. MSCs: mesenchymal stem cells; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; FGF, fibroblast growth factor; TGF‐β, transforming growth factor‐β; TNF‐α, tumor necrosis factor‐α; MSC‐exo, exosomes.
1. INTRODUCTION
In December 2019, an outbreak of unidentifiable pneumonia cases was first officially reported in Wuhan, China. It was subsequently confirmed that the pneumonia is an acute respiratory infectious disease caused by infection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel β‐coronavirus which had never been reported before. 1 , 2 As the global epidemic grew and spread rapidly, the World Health Organization (WHO) officially named the new type of disease as coronavirus disease 2019 (COVID‐19). COVID‐19 was confirmed to be more contagious than either the severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), with a confusing manifestation ranging from asymptomatic patients to severely ill patients with acute respiratory distress syndrome (ARDS) and pulmonary fibrosis, amongst several potential health problems, and has had disastrous consequences for public health management.
2. PATHOGENESIS OF COVID‐19
2.1. The novel coronavirus and its infection pathways
Initially named 2019‐nCoV, SARS‐CoV‐2 belongs to the subfamily Coronavirinae in the family Coronaviridae of the order Nidovirales. As a single‐stranded positive‐stranded RNA virus of the β subclass of the coronavirus genus, it is only the 7th human coronavirus to be discovered. The virus has a diameter of about 80‐120 nm and a full‐length genome of approximately 29.9 kb. It can encode 29 proteins and has 79% homology to the SARS virus sequence. The spike glycoprotein (S protein) on its surface is an essential structural protein that mediates its invasion into human cells. Through the host cell receptor‐angiotensin converting enzyme 2 (ACE2), SARS‐CoV‐2 adsorbs onto and enters the host, then replicates, assembles, and releases a large number of viral particles. 3 ACE2 is expressed on the surface membrane of alveolar, tracheal and bronchial epithelial cells in the lung, and monocytes and macrophages in the immune system. It can also be expressed in the heart, kidney and intestines. ACE2 lowers blood pressure and regulates the renin‐angiotensin system by inactivating angiotensin II (Ang II) produced by ACE, and serves as a crucial regulator of pulmonary oedema. SARS‐CoV‐2 utilizes a highly glycosylated homotrimeric S protein to enter the host cell, and its affinity for ACE2 is 10‐20 times that of SARS virus, thus enhancing its transmissibility. 4 In fact, although the fatality rate of COVID‐19 is lower than SARS (9.6%) and MERS (34.4%), its higher infectious rate has led to a much wider outbreak with significantly more complications in epidemic prevention and control, due to a large number of asymptomatic and mild patients (Figure 1).
FIGURE 1.
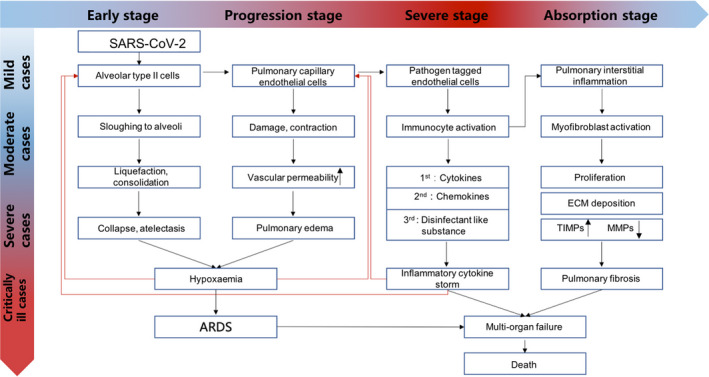
COVID‐19 pathogenic progression
For patients with symptoms, the incubation period (time from exposure to onset of symptoms) has a wide range but averages to ~4‐5 days. 5 The most common symptoms include dry cough, fever and shortness of breath. Other common symptoms are myalgia, fatigue, sore throat, nausea, vomiting, diarrhoea, conjunctivitis, anorexia and headache (cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html). For a small number of severely ill patients, the disease begins to worsen about 5‐10 days after the onset of symptoms, and complications such as acute respiratory distress syndrome (ARDS) and other end‐organ failures could occur. 6 The mortality rate is significantly higher amongst elderly adults over 65. Adults with underlying cardiovascular disease, respiratory disease, endocrine metabolic disease, diabetes or a weakened immune system are the most vulnerable to serious complications of COVID‐19. 7
2.2. Cytokine release syndrome
One of the reasons for aggravated severe illness in COVID‐19 patients aggravation is the excessive immune response associated with the cytokine release syndrome (CRS), which in turn leads to lung tissue damage, repair imbalance and respiratory failure. The patient could also eventually die from multiple organ failure. 8 CRS, also known as a 'cytokine storm', is an aberrant systemic inflammatory response that can be triggered by multiple factors such as severe infections and certain drugs. The clinical manifestation is the sharp rise of a large number of cytokines within a short time frame. Analysis of plasma cytokine levels in 41 confirmed cases of COVID‐19 in China revealed that, compared with healthy adults, ICU and non‐ICU hospitalized patients’ levels of IP‐10, MCP‐1, MIP‐1A, MIP1‐B, PDGF, TNF‐α and VEGF were significantly increased. 9 Extremely high concentrations of IL‐6, GCSF, CRP and TNF‐α have also been recorded in COVID‐19 patients. 10 The excessive inflammatory CRS could also promote thrombosis and deaths by thromboembolism in critically ill patients. 11 , 12 , 13 At present, there is no specific treatment for CRS. In clinical practice, glucocorticoid injections for systemic immune suppression and cytokine inhibitors have been the primary methods. However, the use of glucocorticoids in viral pneumonia carries additional risks of steroid treatment sequelae such as diabetes and osteonecrosis, so there has been controversy in the academic community.
2.3. Acute respiratory distress syndrome
ARDS refers to acute progressive hypoxic respiratory failure caused by various pulmonary and extrapulmonary pathogenic factors other than cardiogenic. 14 According to the Berlin definition, patients with less severe hypoxaemia (as defined by a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of 300 or less) are considered to have acute lung injury (ALI), and those with more severe hypoxaemia (as defined by a ratio of 200 or less) are considered to have the ARDS. 15 , 16 ARDS is a continuous pathological process, and its early stage is ALI. The main manifestations are sudden progressive dyspnoea, varying degrees of cough, less sputum, late cough and bloody sputum. Arterial hypoxaemia is a characteristic feature, which is irresistible to oxygen therapy. If PaCO2 increased, it indicated that the patient is in critical condition. Early chest X‐ray is often negative, and then, interstitial pulmonary oedema occurs, manifested as two lungs scattered in different sizes and fuzzy edge of the patchy increased density shadow. Pulmonary interstitial fibrosis may occur in the late stage. Multiple organ failure may occur after the disease develops. 15
2.4. Pulmonary fibrosis
Although many patients who develop ARDS survive the acute phase of the disease, and might even be discharged, a large proportion of them die subsequently from progressive pulmonary fibrosis. 17 Dysregulation of matrix metalloproteinases in the inflammatory phase of ARDS could lead to a complex combination of epithelial and endothelial damage, and thus uncontrolled fibrosis. 18 Continuous and aberrant activation of epithelial cells could lead to cellular senescence and overactive secretion of pro‐fibrotic growth factors, chemokines, vascular inhibitors and procoagulant mediators. These factors are collectively referred to as senescence‐associated secretory phenotype (SASP) factors. 19 SASP factors can lead to abnormal wound healing, which is characterized by a dysregulated crosstalk between epithelial cells and mesenchymal cells, and the consequent accumulation of myofibroblasts. Fibroblasts and myofibroblasts in fibrotic lungs exhibit markers of stress and senescence, including resistance to apoptosis and excessive production of extracellular matrix components. 20 The resultant increase in matrix stiffness could affect the microenvironment and thus the crosstalk between fibroblasts and epithelial cells, resulting in irreversible damage and fibrosis. 21
2.5. Pathological anatomy
Studies have found that COVID‐19 can cause multiple organ and tissue damage, especially in the respiratory system. 22 , 23 The tracheal and bronchial mucosa exhibited hyperaemia and increased secretions. 13 Although the pathological characteristics of lung lesions caused by SARS‐CoV‐2 are similar to SARS, there were also significant differences. Parenchymal areas contain diffuse alveolar injury and exudative inflammation. 24 The alveolar cavity is often filled with serum, fibrin exudate and extensive transparent hyaline membrane formations, as observed in autopsies. 22 , 25 , 26 , 27 White blood cells that infiltrate the alveoli are mainly monocytes and macrophages. Type II lung alveolar cell proliferation and focal lung cell shedding can be observed. Pulmonary interstitial fibrosis is frequently observed in cases with a long duration of the disease.
COVID‐19 can also affect multiple organs with varying degrees of acute damage. SARS‐CoV‐2 was also detected in the lymph nodes, spleen, heart, liver, gallbladder, kidney, stomach, breast, skin and testis, through qRT‐PCR‐based viral nucleic acid detection, electron microscopy and immunohistochemical staining. 23 A study noted lesions in the lymphoid hematopoietic organs. 28 Lymphocytes in the spleen and lymph nodes, especially CD4+ and CD8+ T cells, were significantly reduced. Lymphocyte degeneration, necrosis and macrophage proliferation were frequently observed. The myocardium also exhibits cellular degeneration, occasional necrosis, interstitial oedema, and mild infiltration of monocytes, lymphocytes and/or neutrophils. Hepatocyte degeneration, spot necrosis, and small, bridging or large necrosis of neutrophil infiltration are found in the liver. In the kidney, hyperaemia, segmental hyperplasia or necrosis, and protein exudation in the glomerulus were observed. Sometimes pancreatic islet cell degeneration and lysis are detected. The oesophagus, stomach and intestinal mucosal epithelium showed varying degrees of degeneration, necrosis and exfoliation. The testes also showed different degrees of reduction and damage of spermatogenic cells. Brain congestion and oedema, some neuronal degeneration, and ischaemic changes were also detected.
3. POTENTIAL MECHANISMS OF STEM CELL THERAPY IN COVID‐19
Stem cells are endowed with the properties of self‐renewal and multi‐lineage differentiation potential, thus making them an attractive modality for cell therapy in the clinic. However, due to many ethical and legal restrictions, clinical development and progression of stem cell therapies have been relatively slow. 29 Because adult stem cells are exempt from the aforementioned ethical and legal restrictions, while possessing excellent tissue repair capabilities, usage of adult stem cells has been more popular than embryonic or pluripotent stem cells in the clinic. 30 Accumulating studies have shown that stem cell therapy is becoming one of the emerging treatment strategies for several refractory diseases with no known treatments, including viral infections. 31 Newly emerging viral pandemic, which could cause multi‐organ damage and for which there are no particular therapies, drugs or vaccines available, is especially amenable to stem cell therapy. With the COVID‐19 pandemic, stem cell therapies and especially mesenchymal stem cell (MSC)‐related therapies have demonstrated their therapeutic potential for newly emerging diseases with no available treatments (Figure 2).
FIGURE 2.
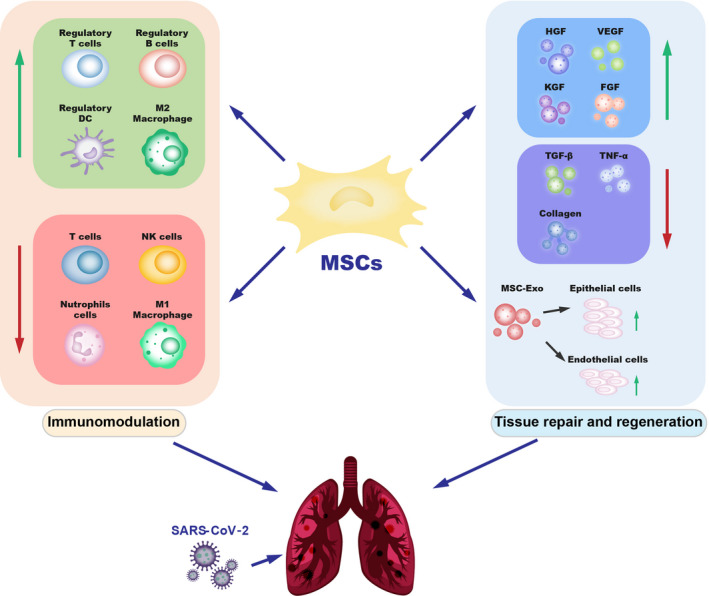
The potential mechanisms of MSCs therapy for COVID‐19. MSCs have great therapeutic potential in immunomodulation and tissue repair through secretion of soluble paracrine protein factors and exosomes. MSCs can regulate the functions of a variety of immune cells, secrete several cytokines, promote tissue repair and regeneration, and may play important therapeutic roles in patients with COVID‐19. MSCs: mesenchymal stem cells; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor; KGF, keratinocyte growth factor; FGF, fibroblast growth factor; TGF‐β, transforming growth factor‐β; TNF‐α, tumour necrosis factor‐α; MSC‐exo, exosomes
3.1. MSC‐related cells
MSCs are derived from the mesoderm and ectoderm of early embryonic development. They express specific cell surface markers such as CD73, CD90, CD105, CD29, CD44, CD146 and CD166, while being negative for CD45, CD31 and CD34. 32 , 33 , 34 MSCs are also known as mesenchymal stromal cells, and these matrix‐derived cells are capable of self‐renewal and differentiation into chondrocytes, osteoblasts and adipocytes. 35 MSCs were initially found and isolated from the bone marrow (BM), but were subsequently also discovered in various tissues such as the adipose fat pads, dental pulp, umbilical cord and placenta. 36 Currently, MSCs from different tissues are being tested for their therapeutic effects in COVID‐19. 37
MSCs express low levels of human leucocyte antigen (HLA) class I molecules, and do not express HLA class II molecules or costimulatory molecules such as CD40, CD40L, CD80 and CD86. This expression profile allows MSCs to escape the cytotoxic effects of lymphocytic T cells, B cells and NK cells, were thus termed 'immune‐privileged' cells. 38 , 39 , 40 , 41 In addition, MSCs possess immunomodulatory and anti‐inflammatory effects, and can detect microenvironmental injury signals to direct pro‐regenerative signalling processes, 42 , 43 , 44 thus making them attractive candidates for therapeutic use in various diseases. For clinical allotransplantation, their hypo‐immunogenicity and short lifespan in vivo make them especially suitable for clinical research. Therefore, MSCs are a promising tool for the treatment of disorders involving immune dysregulation and extensive tissue damage, as is the case with COVID‐19, and multiple clinical trials have been launched. 45
Immunity‐ and matrix‐regulatory cells (IMRCs) are a new type of hESC‐derived MSC‐like cells that resemble MSCs in their capacity for self‐renewal and tri‐lineage mesenchymal differentiation. Moreover, compared to standard adult MSCs, they show enhanced immunomodulatory and anti‐fibrotic functions, and significantly extended lifespans in vitro for consistent quality in production. A recent report showed that intravenously delivered IMRCs could home into the lungs and inhibit both pulmonary inflammation and fibrosis after bleomycin‐induced acute lung injury in mouse models in vivo. 46 Moreover, a pilot study for compassionate use of IMRCs showed that they could ameliorate the ARDS in two severely ill COVID‐19 patients. IMRCs’ hyper‐immunomodulatory function, pro‐regenerative paracrine signals and functional inhibition of TGF‐β1‐induced fibrosis were potential mechanisms for amelioration of pulmonary injury. Clinical trials for IMRCs are now in progress as well.
3.2. Immunomodulatory function of MSCs
MSCs have been widely used in basic research and clinical studies on immune‐mediated inflammatory diseases, such as graft‐versus‐host disease (GvHD), Crohn's disease, inflammatory bowel disease, rheumatoid arthritis and ARDS. MSCs’ properties in immunomodulatory and anti‐inflammatory signalling make them uniquely suited for these complex multifactorial diseases, including COVID‐19. 28 As such, several clinical trials have launched for these diseases. 47 , 48 MSCs can activate immune regulatory responses through interactions with a wide repertoire of immune cells and participate in both innate immunity and adaptive immunity regulation. 49 Below, we outline some of the host immune cells that MSCs interact with, either by direct contract or indirectly through paracrine secretion of various cytokines (Figure 1) to modulate the immune cells. 50 , 51
3.2.1. T cells
MSCs broadly suppress T‐cell activation and proliferation in vitro via a plethora of soluble and cell contact‐dependent mediators. These mediators may act directly upon T cells or indirectly via modulation of antigen‐presenting cells and other accessory cells. 52 MSCs can decrease the secretion of IFN‐γ and TNF‐α of T cells, and upregulate the secretion of IL‐4, so that the cells are transformed from a pro‐inflammatory state to an anti‐inflammatory state. In addition, MSCs can inhibit abnormally activated Th1 cells, restore the Th1/Th2 balance inhibit excessive proliferation of T cells and suppress the activity of cytotoxic CD8+ T lymphocytes via the NKG2D pathway. 53 Thus, MSCs can improve the immune status by regulating the function of multiple subtypes of T cells. 54 , 55 Lymphopenia is also a prominent feature of COVID‐19, suggesting that SARS‐CoV‐2 infection causes T‐cell imbalance. 56 , 57 , 58 , 59 In fact, in severely ill COVID‐19 patients, the number of CD4+ and CD8+ T cells in the peripheral blood is often significantly reduced, while the overall immune system is abnormally activated and dysregulated by the cytokine storm during ARDS, suggesting a severe immune imbalance. Therefore, the immunomodulatory effects of MSCs and IMRCs on T cells may have potential therapeutic significance for patients with COVID‐19 and ARDS.
3.2.2. Antigen‐presenting cells
MSCs can interfere with the antigen presentation functions, differentiation and maturation of dendritic cells (DC), thereby reducing DC activation and inflammatory factor secretion. 60 MSCs regulate the differentiation of CD11c + B220‐DC precursors into regulatory DCs via prostaglandin E2 and PI3K signalling. 61 MSCs vigorously promote the proliferation of mature DCs and drive mature DCs to transdifferentiate into a novel regulatory DC population to escape their apoptotic fate. 62 In addition, MSCs can prevent DCs from secreting IFNγ and promoting T‐cell expansion in tumours. 63 Since SARS‐CoV‐2 infection also results in DC reduction, 64 MSCs could rescue DCs for the treatment of COVID‐19.
Macrophages are the other major antigen‐presenting cell type, and they are one of the cell types considered to play an essential role in ARDS. 65 MSCs can regulate macrophage polarization via secretory exosomes to suppress chronic inflammation and promote tissue healing after injury. 66 MSCs also secrete the TSG‐6 factor and IL‐10 to inhibit NF‐κB signalling and other pro‐inflammatory pathways, thereby driving the polarization of pro‐inflammatory M1 macrophages into anti‐inflammatory M2 macrophages. 54 , 67 Thus, MSCs could regulate macrophage polarization and their related signalling molecules, to modulate ARDS, the anti‐viral immunity, and tissue healing in COVID‐19 patients. 68
3.2.3. Neutrophils
Neutrophils can kill pathogens (bacteria, fungi and viruses) through an oxidative burst of reactive oxygen species (ROS) and phagocytosis, and they are recruited early to sites of infection to perform their defensive functions. 69 Paradoxically, it has been reported that excessive neutrophil recruitment might exacerbate COVID‐19 immunopathology. 70 Clinical studies have found that the number of neutrophils in the bronchoalveolar lavage fluid of ARDS patients is positively correlated with the severity of COVID‐19 and the cytokine storm. 71 In fact, the neutrophil to lymphocyte ratio (NLR) can be used as an independent risk factor for severe disease in COVID‐19 patients. 72 Intravenous injection of bone marrow mesenchymal stem cells (BMSCs)‐derived exosomes into severe COVID‐19 patients with ARDS can significantly reduce the production of neutrophils by 32%, thereby reducing their NLR levels and improving their clinical oxygenation index. 73
3.2.4. Other immune cells
MSCs can also inhibit excessive proliferation of B cells, prevent their differentiation into plasma cells and reduce excessive levels of immunoglobulin secretion by downregulating the expression of Blimp‐1. 55 After MSC treatment, overactivated CXCR3+ NK cells also disappear in 3‐6 days, showing that MSCs have a potential regulatory effect on NK cells as well. 74
3.2.5. Tissue repair and regeneration capabilities of MSCs
Severely ill COVID‐19 patients often present severe pneumonia, respiratory failure, ARDS and pulmonary fibrosis. During this complex inflammatory pathogenic process, 55 , 56 the integrity of the lung alveolar capillary membrane is gradually destroyed, contributing to the formation of pulmonary oedema, lung tissue degeneration and fibrosis. MSCs can secrete a variety of growth factors and cytokines to improve the microenvironment of the lung tissue and promote endogenous lung repair, with potential benefits for COVID‐19 patients (Figure 2). For example, MSCs can promote cell proliferation and tissue damage repair by secreting hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF) and fibroblast growth factor (FGF). 75 MSCs secrete HGF through extracellular vesicles, reduce inflammatory damage and increase autophagy, thereby retaining or restoring the alveolar epithelium and the pulmonary vascular endothelial lining. 76 , 77 The HGF, KGF and angiopoietin‐1 secreted by MSCs also possess pro‐angiogenic, anti‐inflammatory and pro‐proliferation effects. 42 It has been reported that MSCs can reduce apoptosis of the alveolar epithelial cells and endothelial cell by secretion these three growth factors. 77 , 78 , 79 The VEGF and FGF secreted by MSCs can also promote lung tissue repair. 80 , 81
In addition, MSCs can reduce the levels of pro‐fibrotic factors to improve the microenvironment of lung cells and prevent pulmonary fibrosis, especially in patients with COVID‐19. One possible mechanism for this effect is exosome regulation. Exosomes are membrane‐bound extracellular vesicles of nanometre size (70‐150 nm), which not only participate in the communication between cells, but also participate in tissue damage repair. 82 It has been demonstrated that MSCs and their exosomes (MSC‐Exo) considerably improved lung inflammation and pathological damage resulting from different types of lung injuries. MSC exosomes can promote the regeneration of alveolar epithelial cells, exert anti‐alveolar inflammation, prevent endothelial cell apoptosis, inhibit early epithelial‐mesenchymal trans‐differentiation and prevent myofibroblast growth by reducing the levels of TGF‐β, TNF‐α, type I collagen, type III collagen, hydroxyproline and serum ceruloplasmin in lung tissues, thereby alleviating pulmonary fibrosis. 83 The MSC‐like IMRCs could also reverse pulmonary fibrosis by overexpressing the matrix metalloproteinase MMP1 and reducing collagen I levels during fibrogenesis induced by TGF‐β1. 84
MSCs can also promote the repair of other damaged tissues in COVID‐19 patients. Pathological results show that SARS‐CoV‐2 virus can also affect the kidneys, causing severe acute tubular necrosis. 85 Studies have shown that MSCs secrete cytokines to activate a variety of repair mechanisms in acute kidney injury, including anti‐inflammatory, anti‐apoptotic and pro‐angiogenic pathways, thereby promoting the repair of kidney injury. 86 , 87 MSCs can also treat COVID‐19‐related intestinal injury through mucosal repair and epithelial regeneration. 88
4. STEM CELL THERAPY FOR COVID‐19 IN THE CLINIC
4.1. Guiding principles and management methods in China
According to 'The expert opinion for the prevention and therapy of novel coronavirus pneumonia' guidelines, the clinical use of stem cell therapies for COVID‐19 is still in the exploratory stage in China. Treatments should be carried out only within the scope of the emergency project managed by the Ministry of Science and Technology of the People's Republic of China, in accordance with the 'The Expert Guidance on Clinical Research and Application of Stem Cell Therapy for Novel Coronavirus Pneumonia (COVID‐19)' guidelines. Clinical research and clinical trials must be performed according to 'The Stem Cell Clinical Research Management Methods' guidelines jointly issued by the National Health Protection Commission and the National Medical Products Administration in 2015, and 'The Guidelines for Quality Control and Preclinical Study of Stem Cell Preparations (Trial)' to ensure stem cell therapies for COVID‐19 are tested in a scientifically and medically rigorous manner consistent with internationally accepted standards. 89
For example, researchers are required to work out a detailed clinical research plan and pass the scientific review of the academic committee of clinical research institutions and the ethical review of the ethics committee. All participating units should carry out clinical research under the conditions of compliance with ethics, informed consent, project filing and clinical registration. Moreover, the preparation of stem cells must be carried out according to 'The Guidelines for Quality Control and Preclinical Study of Stem Cell Preparations (Trial)', and the quality of stem cells must meet the required standards for human clinical trials of stem cell drugs and receive official approval from the National Medical Products Administration before clinical trials can be initiated.
More specifically, indications allowed for stem cell therapy in COVID‐19 include severe or critical illness from COVID‐19‐related pneumonia. Patients should receive no more than 3 rounds of stem cell infusion. The dose of stem cell injection for each round should be 1 to 5 × 106 cells/kg body weight, and the interval time between each round is recommended to be no less than 3 days. A proper clinical research programme must be designed according to the specific goals of the clinical research and the actual working conditions for clinical implementation. Multi‐centre, randomized controlled and double‐blinded trials are recommended. Patients in both the stem cell treatment arm and the control arm should receive conventional treatments recommended by the above guidelines. The placebo used in the control arm should contain only normal saline plus human serum albumin without stem cells. Follow‐up after treatment is strictly required according to the clinical protocol guidelines.
4.2. Clinical trials for COVID‐19 stem cell therapies
4.2.1. Overview
Clinical trials for stem cell therapies against COVID‐19 were searched by using the terms 'COVID‐19' and 'stem cells' in the ClinicalTrials.gov database (https://clinicaltrials.gov), the World Health Organization International Clinical Trials Registry Platform (Chinese Clinical Trial Registry, http://www.chictr.org.cn) and the European Union Clinical Trials Register (https://www.clinicaltrialsregister.eu) (September 2020) (Table S1). All observational studies and 6 withdrawn clinical studies were excluded from the list. Eventually, 88 clinical trials related to stem cells were found to be registered in different countries. In these clinical studies, the therapeutic efficacy (60 trials) and the safety (32 trials) of stem cells and their derivatives for treating COVID‐19 were being investigated.
4.2.2. Indications and phases
In total, 88 trials were found to be registered to investigate the safety and efficacy of transplantation therapy of stem cells or stem cell‐derived exosomes for COVID‐19 patients. Indications under investigation include COVID‐19 with severe/critical pneumonia, respiratory failure, ARDS and pulmonary fibrosis (Figure 3). Most studies were registered to treat patients with 'COVID‐19' (19 out of 88) and 'severe/critical pneumonia' (37 out of 88). According to a meta‐analysis of 50 466 hospitalized patients with COVID‐19, 14.8% of COVID‐19 patients developed ARDS. 90 Treatment of patients with 'ARDS' was being investigated in 24 of 88 studies. Although ARDS patients often manifest pulmonary fibrosis after hospital discharge, 90 , 91 only 2 of 88 studies were registered to investigate the efficacy of stem cell therapies in patients with 'pulmonary fibrosis', one of which uses 'MSCs derived from human embryonic stem cells' (ChiCTR2000031139). Interestingly, only 1 of 88 studies is using 'extracorporeal stromal cell therapeutics' to treat COVID‐19 patients with acute kidney injury (NCT04445220). The vast majority of these clinical trials (63 out of 88) are testing the safety of stem cell therapies for feasibility, tolerance, and severe adverse events (19 trials for Phase I, 24 for Phase I/II, and 20 for Phase II). Few clinical trials have progressed beyond Phase II (3.4%), with only 2 trials in Phase II/III and only 1 trial in Phase III. In 22 studies, the clinical phase is unclear or 'Not Applicable' (Figure 3).
FIGURE 3.
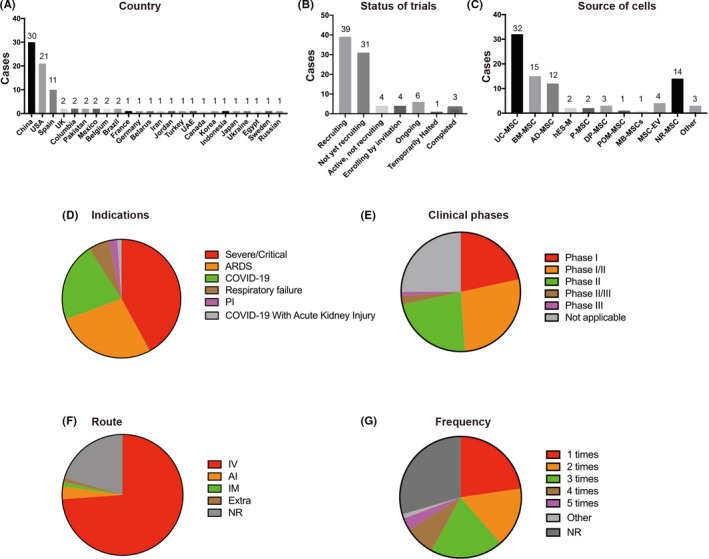
Statistical summary of countries, status of trials, source of cells, indications, clinical phases, administrative routes and frequency of doses used by stem cell therapies against COVID‐19. UC‐MSCs, umbilical cord‐derived MSCs; BM‐MSCs, bone marrow‐derived MSCs; AD‐MSCs, adipose‐derived MSCs; hES‐M, MSC‐like cells derived from human embryonic stem cells; P‐MSCs, placenta‐derived MSCs; DP‐MSC, dental pulp‐derived MSCs; MSC‐EV, extracellular vesicles derived from MSCs; POM‐MSC, pooled olfactory mucosa MSCs; MB‐MSCs, menstrual blood‐derived MSCs; ARDS, acute respiratory distress syndrome; PI, pulmonary interstitial fibrosis; IV, intravenous; IM, intramuscular; AI, aerosol inhalation; Extra, extracorporeal stromal cell therapeutic; NR, not reported
4.2.3. Country and status
Because COVID‐19 was first officially reported in China, it is unsurprising that 34% of these trials were registered in China. In China, a total of 30 clinical studies have been registered, with 18 clinical trials in the Chinese Clinical Trial Registry and 12 clinical trials in the US National Institutes of Health (Figure 3A). One clinical trial has been completed, and 16 trials are still 'Recruiting'. In China, strict quarantine rules and epidemic control measures led to rapid suppression of the COVID‐19 outbreak before the summer of 2020. As a result, many registered clinical trials could not be completed due to insufficient patients, and 13 clinical trials are 'Not yet recruiting'. Numerous other countries like the United States (21 out of 88), Spain (11 out of 88), UK (2 out of 88), France (1 out of 88), Columbia (2/88), Belgium (2 out of 88), Brazil (2 out of 88), Mexico (2 out of 88) and Pakistan (2/88) are also carrying out clinical trials for stem cell therapies against COVID‐19 (Figure 3A). Globally, the clinical trials are mainly concentrated in China, the United States and Europe, which may be related to the severity of the epidemic and the strength of their scientific research infrastructure. Most of these studies are 'Recruiting' or 'Ongoing' (29 out of 58), although 3 clinical trials have been completed (Figure 3B).
4.2.4. Source and dosing of stem cells
The source of stem cells used in these trials is a major point of variability amongst the searched studies (Figure 3C). Because of the aforementioned advantages of MSCs in immune regulation and tissue repair, it is not surprising that MSCs have become the predominant stem cell type in COVID‐19 clinical trials. The most common source of MSCs was the umbilical cord/Wharton's jelly (32 out of 88), followed by bone marrow (15 out of 88), adipose tissue (12 out of 88), dental pulp (3 out of 88), placenta (2 out of 88) and menstrual blood (1 out of 88). In 14 studies, the MSCs source was unclear. Interestingly, in 2 studies, human embryonic stem cell‐derived MSCs (hES‐M) were being used (ChiCTR2000031139, NCT04331613), likely due to the indefinite expansion potential and consistent quality associated with embryonic stem cells. Considering the limited expansion potential of adult MSCs, extracellular vesicles derived from MSCs (MSC‐EV) were also being used as an alternative in 4 studies. Pooled olfactory mucosa MSCs were also used in 1 study (NCT04382547). Finally, only 3 studies used non‐MSCs, namely cord blood stem cells, autologous immune cells and autologous non‐hematopoietic peripheral blood stem cells (NHPBSCs).
Cell dose and proposed regimens also varied greatly amongst studies. While the MSC infusion dosage ranged over an order of magnitude between 0.5 × 106 and 10 × 106 cells/kg (Table S1), the most commonly used infusion dosage was 1 × 106 cells/kg. In some studies, stem cells are infused regardless of the weight of the patient. In these cases, the proposed infusion dosage ranged from 1.5 × 107 to 75 × 107 cells per round regardless of the weight of the patient, with 10 × 107 cells per round as the most common dose (Table S1). The highest dose of 75 × 107 MSCs per round was used for the 'extracorporeal stromal cell therapeutics' against COVID‐19‐related acute kidney injury (NCT04445220). Of note, higher cell doses will likely bring higher treatment risks. Therefore, it is necessary to find a balance between therapeutic efficacy and safety concerns.
4.2.5. Route and frequency of administration
Intravenous injection of MSCs may produce a first‐order lung effect, 92 which leads to significant cell retention in the lungs, thus providing an advantage for lung tissue repair in COVID‐19, ARDS and pulmonary fibrosis. Therefore, most of the ongoing clinical trials proposed to perform intravenous cell infusion (65 out of 88; Figure 3F). Three studies focused on the administration of MSCs‐derived exosomes via the inhalation route. Intramuscular injection of MSCs was used in one study (NCT04389450). The 'extracorporeal stromal cell therapeutics' was used in COVID‐19 subjects with acute kidney injury in a study (NCT04445220). However, in 18 studies, the route of MSCs administration was not clearly stated.
Although a single round of MSC infusion, as proposed in 20 out of 88 trials, has been shown to provide therapeutic benefits, more than one round may be required to induce complete tissue repair or even to maintain therapeutic benefits (Figure 3G). In some studies, the mentioned MSC doses would be injected two (14 out of 88), three (17 out of 88), four (7 out of 88) and even five rounds (3 out of 88) with short time intervals of 2 or 3 days (Figure 3G; Table S1).
4.3. The safety and efficacy of stem cell therapy in COVID‐19
Recently, some studies have been published to report the safety and efficacy of stem cell therapy for COVID‐19 (Table 1). In these published reports, MSCs derived from the umbilical cord (UC), adipose tissue (AD), bone marrow (BM), menstrual blood (MB), dental pulp (DP), human embryonic stem cells (hESCs) and exosomes derived from MSCs were used. While several biotech companies including Athersys, Cynata, Mesoblast and Pluristem have also initiated clinical trials with MSC‐like cells for COVID‐19, some with announcements of preliminary success, we will not cover them below because not all the details of their clinical trials are available yet.
TABLE 1.
Published clinical trials of stem cell therapies against COVID‐19
| Trial ID no. | Indications | Patient population | Source of cells | Dose of cells | Route | Frequency | Clinical phases | Reference |
|---|---|---|---|---|---|---|---|---|
| ChiCTR2000029990 a | Moderate/Severe/Critical | 7 | MSCs | 1 × 106 cells/kg/round | IV | 1 round | Phase I/II | 74 |
| NR | Severe/Critical | 25 | MSCs | 1 × 106 cells/kg/round | IV | 2 or 3 rounds (interval 5 d) | NR | 97 |
| NCT 03042143 b | ARDS | 60 | UC‐MSCs | 40 × 107 cells/round | IV | 1 round | Phase I/II | 112 |
| ChiCTR2000031319 NCT04336254 | Severe/Critical | 20 | DP‐MSCs | 3 × 107 cells/round | IV | 3 rounds at day 1, 4 and 7 | Phase I/II | 113 |
| NCT04331613 | ARDS | 2 | hESC‐IMRCs (CAStem) | 3, 5, 10 × 106 cells/kg/round | IV | 1 round | Phase I | 84 |
| NR | ARDS | 24 | BM‐MSCs‐EV | 15 mL | IV | 1 round | NR | 73 |
| NCT04348461 | Severe/Critical | 13 | AD‐MSCs | 1 × 106 cells/kg/round | IV | 1, 2 or 3 rounds | Phase II | 101 |
| ChiCTR2000029606 | ARDS | 2 | MB‐MSCs | NR | IV | 3 rounds | Not Applicable | 106 |
| ChiCTR2000031494 | Severe/Critical | 41 | UC‐MSCs | 2 × 106 cells/kg/round | IV | 1 round | Phase I | 98 |
| NR | Severe/Critical | 1 | UC‐MSCs | 5 × 107 cells/round | IV | 3 rounds (interval 3 d) | NR | 99 |
| NCT04288102 | Severe/Critical | 18 | UC‐MSCs | 3 × 107 cells/round | IV | 3 rounds (on days 0, 3 and 6) | Phase I | 100 |
Abbreviations: MSCs, mesenchymal stem cells; UC‐MSCs, umbilical cord‐derived MSCs; BM‐MSCs, bone marrow‐derived MSCs; AD‐MSCs, adipose‐derived MSCs; DP‐MSCs, dental pulp‐derived MSCs; BM‐MSCs‐EV, exosomes derived from bone marrow‐derived MSCs; IV, intravenous; MB‐MSCs, menstrual blood‐derived MSCs; hESC‐IMRCs, immunity‐ and matrix‐regulatory cells from human embryonic stem cells; NR, not reported.
4.3.1. MSCs
The first study for stem cell treatment of COVID‐19 by Dr Zhao (ChiCTR2000029990) 74 reported that intravenous administration of clinical‐grade human mesenchymal stem cells (MSCs) into 7 COVID‐19 patients resulted in improved functional outcomes and facilitated recovery. In this study, 7 enrolled patients (1 critical illness, 4 severe illness, 2 moderate illness) received intravenous infusions of MSCs. After 14 days, MSCs could significantly improve the functional outcomes of all 7 patients without any adverse effects observed. The pulmonary function and symptoms of these 7 patients were significantly improved within 2 days after MSC transplantation. After treatment, the peripheral lymphocytes were increased, the C‐reactive protein decreased, and the overactivated cytokine‐secreting immune cells disappeared in 3‐6 days. Moreover, their gene expression profile showed that MSCs were negative for both ACE2 and TMPRSS2, which are required by the SARS‐CoV‐2 virus to enter host cells, 93 , 94 , 95 , 96 thus suggesting MSCs do not carry risks for SARS‐CoV‐2 cross‐contamination.
Another retrospective study evaluated the treatment efficacy and side effects of MSC therapy on severe COVID‐19. 97 In total, 25 patients were enrolled according to their inclusion/exclusion criteria. A total of 7 patients received 1 round, 7 patients received 2 rounds, and 11 patients received 3 rounds of MSCs therapy. After MSC therapy, 16 patients (64%) showed improvements by chest CT scans and all patients showed clinical improvements. No fatalities occurred during hospitalization. However, no significant changes in inflammation indices, IgG and IgM were found, and the serum levels of lactate (LAC), cardiac troponin T (cTnT) and creatine kinase MB (CK‐MB) elevated significantly after MSC therapy, suggesting little improvement in immunomodulation and some cardiotoxicity after MSC therapy. While the reasons are unclear, possibly related to a mild cytokine storm, missing the optimal detection time window and limited analysis of inflammation indices, these results serve as a reminder that stem cell clinical trials should be extremely cautious on patients with underlying metabolic diseases and their inflammatory marker analyses.
Umbilical cord‐derived MSCs (UC‐MSCs)
Shu et al reported that infusion of UC‐MSCs was effective and safe for the treatment of severe COVID‐19 (ChiCTR2000031494). 98 In this study, 12 COVID‐19 patients with severe illness received intravenous infusions of UC‐MSCs. Results showed that the 28‐day mortality rate was zero in the UC‐MSCs treatment arm, whereas the mortality rate was 10.34% in the control arm. After MSCs transplantation, the time to clinical improvement was shorter than that in the control arm. UC‐MSC infusion improved clinical symptoms, including weakness and fatigue, shortness of breath, and low oxygen saturation. UC‐MSCs could reduce inflammatory CRP and IL‐6 levels, accelerate the recovery of lymphocyte count and shorten the lung inflammation absorption period. Intravenous UC‐MSCs was found to be a safe and effective treatment option for severe COVID‐19.
Liang et al reported a case report of a critically ill COVID‐19 patient treated by UC‐MSCs. 99 The patient received 3 rounds of allogeneic UC‐MSCs (5 × 107 cells each round), together with thymosin a1 and antibiotics via daily injection. UC‐MSC infusion improved most of the laboratory indices and relieved the inflammation symptoms. Throat swab tests for SARS‐CoV‐2 turned negative on both day 21 and day 23. On day 30, the previously critically ill patient was discharged from hospital after recovery.
Wang et al reported a Phase I clinical trial of UC‐MSCs for COVID‐19 (NCT04288102). 100 COVID‐19 patients with moderate and severe pulmonary disease received 3 rounds of intravenous infusions of UC‐MSCs (3 × 107 cells per infusion) on days 0, 3 and 6, respectively. No serious infusion‐associated adverse events were observed. In most severe patients, the PaO2/FiO2 ratio improved after UC‐MSC treatment. In the UC‐MSC treatment arm, 2 moderate and 2 severe patients with high baseline IL‐6 levels showed a decrease in IL‐6 within 3 days after UC‐MSC infusion and remained stable in the following 4 days. Chest CT scans showed that the lung lesions of patients receiving UC‐MSC infusions were well controlled within 6 days, and completely disappeared within 2 weeks after UC‐MSC infusion. In the control arm, 1 severe patient still had obvious pulmonary lesions at the point of discharge.
Adipose tissue‐derived MSCs
Sanchez‐Guijo et al reported a study of Adipose tissue‐derived MSCs (AD‐MSCs) for the treatment of patients with severe COVID‐19‐related pneumonia (NCT04348461). 101 A total of 13 severe COVID‐19 pneumonia patients under mechanical ventilation received intravenous administration of AD‐MSCs. No adverse events related to cell therapy were observed. Clinical improvement was observed in 9 patients (69.2%), 7 patients were extubated and discharged from ICU, and 4 patients remained intubated. Administration of AD‐MSCs reduced the levels of inflammatory markers C‐reactive protein, IL‐6, ferritin, LDH and D‐dimer, and increased the lymphocyte counts.
Menstrual blood‐derived MSCs
In 2007, MSCs were identified in the endometrial tissues shed along with menstrual blood. 102 Since then, this subtype of MSCs has been studied in basic medical research and clinical trials. 103 , 104 , 105 Li et al reported a clinical study using Menstrual blood‐derived MSCs (MB‐MSCs) for the treatment of COVID‐19 patients with severe illness (ChiCTR2000029606). 106 MB‐MSC transplantation increased CD4 + lymphocyte counts and decreased the levels of inflammatory markers IL‐6 and C‐reactive protein. After MB‐MSC transplantation, the oxygen saturation (SaO2) and partial pressure of oxygen (PO2) improved. Additionally, the chest CT scans indicated that the patients’ bilateral lung exudate lesions were adsorbed after MB‐MSC infusion.
MSC‐like stem cells derived from hESCs
Despite their safety and efficacy, clinical applications of primary MSCs derived from the umbilical cord, bone marrow or adipose tissue have been hampered by the lack of available donor tissues, limited cell numbers from each donor, donor and tissue heterogeneity, inconsistent cell quality and the lack of standardized cell preparations. Wu et al reported a cell population derived from hESCs and named it as immunity‐ and matrix‐regulatory cells (IMRCs, also called CAStem). 84 IMRCs resembled MSCs in their capacity for self‐renewal and tri‐lineage mesenchymal differentiation, but displayed an even higher consistency in quality, even stronger immunomodulatory and anti‐fibrotic functions, and a robust ability to treat lung injury and fibrosis in vivo. On this basis, IMRCs were approved for compassionate use in a pilot study and subsequently a Phase I trial (NCT04331613), in response to the emergency of the COVID‐19 crisis in China. After patient consent, IMRCs were administered intravenously in 2 severely ill COVID‐19 patients. After IMRC transfusion, both the severely ill COVID‐19 patients showed significant recovery from pneumonia, tested negative for SARS‐CoV‐2 and were recommended for discharge within 14 days. Many pro‐inflammatory cytokines were suppressed after IMRC infusion, including GRO‐α, IFN‐α2, IL‐3, IL‐9, IL‐13, MCP‐3, M‐CSF, sCD40L and TNF‐α by day 4‐8. This is the first clinical trial for COVID‐19 treatment using hESC‐derived cells, and the preliminary results suggest both efficacy and at least short‐term safety in COVID‐19 patients with severe illness.
4.3.2. Exosomes Derived from MSCs
MSCs transplantation is limited by safety, cell survivability, scalability, consistency and regulatory issues that make it difficult to meet the needs of millions of SARS‐CoV‐2 infected patients worldwide. 74 , 99 , 107 Exosomes or extracellular vesicles (EVs) derived from MSCs present another potential option for the large numbers of COVID‐19 patients, since MSCs cultured in vitro can continuously shed large amounts of exosomes into the conditioned media instead of dying shortly after transplantation in vivo. Multiple preclinical studies have shown that exosomes from MSCs exert favourable therapeutic effects in animal models of acute lung injury (ALI), ARDS, fibrosis and other inflammatory diseases. Exosomes function by reducing inflammation, enhancing oedema clearance, promoting restoration of leaky epithelial membranes and reducing other sequelae of the cytokine storm. 108 , 109 , 110 , 111 Sengupta et al reported that exosomes (ExoFloTM) derived from BM‐MSCs could work in severe COVID‐19. 73 In this study, 24 SARS‐CoV‐2 PCR positive patients received a single 15 mL intravenous dose of ExoFlo derived from allogeneic BM‐MSCs. No adverse events were observed within 72 hours of ExoFlo administration. 83% of the patients survived, and 71% of the patients recovered. Moreover, patients’ clinical status and oxygenation index improved after just one treatment. Meanwhile, reductions of absolute neutrophil count and increases of absolute lymphocyte count were observed, that is, the NLR decreased. Likewise, ExoFlo reduced the inflammatory markers C‐reactive protein, Ferritin and D‐dimer. This study demonstrated that only a single intravenous dose of BM‐MSC‐derived exosomes could effectively and safely treat patients with severe COVID‐19. Indirectly, the clinical success of MSC‐derived exosomes also supports the idea that MSCs likely treat COVID‐19 pulmonary disease via a paracrine and secretory mechanism.
5. FUTURE PROSPECTS
Of all the stem cell therapies touted for COVID‐19 treatment, MSCs or MSC‐like derivatives have been the most promising in preclinical studies and clinical trials so far. Intravenously infused MSCs have been found to migrate directly to the lungs, where they can secrete numerous factors that play an important role in immunomodulation, protecting alveolar epithelial cells, resisting pulmonary fibrosis and improving lung function, which is a great benefit for treating severe pulmonary disease in COVID‐19. The main mechanism of their therapeutic effect is through the secretion of soluble factors, such as cytokines, chemokines, angiogenic factors, growth factors, and exosomes and extracellular vesicles. While multifactorial in nature, a large corpus of research work suggests that it is these complex mechanisms that make them suitable for treating complex and multifactorial diseases for which no other reductionistic drug treatments are available yet, such as COVID‐19‐related ARDS and other similar inflammatory diseases that involve a cytokine storm (CRS). The extraordinarily rapid global spread of SARS‐CoV‐2 and the rapidly escalating public health emergency of the COVID‐19 pandemic had essentially forced multiple institutions across the world to take the leap of faith and put multiple types of stem cell therapies into pilot studies and clinical trials. Fortunately, MSCs and MSC‐like derivatives have shown some promising results in safety and efficacy.
However, more work remains to be done. Although several clinical trials have preliminarily demonstrated the safety and efficacy of intravenous MSCs in patients with COVID‐19‐related lung diseases, the unclear heterogeneity of the sources of MSCs and thus their secretory and immunomodulatory capabilities make it difficult to compare and learn from the clinical trial results from different studies. Moreover, the consistency and uniformity of stem cell quality cannot be quantified nor guaranteed at this point. Therefore, overcoming the heterogeneity of stem cells is one of the most pressing issues of stem cell therapy in the clinic. Future work should focus on the development, dissemination and international agreement on clinical standards to quantify the quality and consistency of stem cell therapies, proper completion and publication of existing clinical trials for the COVID‐19 crisis, and the development of scalable technologies and resources for producing the large numbers of stem cells needed in a public health crisis. For nobody knows yet when the current COVID‐19 crisis will end, and when the next crisis will come.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
YT, JW and JH conceived the project and supervised the manuscript. ZL, SN, B.G and TG contributed equally to this work and wrote the manuscript with help from all the authors. ZL, SN, BG, TG, LW, YW, L.W., YT, JW and JH participated in the experiments and data analysis.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This work was supported by National Key Research and Development Program (2020YFC0843900 to QZ, 2018YFA0108400 and 2017YFA0104403 to JH, 2016YFA0101502 and 2017YFA0105000 to LW,), Beijing Municipal Science & Technology Commission (Z181100003818005 to QZ), International Partnership Program of Chinese Academy of Sciences (152111KYSB20160004 to QZ), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16030601 to LW and XDA16040502 to JH), the National Natural Science Foundation of China (31621004 to QZ), and the Key Research Projects of the Frontier Science of the Chinese Academy of Sciences (QYZDY‐SSW‐SMC002 to QZ).
Li Z, Niu S, Guo B, et al. Stem cell therapy for COVID‐19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;53:e12939 10.1111/cpr.12939
Li, Niu, Guo and Gao are contributed equally.
Contributor Information
Yuanqing Tan, Email: haojie@ioz.ac.cn, Email: wuxf@ioz.ac.cn, Email: 357769970@qq.com.
Jun Wu, Email: haojie@ioz.ac.cn, Email: wuxf@ioz.ac.cn, Email: 357769970@qq.com.
Jie Hao, Email: haojie@ioz.ac.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Table S1 of this article.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wrapp D, Wang N, Corbett KS. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walls AC, Park Y‐J, Tortorici MA, et al. Structure, Function, and Antigenicity of the SARS‐CoV‐2 Spike Glycoprotein. Cell. 2020;181:281‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus‐Infected Pneumonia. N. Engl. J. Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID‐19‐Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China (vol 395, pg 497, 2020). Lancet. 2020;395:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 11. Klok FA, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: An updated analysis. Thromb Res. 2020;191:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beyrouti R, Adams ME, Benjamin La, et al. Characteristics of ischaemic stroke associated with COVID‐19. J Neurol Neurosurg Psychiatry. 2020;91:889‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334‐1349. [DOI] [PubMed] [Google Scholar]
- 16. Bernard GR, Artigas A, Brigham Kl, et al. Report of the American‐European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intens Care Med. 1994;20:225‐232. [DOI] [PubMed] [Google Scholar]
- 17. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID‐19: the potential role for antifibrotic therapy. Lancet Resp Med. 2020;8:807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zemans RL, Colgan SP, Downey GP. Transepithelial Migration of Neutrophils Mechanisms and Implications for Acute Lung Injury. Am J Respir Cell Mol Biol. 2009;40:519‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopes‐Paciencia S, Saint‐Germain E, Rowell M‐C, Ruiz AF, Kalegari P, Ferbeyre G. The senescence‐associated secretory phenotype and its regulation. Cytokine. 2019;117:15‐22. [DOI] [PubMed] [Google Scholar]
- 20. Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic Pulmonary Fibrosis (IPF): an overview. J Clin Med. 2018;7:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Z, Lei S, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Resp Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bian X‐W. Autopsy of COVID‐19 victims in China. Nat Sci Rev. 2020;7(9):1414‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabaan AA, Al‐Ahmed S, Haque S, et al. SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: A comparative overview. Le infezioni in Medicina. 2020;28:174‐184. [PubMed] [Google Scholar]
- 25. Garvin MR, Alvarez C, Izaal Miller J, et al. A mechanistic model and therapeutic interventions for COVID‐19 involving a RAS‐mediated bradykinin storm. eLife. 2020;9:e59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adachi T, Chong J‐M, Nakajima T, et al. Clinicopathologic and Immunohistochemical findings from autopsy of patient with COVID‐19, Japan. Emerg Infect Dis. 2020;26(9):2157‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mong MA, Awkal JA, Marik PE. Accelerated hyaluronan concentration as the primary driver of morbidity and mortality in high‐risk COVID‐19 patients: with therapeutic introduction of an oral hyaluronan inhibitor in the prevention of Induced Hyaluronan Storm Syndrome. medRxiv. 2020. 10.1101/2020.04.19.20071647 [Epub ahead of print]. [DOI] [Google Scholar]
- 28. Bian X‐W. Autopsy of COVID‐19 patients in China. Nat Sci Rev. 2020;7:1414‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cogle CR, Guthrie SM, Sanders RC, Allen WL, Scott EW, Petersen BE. An overview of stem cell research and regulatory issues. Mayo Clin Proc. 2003;78:993‐1003. [DOI] [PubMed] [Google Scholar]
- 30. Esquivel D, Mishra R, Soni P, Seetharaman R, Mahmood A, Srivastava A. Stem cells therapy as a possible therapeutic option in treating COVID‐19 patients. Stem Cell Rev Rep. 2020:1‐9. 10.1007/s12015-020-10017-6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang D, Li H, Lian J, Zhu X, Qiao L, Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus‐induced acute lung injury. Stem Cell Res Ther. 2020;11:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells ‐ current trends and future prospective. Biosci Rep. 2015;35(2):e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoogduijn MJ. Are mesenchymal stromal cells immune cells? Arthritis Res Ther. 2015;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dominici M, Le Blanc M, Muller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 35. Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis‐Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative Med. 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Can A, Coskun H. The rationale of using mesenchymal stem cells in patients with COVID‐19‐related acute respiratory distress syndrome: What to expect. Stem Cells Transl Med. 2020. 10.1002/sctm.20-0164 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32:16‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169‐2179. [DOI] [PubMed] [Google Scholar]
- 40. Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69:1‐9. [DOI] [PubMed] [Google Scholar]
- 41. Haddad R, Saldanha‐Araujo F. Mechanisms of T‐cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11(10):707‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666‐1669. [DOI] [PubMed] [Google Scholar]
- 44. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383. [DOI] [PubMed] [Google Scholar]
- 45. Chen SJ, Wang SC, Chen YC. Novel antiviral strategies in the treatment of COVID‐19: A Review. Microorganisms. 2020;8(9):E1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu J, Song D, Li Z, et al. Immunity‐and‐matrix‐regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 2020;30(9):794‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493‐507. [DOI] [PubMed] [Google Scholar]
- 49. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726‐736. [DOI] [PubMed] [Google Scholar]
- 50. Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy. 2017;19:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T‐cell effector pathways. Stem Cell Res Ther. 2011;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Esquive D, Mishra R, Srivastava A. Stem cell therapy offers a possible safe and promising alternative approach for treating vitiligo: A review. Curr Pharm Des. 2020;26 10.2174/1381612826666200730221446 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 54. Fayyad‐Kazan H, Faour WH, Badran B, Lagneaux L, Najar M. The immunomodulatory properties of human bone marrow‐derived mesenchymal stromal cells are defined according to multiple immunobiological criteria. Inflamm Res. 2016;65:501‐510. [DOI] [PubMed] [Google Scholar]
- 55. Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114‐126. [DOI] [PubMed] [Google Scholar]
- 56. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex Immune Dysregulation in COVID‐19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dokic JM, Tomic SZ, Colic MJ. Cross‐Talk Between Mesenchymal Stem/Stromal Cells and Dendritic Cells. Curr Stem Cell Res T. 2016;11:51‐65. [PubMed] [Google Scholar]
- 61. Zhang Y, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria‐induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. 2014;59:671‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged‐2‐dependent regulatory dendritic cell population. Blood. 2009;113:46‐57. [DOI] [PubMed] [Google Scholar]
- 63. Ghosh T, Barik S, Bhuniya A, et al. Tumor‐associated mesenchymal stem cells inhibit naive T cell expansion by blocking cysteine export from dendritic cells. Int J Cancer. 2016;139:2068‐2081. [DOI] [PubMed] [Google Scholar]
- 64. Zhou R, Kai‐Wang To K, Wong Y‐K, et al. Acute SARS‐CoV‐2 infection impairs dendritic cell and T Cell Responses. Immunity. 2020;53(4):864‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Otsuka R, Seino KI. Macrophage activation syndrome and COVID‐19. Inflamm Regen. 2020;40:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ti D, Hao H, Tong C,et al. LPS‐preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome‐shuttled let‐7b. J Transl Med. 2015;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang G, Cao K, Liu K, et al. Kynurenic acid, an IDO metabolite, controls TSG‐6‐mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schonrich G, Raftery MJ. Neutrophil Extracellular Traps Go Viral. Front Immunol. 2016;7:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Didangelos A. COVID‐19 Hyperinflammation: What about Neutrophils? mSphere. 2020;5(3):e00367‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perrone LA, Plowden JK, Garcia‐Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mikacenic C, Moore R, Dmyterko V, et al. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator‐associated pneumonia. Crit Care. 2018;22:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29:747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(‐) mesenchymal stem cells improves the outcome of patients with COVID‐19 Pneumonia. Aging Dis. 2020;11:216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kuraitis D, Giordano C, Ruel M, Musaro A, Suuronen EJ. Exploiting extracellular matrix‐stem cell interactions: a review of natural materials for therapeutic muscle regeneration. Biomaterials. 2012;33:428‐443. [DOI] [PubMed] [Google Scholar]
- 76. Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7:615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meng SS, Guo F‐M, Zhang X‐W, et al. mTOR/STAT‐3 pathway mediates mesenchymal stem cell‐secreted hepatocyte growth factor protective effects against lipopolysaccharide‐induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem. 2019;120:3637‐3650. [DOI] [PubMed] [Google Scholar]
- 78. Bernard O, Jeny F, Uzunhan Y, et al. Mesenchymal stem cells reduce hypoxia‐induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am J Physiol Lung Cell Mol Physiol. 2018;314:L360‐L371. [DOI] [PubMed] [Google Scholar]
- 79. Chen XX, Tang L, Han ZH, Wang WJ, Meng JG. Coculture with bone marrow‐derived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharide‐stimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin‐1 modulating the Toll‐like receptor‐4 signal pathway. Mol Med Rep. 2019;19:1891‐1902. [DOI] [PubMed] [Google Scholar]
- 80. Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chu X, Chen C, Chen C, Zhang J‐S, Bellusci S, Li X. Evidence for lung repair and regeneration in humans: key stem cells and therapeutic functions of fibroblast growth factors. Front Med. 2020;14:262‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao T, Sun F, Liu J, et al. Emerging role of mesenchymal stem cell‐derived exosomes in regenerative medicine. Curr Stem Cell Res Ther. 2019;14:482‐494. [DOI] [PubMed] [Google Scholar]
- 83. Wang HY, Liu C, Wang Y, et al. Experimental treatment of pulmonary interstitial fibrosis with human umbilical cord blood mesenchymal stem cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013;31:675‐680. [PubMed] [Google Scholar]
- 84. Wu J, Song D, Li Z, et al. Immunity‐and‐matrix‐regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 2020;30:794‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Martinez‐Rojas MA, Vega‐Vega O, Bobadilla NA. Is the kidney a target of SARS‐CoV‐2? Am J Physiol Renal Physiol. 2020;318:F1454‐F1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rota C, Morigi M, Imberti B. Stem Cell Therapies in Kidney Diseases: Progress and Challenges. Int J Mol Sci. 2019;20:2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barnes CJ, Distaso CT, Spitz KM, Verdun VA, Haramati A. Comparison of stem cell therapies for acute kidney injury. Am J Stem Cells. 2016;5:1‐10. [PMC free article] [PubMed] [Google Scholar]
- 88. Shi X, Chen Q, Wang F. Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta‐analysis of experimental and clinical studies. Stem Cell Res Ther. 2019;10:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. MacPherson A, Kimmelman J. Ethical development of stem‐cell‐based interventions. Nat Med. 2019;25:1037‐1044. [DOI] [PubMed] [Google Scholar]
- 90. Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: A single arm meta‐analysis. J Med Virol. 2020;92:612‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ngai JC, Ko FW, Ng SS, et al. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first‐pass effect. Stem Cells Dev. 2009;18:683‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission, Science China. Life Sci. 2020;63:457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iwata‐Yoshikawa N, Okamura T, Shimizu Y, et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6):e01815‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen X, Shan Y, Wen Y, Sun J, Du H. Mesenchymal stem cell therapy in severe COVID‐19: A retrospective study of short‐term treatment efficacy and side effects. J Infect. 2020;81(4):647‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shu L, Niu C, Li R, et al. Treatment of severe COVID‐19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID‐19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;99:e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meng F, Xu R, Wang S, et al. Human umbilical cord‐derived mesenchymal stem cell therapy in patients with COVID‐19: a phase 1 clinical trial. Signal Transduct Target Therap. 2020;5:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sánchez‐Guijo F, Garcia‐Arranz M, Lopez‐Parra M, et al. Adipose‐derived mesenchymal stromal cells for the treatment of patients with severe SARS‐CoV‐2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25(100454). 10.1016/j.eclinm.2020.100454 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meng X, Ichim T, Zhong J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khanmohammadi M, Khanjani S, Edalatkhah M, et al. Modified protocol for improvement of differentiation potential of menstrual blood‐derived stem cells into adipogenic lineage. Cell Prolif. 2014;47:615‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood‐derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen L, Zhang C, Chen L, et al. Human menstrual blood‐derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. 2017;6:272‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tang L, Jiang Y, Zhu M, et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID‐19. Front Med. 2020:1‐10. 10.1007/s11684-020-0810-9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Metcalfe SM. Mesenchymal stem cells and management of COVID‐19 pneumonia. Med Drug Dis. 2020;5:100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Katsha AM, Ohkouchi S, Xin H. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase‐induced emphysema model. Mol Ther. 2011;19:196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee JH, Park J, Lee JW. Therapeutic use of mesenchymal stem cell‐derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tang XD, Shi L, Monsel A, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang‐1 mRNA. Stem Cells. 2017;35:1849‐1859. [DOI] [PubMed] [Google Scholar]
- 111. Wang M, Yuan Q, Xie L. Mesenchymal stem cell‐based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gorman E, Shankar‐Hari M, Hopkins P, et al. Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration in COVID‐19 (REALIST‐COVID‐19): A structured summary of a study protocol for a randomised, controlled trial. Trials. 2020;21:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ye Q, Wang H, Xia X, et al. Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID‐19: structured summary of a study protocol for a randomized controlled trial (Phase I/II). Trials. 2020;21:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available in the Table S1 of this article.


