Dear Editor,
We have read with great interest the correspondence of Kahraman et al (2020) on the emergence of oral mucosal changes adjacent to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection; hereby we demonstrate the characteristics of 13 laboratory‐confirmed coronavirus disease (COVID‐19) patients with oral mucositis according to the CARE guidelines. 1 , 2
The referenced patients sought care at our department from April to August 2020 due to generalized pain and soreness within the oral cavity related mainly to nonkeratinized mucosa without a specific cause (Table 1). All included patients had previously undergone polymerase chain reaction (PCR) testing for SARS‐CoV‐2, which confirmed their infection with a mean cycle threshold (Ct) value of 18.46 ± 3.8 (12‐26). Their mean age was 51.08 ± 8.79 (34‐62) years old, and eight of them (62.5%) were females. Regarding their COVID‐19 symptoms, two patients (15.4%) had persistent fever, four (30.8%) had ageusia, and two (15.4%) had anosmia. The majority of them (69.2%) had a mild course of SARS‐CoV‐2 infection and were prescribed paracetamol (PCM); contrarily, four patients experienced a moderate course of infection—two patients (15.4%) were prescribed chloroquine and other two (15.4%) were prescribed dexamethasone. 3
TABLE 1.
Demographic, clinical, and laboratory characteristics of COVID‐19 patients with oral mucositis
| No | Gender | Age | Comorbidities | Smoking | Hygiene | Ct | Fever | Ageusia | Anosmia | Severity | COVID‐19‐MED | Pain | Location | Onset | Duration | Mucositis‐MED |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 50 | Diabetes | Yes | Fair | 16 | Yes | Yes | Yes | Moderate | Dexamethasone | 8 | All over the mouth | 0 | 14 | Paracetamol |
| 2 | Male | 34 | Hypertension & Diabetes | No | Fair | 18 | No | No | No | Mild | Paracetamol | 3 | Palate and buccal mucosa | 1 | 7 | Magic MW |
| 3 | Female | 56 | N/A | No | Good | 24 | No | No | No | Mild | Paracetamol | 6 | Hard and soft palate | 1 | 7 | Magic MW |
| 4 | Male | 62 | N/A | No | Poor | 26 | No | No | No | Mild | Paracetamol | 3 | All over the mouth | 2 | 7 | Magic MW |
| 5 | Female | 45 | Asthma | No | Poor | 19 | No | No | No | Mild | Paracetamol | 3 | All over the mouth | 1 | 7 | Magic MW |
| 6 | Female | 57 | N/A | Yes | Good | 20 | No | No | No | Mild | Paracetamol | 4 | Buccal mucosa | 0 | 7 | Magic MW |
| 7 | Male | 49 | N/A | No | Poor | 18 | No | Yes | No | Mild | Paracetamol | 3 | All over the mouth | 0 | 7 | Magic MW |
| 8 | Female | 39 | Hypertension | No | Fair | 13 | No | Yes | Yes | Moderate | Chloroquine | 9 | All over the mouth | 0 | 14 | Paracetamol |
| 9 | Male | 46 | N/A | No | Good | 17 | No | No | No | Mild | Paracetamol | 3 | Buccal mucosa | 2 | 7 | Magic MW |
| 10 | Female | 62 | N/A | No | Good | 18 | No | No | No | Mild | Paracetamol | 4 | All over the mouth | 1 | 7 | Magic MW |
| 11 | Male | 55 | Diabetes | No | Fair | 19 | No | No | No | Moderate | Dexamethasone | 8 | Gingiva | 2 | 7 | Paracetamol |
| 12 | Female | 61 | N/A | No | Good | 12 | Yes | Yes | No | Moderate | Chloroquine | 8 | Buccal mucosa | 0 | 14 | Paracetamol |
| 13 | Female | 48 | Asthma | Yes | Fair | 20 | No | No | No | Mild | Paracetamol | 4 | All over the mouth | 1 | 7 | Magic MW |
Abbreviations: Ct, Cycle threshold value of polymerase chain reaction (PCR) test for SARS‐CoV‐2; COVID‐19‐MED, medication prescribed for COVID‐19; mucositis‐MED, Medication prescribed for mucositis.
The mean onset of mucositis emergence was 0.85 ± 0.8 (0‐2) days calculated since the day of PCR testing, while its mean duration was 8.62 ± 3.07 (7‐14) days. An 11‐item numerical rating scale (NRS) was used to evaluate the manifested intraoral pain where “0” denotes “no pain” and “10” denotes “pain as bad as you can imagine.” 4 The mean score of pain intensity was 5.08 ± 2.36. 3 , 4 , 5 , 6 , 7 , 8 , 9 On intraoral examination, sporadic erythema with minor irritations was found all over the mouth (53.8%), on the buccal mucosa (30.8%), palate (15.4%), and gingiva (7.7%). Depapillation of the tongue was observed in all cases with a tendency to be more localized at the borders (Figure 1).
FIGURE 1.
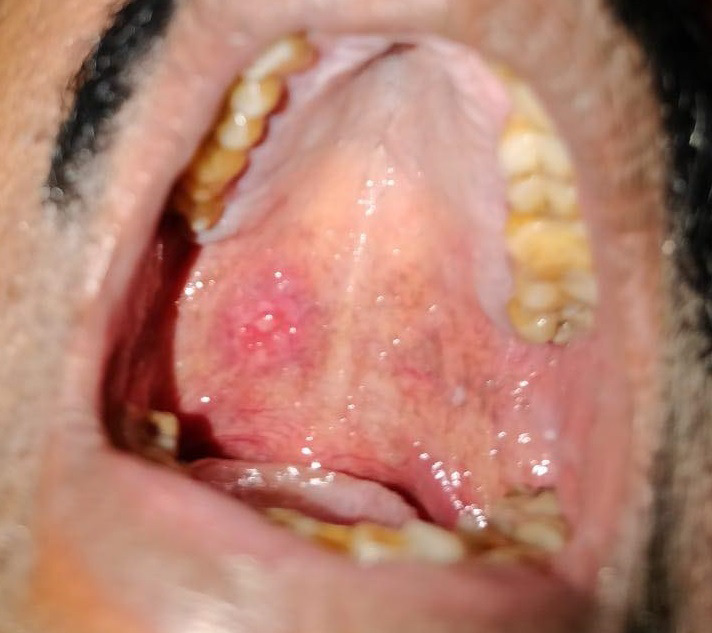
Mucositis in palate of a laboratory‐confirmed COVID‐19 patient
While nine patients (69.2%) were prescribed “Magic mouthwash” containing lidocaine 1%, chlorhexidine 2%, and prednisolone 20 mg in 100 mL, four patients (30.8%) were prescribed PCM to relieve their symptoms of mucositis. Mann‐Whitney test yielded a statistically significant difference favoring “Magic mouthwash” in reducing the duration of mucositis, U (N Magic = 9, N PCM = 4) = 4.5, z = −2.85, P = .034.
Inferential statistics revealed that COVID‐19 severity was significantly associated with duration of mucositis and its pain (H = 5.76 and 9.29; P = .016 and .002, respectively). Ageusia was also significantly associated with Ct value, mucositis duration, and onset (U = 2, 4.5, and 1.5; P = .013, .034, and .018, respectively).
Our findings support the suggested role of oral mucosa in providing an entry for SARS‐CoV‐2 due to the high expression of angiotensin‐converting enzyme II (ACE2) receptors. 5 According to Sonis theory, oral mucositis is primarily initiated by oxidative stress and the formation of reactive oxygen species (ROS) in response to somatotoxic doses of nonsurgical oncologic treatment. 6 The excessive production of ROS in the mucosal tissues of severely ill COVID‐19 patients may explain the significant direct association observed in our cases between severity of COVID‐19 and mucositis duration and pain intensity. 7 Secondary infections and drug reactions cannot be ruled out entirely, especially with severely ill patients due to immune dysregulation. 8 In addition, oral mucosal changes were consistently observed in children with pediatric multisystem inflammatory syndrome temporally associated with SARS‐COV‐2 (PIMS‐TS), which is suggested to be linked to IgG antibody‐mediated enhancement. 9
In conclusion, oral mucositis may occur in COVID‐19 patients either as a direct manifestation of cellular damage triggered by SARS‐CoV‐2 or as an opportunistic infection due to immune dysregulation. This case‐series warrants larger epidemiologic studies to verify the etiology and prevalence of oral mucositis among COVID‐19 patients.
PATIENT CONSENT
All patients agreed to use their clinical and laboratory results for academic purposes while concealing their identifying personal data.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Abanoub Riad: Writing‐original draft. Islam Kassem: Data curation; Investigation. Mai Badrah: Formal analysis. Miloslav Klugar: Supervision; Writing‐review & editing.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cebeci Kahraman F, Çaşkurlu H. Mucosal involvement in a COVID‐19‐positive patient: a case report. Dermatol Ther. 2020;33(4):e13797. 10.1111/dth.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus‐based clinical case reporting guideline development. BMJ Case Rep. 2013;2013;2013:bcr2013201554. 10.1136/bcr-2013-201554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NHMRC . Australian Guidelines for the clinical care of people with COVID‐19. Australian Clinical Practice Guidelines. https://www.clinicalguidelines.gov.au/register/australian-guidelines-clinical-care-people-covid-19. Accessed September 17, 2020.
- 4. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798‐804. 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 5. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1‐5. 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4(4):277‐284. 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 7. Laforge M, Elbim C, Frère C, et al. Tissue damage from neutrophil‐induced oxidative stress in COVID‐19. Nat Rev Immunol. 2020;20(9):515‐516. 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riad A, Klugar M, Krsek M. COVID‐19 related oral manifestations, early disease features? Oral Dis. 2020;13516. 10.1111/odi.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riad A, Boccuzzi M, Sagiroglu D, Klugar M, Krsek M. Pediatric multisystem inflammatory syndrome temporally associated with SARS‐COV‐2: Oral manifestations and implications. Int J Paediatr Dent. 2020;12694. 10.1111/ipd.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


