Abstract
Background
The coronavirus disease 2019 (COVID‐19) shows high morbidity and mortality, particularly in patients with concomitant cardiovascular diseases. Some of these patients are under oral anticoagulation (OAC) at admission, but to date, there are no data on the clinical profile, prognosis and risk factors of such patients during hospitalization for COVID‐19.
Design
Subanalysis of the international ‘real‐world’ HOPE COVID‐19 registry. All patients with prior OAC at hospital admission for COVID‐19 were suitable for the study. All‐cause mortality was the primary endpoint.
Results
From 1002 patients included, 110 (60.9% male, median age of 81.5 [IQR 75‐87] years, median Short‐Form Charlson Comorbidity Index [CCI] of 1 [IQR 1‐3]) were on OAC at admission, mainly for atrial fibrillation and venous thromboembolism. After propensity score matching, 67.9% of these patients died during hospitalization, which translated into a significantly higher mortality risk compared to patients without prior OAC (HR 1.53, 95% CI 1.08‐2.16). After multivariate Cox regression analysis, respiratory insufficiency during hospitalization (HR 6.02, 95% CI 2.18‐16.62), systemic inflammatory response syndrome (SIRS) during hospitalization (HR 2.29, 95% CI 1.34‐3.91) and the Short‐Form CCI (HR 1.24, 95% CI 1.03‐1.49) were the main risk factors for mortality in patients on prior OAC.
Conclusions
Compared to patients without prior OAC, COVID‐19 patients on OAC therapy at hospital admission showed lower survival and higher mortality risk. In these patients on OAC therapy, the prevalence of several comorbidities is high. Respiratory insufficiency and SIRS during hospitalization, as well as higher comorbidity, pointed out those anticoagulated patients with increased mortality risk.
Keywords: anticoagulant, atrial fibrillation, Coronavirus disease 2019, SARS‐CoV‐2, thrombosis, venous thromboembolism
1. INTRODUCTION
On 12 March 2020, the World Health Organization (WHO) declared the novel coronavirus disease 2019 (COVID‐19), a condition caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), as a pandemic. 1
COVID‐19 is characterized by high morbidity and mortality, and there is increasing evidence showing interactions between cardiovascular system and poor prognosis in patients with pre‐existing cardiovascular pathologies. 2 , 3 , 4 In fact, cardiovascular manifestations include a variety of clinical presentations such us acute cardiac injury with cardiomyopathy, ventricular arrhythmias and haemodynamic instability. 4 , 5
COVID‐19 poor prognosis is also more frequent between elderly patients with underlying medical conditions. 6 , 7 This is the case of patients with atrial fibrillation (AF), who are usually elderly, with multiple comorbidities increasing their risk and making them more vulnerable. 8 , 9 On the other hand, previous studies showed that COVID‐19 leads to vascular inflammation, contributing to hypercoagulable state and endothelial dysfunction, manifested by alteration in D‐dimer and fibrinogen levels, prothrombin time and platelet count, thus increasing the risk of disseminated intravascular coagulation and venous thromboembolism (VTE). 7 , 10 , 11 , 12 , 13 In fact, pulmonary embolism (PE) may be more frequent than expected, based on reports from autopsy studies. 14 , 15 Specifically, it has been suggested that disseminated intravascular coagulation followed by microthrombosis in COVID‐19 patients is mainly determined by activation of the clotting system, platelet activation, and impaired artery dilatation or artery vasoconstriction. 16
As such, patients with previous VTE could be even at higher risk of recurrent events and mortality after SARS‐CoV‐2 infection.
Interestingly, anticoagulation with low‐molecular‐weight heparin (LMWH) seems to decrease mortality in patients with COVID‐19. 17 If this fact is confirmed, it may suggest that anticoagulated patients would have a better prognosis. However, there are not currently available data on the prognosis and risk factors of patients requiring oral anticoagulation (OAC) prior to the admission for COVID‐19. In the present study, our aim was to characterize the clinical profile and short‐term prognosis of patients on OAC therapy for diverse indications admitted for COVID‐19 from the wide and multicentre International COVID‐19 Clinical Evaluation Registry (HOPE COVID‐19). Additionally, we also aimed to investigate risk factors for poor prognosis in this population.
2. METHODS
This is a subanalysis of the first 1002 patients included in the HOPE Registry. 18 In brief, the HOPE Registry is an international initiative without conflicts of interest, designed as a retrospective cohort registry, a ‘real‐world’ all‐comers type, with voluntary participation and no financial remuneration.
All patients discharged (deceased or alive) after hospital admissions for COVID‐19 were suitable for the study. There were no exclusion criteria, except for patients’ explicit refusal to participate. From 23 March 2020 to 2 April 2020, all patients fulfilling inclusion criteria from 23 centres in 19 cities and 4 countries (Ecuador, Germany, Italy and Spain) were assessed in the present manuscript. Clinical and demographic data were collected at inclusion and during the hospitalization.
The Charlson Comorbidity Index (CCI) is a list of 19 comorbid conditions described to predict 10‐year survival in patients with multiple comorbidities. 19 In this study, a modification (Short‐Form) of the original CCI has been used (1 point for each of the following: cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, heart failure/coronary artery disease, dementia; and peripheral artery disease; and 2 points for each of the following: chronic kidney disease and cancer). 20
As there was no anticoagulation management protocol in any of the participating centres, in many of them, OAC was switched to LMWH following the local attending physician criteria.
Reporting of the study conforms to broad EQUATOR guidelines. The study was performed according to the ethical principles of Declaration of Helsinki and Good Clinical Practice Guidelines and has been approved by Ethics Research Committee from the Hospital Clínico San Carlos (Madrid, Spain) (20/241‐E) and the Spanish Agency for Medicines and Health Products (classification: EPA‐0D). Written informed consent was waived because of the characteristics of the anonymized registry and the severity of the situation. However, at least verbal authorization from the patient (or familiar or caregiver when unavailable) was required.
2.1. Laboratory analyses
Laboratory parameters were considered elevated as defined by local laboratory cut‐off levels. However, the HOPE Registry protocol suggested the cut‐off levels for the laboratory parameters as follows: D‐dimer (≥0.5 mg/L), procalcitonin (≥0.5 ng/mL), C‐reactive protein (≥10 mg/L), troponins (>99th percentile), transaminases (≥40 U/L), ferritin (≥336 ng/mL) and lactate dehydrogenase (≥280 U/L).
2.2. Study outcomes
The primary endpoint for this analysis was all‐cause mortality, whereas the composite of all‐cause mortality or any thromboembolic event was the secondary endpoint. This combined endpoint was not recorded in the original data set but obtained retrospectively for this analysis.
Although not classified as primary or secondary outcomes, other adverse events during hospitalization were recorded, including renal failure, respiratory insufficiency, upper respiratory tract infection, heart failure, sepsis, systemic inflammatory response syndrome (SIRS) and any relevant bleeding. Bleeding was defined as 'relevant' at the discretion of the attending medical team and classified using the BARC bleeding score as type 2, 3 or 5.
Local researchers identified, confirmed and recorded all adverse events.
2.3. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate according to the Kolmogorov‐Smirnov test, whilst categorical variables were expressed as absolute frequencies and percentages. The Pearson’s chi‐squared test was used to compare proportions. The Student's t or the Mann‐Whitney U tests were used to compare continuous and categorical variables, as appropriate.
A multivariate Cox proportional hazard regression model was performed to determine risk factors independently associated with the primary and secondary endpoints. Given the small sample size, we only included in this model those variables showing a strict P‐value < .05 in the univariate analyses. Results were reported as hazard ratio (HR) with 95% confidence interval (CI). As few patients suffered thromboembolic events, the date of death was used for the Cox analysis of the secondary endpoint.
To compare the risk of the primary outcome among patients on prior OAC therapy and patients without prior OAC therapy, we conducted a propensity score matching (PSM). Those variables that were significantly different between both cohorts were included in the model to adjust for differences. Patients were matched 1:1 across each cohort on a propensity score generated by logistic regressions using the nearest neighbour technique without replacement with a maximum calliper of 0.2, thus avoiding at least 98% of the bias due to the measured confounders. The value of absolute standardized mean difference <10% indicated the balance of matched cohorts. 21 , 22
Survival analyses by Kaplan‐Meier estimates were performed after PSM to assess differences in event‐free (primary outcome) survival between patients on prior OAC therapy and patients without prior OAC therapy.
Two‐sided P‐values < .05 were accepted as statistically significant. Statistical analyses were performed using SPSS v. 22.0 (SPSS, Inc) and STATA v. 12.0 (StataCorp.) for Windows.
3. RESULTS
1002 patients with precise data on the underlying anticoagulation status were explored in this analysis of the HOPE Registry. Of them, 110 (67 [60.9%] male with median age of 81.5 [IQR 75‐87] years) were on OAC therapy at admission, most of them for AF. Compared to COVID‐19 patients without prior OAC therapy at admission, patients on OAC were significantly different in terms of several comorbidities (Table S1).
Focusing on the 110 patients initially on OAC, 74.5% were on vitamin K antagonist (VKA) therapy, whereas 25.5% were on direct‐acting oral anticoagulants (DOACs). For VKA users, the median time in therapeutic range (TTR) in the previous 6 months after inclusion in the study was 67% (IQR 50‐83). The Short‐Form CCI was 1 (IQR 1‐3) in the overall population. The median duration of hospitalization in those patients who survived was 7 (IQR 4‐12) days. A summary of clinical characteristics and laboratory parameters at admission is shown in Table 1.
Table 1.
Baseline clinical characteristics of patients with prior oral anticoagulation therapy
|
Overall N = 110 |
Atrial fibrillation N = 89 |
Venous thromboembolism a N = 13 |
Mechanical heart valve N = 8 |
P‐value | |
|---|---|---|---|---|---|
| Demographic | |||||
| Male sex, n (%) | 67 (60.9) | 57 (64.0) | 6 (46.2) | 4 (50.0) | .376 |
| Age (y), median (IQR) | 81.5 (75.0‐87.0) | 82 (76.5‐88.0) | 74 (68.5‐84.0) | 79.5 (70.0‐81.5) | .015 |
| Race (Hispanic), n (%) | 4 (3.6) | 2 (2.2) | 2 (15.4) | 0 (0) | .052 |
| Body mass index (kg/m2), median (IQR) | 26.6 (24.7‐30.0) | 26.5 (25.2‐30.1) | 27.8 (22.1‐29.0) | 24.3 (18.8‐33.7) | .247 |
| Comorbidities, n (%) | |||||
| Hypertension | 90 (81.8) | 73 (82.0) | 10 (76.9) | 7 (87.5) | .825 |
| Diabetes mellitus | 35 (31.8) | 31 (34.8) | 4 (30.8) | 0 (0) | .128 |
| Heart failure | 6 (5.5) | 5 (5.6) | 1 (7.7) | 0 (0) | .744 |
| Stroke/TIA | 23 (20.9) | 16 (18.0) | 3 (23.1) | 4 (50.0) | .101 |
| Chronic kidney disease | 20 (18.2) | 16 (18.0) | 2 (15.4) | 2 (25.0) | .852 |
| Coronary artery disease | 16 (14.5) | 12 (13.5) | 3 (23.1) | 1 (12.5) | .648 |
| Hypercholesterolaemia | 65 (59.1) | 50 (56.2) | 9 (69.2) | 6 (75.0) | .427 |
| Current smoking habit | 9 (8.2) | 6 (6.7) | 2 (15.4) | 1 (12.5) | .511 |
| COPD/SAHS | 22 (20.0) | 19 (21.3) | 2 (15.4) | 1 (12.5) | .758 |
| Obesity | 20 (27.0) | 15 (25.4) | 3 (30.0) | 2 (40.0) | .760 |
| History of malignant disease | 24 (21.8) | 22 (24.7) | 2 (15.4) | 0 (0) | .225 |
| Parkinson's disease | 3 (2.7) | 3 (3.4) | 0 (0) | 0 (0) | .695 |
| Any demential level | 4 (3.6) | 2 (2.2) | 1 (7.7) | 1 (12.5) | .235 |
| Any dependency level | 32 (29.1) | 28 (31.5) | 3 (23.1) | 1 (12.5) | .464 |
| Concomitant treatment, n (%) | |||||
| Beta‐blockers | 50 (45.5) | 41 (46.1) | 5 (38.5) | 4 (50.0) | .845 |
| Statins | 20 (18.2) | 14 (15.7) | 4 (30.8) | 2 (25.0) | .369 |
| ACEi/ARBs | 61 (55.5) | 49 (55.1) | 8 (61.5) | 4 (50.0) | .862 |
| Antiplatelet therapy | 11 (10.0) | 6 (6.7) | 3 (23.1) | 2 (25.0) | .063 |
| Oral anticoagulation therapy | |||||
| Vitamin K antagonists | 82 (74.5) | 63 (70.8) | 11 (84.6) | 8 (100) | .129 |
| Direct‐acting oral anticoagulants | 28 (25.5) | 26 (29.2) | 2 (15.4) | 0 (0.0) | |
| Laboratory parameters | |||||
| Creatinine (mg/dL), median (IQR) | 1.15 (0.89‐1.55) | 1.13 (0.88‐1.57) | 1.11 (0.86‐1.32) | 1.38 (0.96‐1.55) | .610 |
| Haemoglobin (g/dL), median (IQR) | 13.0 (11.0‐14.0) | 13.0 (11.0‐14.0) | 13.0 (10.3‐14.0) | 12.0 (12.0‐13.0) | .746 |
| Platelet count (×109/L), median (IQR) | 159.0 (132.0‐206.0) | 157.5 (132.2‐192.0) | 187.0 (149.5‐273.5) | 127.5 (103.8‐267.8) | .181 |
| Elevated D‐dimer, n (%) | 56 (50.9) | 46 (51.7) | 4 (30.8) | 6 (75.0) | .136 |
| Elevated procalcitonin, n (%) | 26 (23.6) | 21 (23.6) | 3 (23.1) | 2 (25.0) | .995 |
| Elevated C‐reactive protein, n (%) | 103 (93.6) | 83 (93.3) | 12 (92.3) | 8 (100) | .740 |
| Elevated troponins, n (%) | 11 (10.0) | 8 (9.0) | 1 (7.7) | 2 (25.0) | .337 |
| Elevated transaminases, n (%) | 44 (40.0) | 34 (38.2) | 6 (46.2) | 4 (50.0) | .720 |
| Elevated ferritin, n (%) | 30 (27.3) | 24 (27.0) | 1 (7.7) | 5 (62.5) | .023 |
| Elevated lactate dehydrogenase, n (%) | 83 (75.5) | 67 (75.3) | 12 (92.3) | 4 (50.0) | .091 |
| TTR in VKA users (%), median (IQR) | 67 (50‐83) | 67 (50‐83) | 67 (54‐91) | 33 (17‐59 | .215 |
| Short‐Form CCI, median (IQR) b | 1 (1‐3) | 1 (1‐3) | 1 (0‐3) | 1 (0.3‐2.8) | .817 |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CCI, Charlson Comorbidity Index; COPD/SAHS, chronic obstructive pulmonary disease/sleep apnoea‐hypopnoea syndrome; IQR, interquartile range; TIA, transient ischaemic attack; TTR, time in therapeutic range; VKA, vitamin K antagonists.
2 patients with other indications for oral anticoagulation have been included in this group (1 with antiphospholipid syndrome and 1 with hypercoagulability).
Cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, heart failure/coronary artery disease, dementia and peripheral artery disease (1 point each); chronic kidney disease (2 points); and cancer (2 points).
During hospitalization, 8 (7.3%) patients required admission in the intensive care unit (ICU) (median duration of ICU stay 6.5 [IQR 2.8‐10.3] days). Of them, 4 (3.6%) patients required mechanical intubation and 2 (1.8%) patients not admitted in the ICU also required mechanical intubation. Regarding anticoagulation therapy during hospitalization, quality information was available in 87 patients. Of them, 18.4% (16/87) of patients maintained the same baseline OAC, 79.3% (69/87) switched to LMWH, 1.15% (1/87) switched to unfractionated heparin, and only 1.15% (1/87) received no anticoagulation therapy. The clinical progress of all patients by main indication for OAC group is shown in Table 2, and a brief comparison with patients without prior OAC is shown in Table S2.
Table 2.
Adverse events during hospitalization in patients with prior oral anticoagulation therapy
|
Overall N = 110 |
Atrial fibrillation N = 89 |
Venous thromboembolism a N = 13 |
Mechanical heart valve N = 8 |
P‐value | |
|---|---|---|---|---|---|
| Renal failure, n (%) | 48 (43.6) | 40 (44.9) | 6 (46.2) | 2 (25.0) | .542 |
| Respiratory insufficiency, n (%) | 82 (74.5) | 65 (73.0) | 9 (69.2) | 8 (100) | .220 |
| Upper respiratory tract infection, n (%) | 14 (12.7) | 10 (11.2) | 2 (15.4) | 2 (25.0) | .510 |
| Heart failure, n (%) | 18 (16.4) | 15 (16.9) | 1 (7.7) | 2 (25.0) | .558 |
| Sepsis, n (%) | 31 (28.2) | 26 (29.2) | 5 (38.5) | 0 (0.0) | .145 |
| Systemic inflammatory response syndrome, n (%) | 25 (22.7) | 19 (21.3) | 6 (46.2) | 0 (0.0) | .039 |
| Any relevant bleeding, n (%) b | 8 (7.3) | 5 (5.6) | 1 (7.7) | 2 (25.0) | .129 |
| Primary endpoint (all‐cause mortality), n (%) | 75 (68.2) | 61 (68.5) | 10 (76.9) | 4 (50.0) | .431 |
| Secondary endpoint (all‐cause mortality or any thromboembolic event), n (%) | 76 (69.1) | 61 (68.5) | 10 (76.9) | 5 (62.5) | .760 |
| Pulmonary embolism, n (%) | 2 (1.8) | 1 (1.12) | 0 (0.0) | 1 (12.5) | .084 |
| Stent thrombosis, n (%) | 1 (0.9) | 0 (0.0) | 1 (7.7) | 0 (0.0) |
2 patients with other indications for oral anticoagulation have been included in this group (1 with antiphospholipid syndrome and 1 with hypercoagulability)
As determined by the attending medical team and classified using the BARC bleeding score as 2, 3, or 5 types. Reported in the clinical history as such.
3.1. Analysis of the primary and secondary outcomes in patients on prior OAC
Regarding the primary outcome for this analysis, 75 (68.2%) patients on prior OAC therapy died during hospitalization. Proportionally, this was significantly higher compared to patients without prior OAC (68.2% vs 26.1%, P < .001). However, as previously described, COVID‐19 patients without prior OAC therapy at admission were significantly different compared with COVID‐19 patients on OAC regarding comorbidities and laboratory parameters (Table S1). For this reason, we performed a PSM analysis to balance both cohorts (Table 3). After PSM, 218 patients remained (109:109) and mortality was more frequent in COVID‐19 patients on prior OAC therapy compared with COVID‐19 patients without prior OAC (67.9% vs 51.4%, P = .013). This translated into a significantly higher risk of mortality assessed by Cox regression analysis (HR 1.53, 95% CI 1.08‐2.16). The Kaplan‐Meier analysis also demonstrated that survival was lower in COVID‐19 patients under previous OAC therapy (log‐rank P‐value = .013) (Figure 1).
Table 3.
Baseline clinical characteristics of patients with and without prior oral anticoagulation therapy after propensity score matching
|
Patients on prior OAC N = 109 |
Patients without prior OAC N = 109 |
P‐value | |
|---|---|---|---|
| Demographic | |||
| Male sex, n (%) | 66 (60.6) | 63 (57.8) | .679 |
| Age (y), median (IQR) | 81 (75‐87) | 83 (74‐88) | .599 |
| Race (Hispanic), n (%) | 4 (3.7) | 3 (2.8) | .781 |
| Comorbidities, n (%) | |||
| Hypertension | 89 (81.7) | 90 (82.6) | .860 |
| Diabetes mellitus | 34 (31.2) | 34 (31.2) | 1.000 |
| Heart failure | 6 (5.5) | 4 (3.7) | .167 |
| Stroke/TIA | 22 (20.2) | 20 (18.3) | .731 |
| Chronic kidney disease | 19 (17.4) | 14 (12.8) | .345 |
| Vascular disease (CAD and/or PAD) | 17 (15.6) | 14 (12.8) | .561 |
| Hypercholesterolaemia | 64 (58.7) | 63 (57.8) | .891 |
| Current smoking habit | 8 (7.3) | 1 (0.9) | .057 |
| COPD/SAHS | 21 (19.3) | 19 (17.4) | .726 |
| Obesity | 20 (18.3) | 20 (18.3) | 1.000 |
| History of malignant disease | 24 (22.0) | 18 (16.5) | .303 |
| Parkinson's disease | 3 (2.8) | 3 (2.8) | 1.000 |
| Any demential level | 4 (3.7) | 11 (10.1) | .511 |
| Any dependency level | 32 (29.4) | 34 (31.2) | .768 |
| Concomitant treatment, n (%) | |||
| Beta‐blockers | 49 (45.0) | 28 (25.7) | .003 |
| Statins | 19 (17.4) | 15 (13.8) | .455 |
| ACEi/ARBs | 60 (55.0) | 68 (62.4) | .271 |
| Antiplatelet therapy | 10 (9.2) | 43 (39.4) | <.001 |
| Laboratory parameters | |||
| Creatinine (mg/dL), median (IQR) | 1.15 (0.89‐1.55) | 1.09 (0.83‐1.58) | .711 |
| Haemoglobin (g/dL), median (IQR) | 13.0 (11.0‐14.0) | 14.0 (12.0‐14.0) | .079 |
| Platelet count (×109/L), median (IQR) | 160.0 (132.0‐206.0) | 175.0 (142.5‐237.0) | .056 |
| Elevated D‐dimer, n (%) | 55 (50.5) | 59 (54.1) | .501 |
| Elevated procalcitonin, n (%) | 26 (23.9) | 18 (16.5) | .186 |
| Elevated C‐reactive protein, n (%) | 102 (93.6) | 99 (90.8) | .340 |
| Elevated troponins, n (%) | 11 (10.1) | 14 (12.8) | .590 |
| Elevated transaminases, n (%) | 43 (39.4) | 36 (33.0) | .333 |
| Elevated ferritin, n (%) | 30 (27.5) | 32 (29.4) | .719 |
| Elevated lactate dehydrogenase, n (%) | 82 (75.2) | 69 (63.3) | .105 |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; COPD/SAHS, chronic obstructive pulmonary disease/sleep apnoea‐hypopnoea syndrome; IQR, interquartile range; OAC, oral anticoagulant; TIA, transient ischaemic attack.
Figure 1.
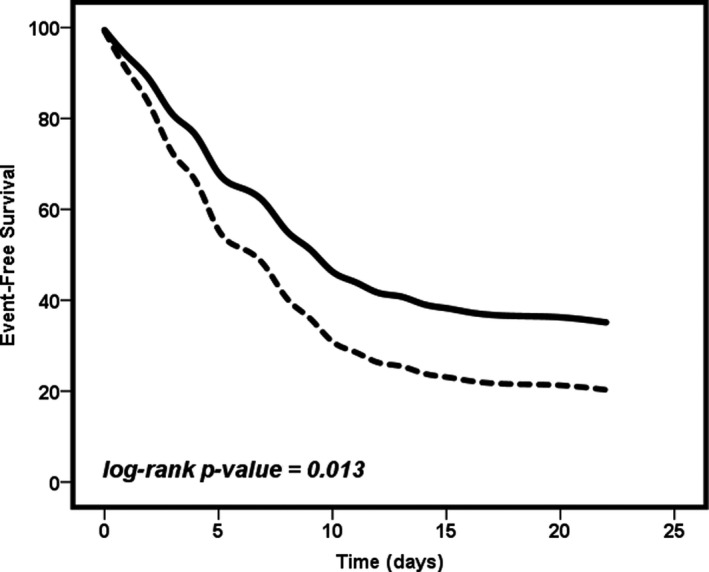
Mortality‐free survival depending on the previous use of oral anticoagulation therapy. Solid line = patients without prior oral anticoagulation at hospital admission. Dashed line = patients on prior oral anticoagulation at hospital admission
In patients on prior OAC, there were no differences in terms of mortality between VKA or DOACs users (69.5% vs 64.3%, P = .608). In 46 (61.3%) cases, death was related to respiratory insufficiency/failure, in 3 (4%) cases, it was related to sepsis, and in 26 (34.7%) cases, it was a combined cause.
We investigated then those factors associated with higher risk of suffering from the primary outcome in the previously anticoagulated cohort. Univariate Cox regression analyses are shown in Table S3. After univariate analyses, we performed a multivariate analysis to found variables independently associated with higher risk. Thus, respiratory insufficiency during hospitalization (HR 6.02, 95% CI 2.18‐16.62), SIRS during hospitalization (HR 2.29, 95% CI 1.34‐3.91) and the Short‐Form CCI (HR 1.24, 95% CI 1.03‐1.49) were found to be the main risk factors for death in this population (Table 4). Cumulative risk of the model is shown in Figure 2.
Table 4.
Multivariate Cox regression analysis for the primary outcome in patients with prior oral anticoagulation therapy
| HR | 95% CI | P‐value | |
|---|---|---|---|
| Heart failure | 0.98 | 0.55‐1.73 | .935 |
| Elevated lactate dehydrogenase | 1.68 | 0.88‐3.22 | .119 |
| Respiratory insufficiency during hospitalization | 6.02 | 2.18‐16.62 | .001 |
| Renal failure during hospitalization | 1.22 | 0.73‐2.03 | .445 |
| SIRS during hospitalization | 2.29 | 1.34‐3.91 | .002 |
| Short‐Form CCI a | 1.24 | 1.03‐1.49 | .025 |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio; SIRS, systemic inflammatory response syndrome.
Cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, heart failure/coronary artery disease, dementia and peripheral artery disease (1 point each); chronic kidney disease (2 points); and cancer (2 points).
Figure 2.
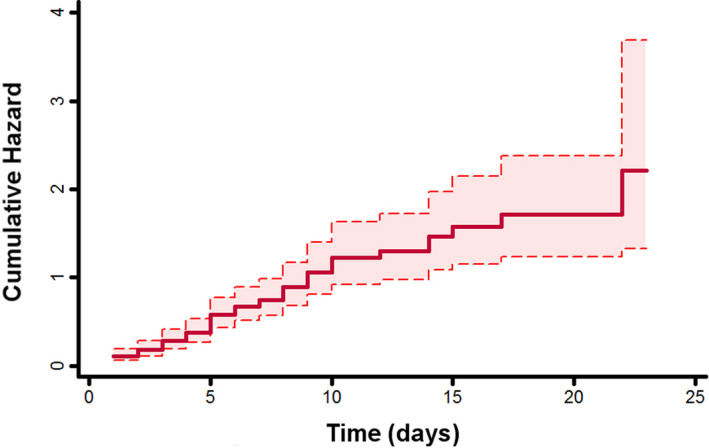
Cumulative hazard with 95% confidence interval of the predictive model for the primary outcome in patients with prior oral anticoagulation therapy
In addition, 76 (69.1%) patients with prior OAC therapy suffered the secondary composite outcome of ‘all‐cause mortality or any thromboembolic event’ during hospitalization. As for the primary endpoint, the rate of this composite outcome was significantly higher in this group in comparison with patients without prior OAC (69.1% vs 26.3%, P < .001). Of these 76 secondary outcomes, 75 (68.2%) were deaths, 2 (1.8%) were PE, and 1 (0.9%) was a stent thrombosis. Of note, 2 patients who suffered a thromboembolic event died during admission: the first one was a patient with PE, and the other was the patient with the stent thrombosis. Both patients were on VKA therapy.
Univariate and multivariate Cox regression analyses of factors associated with higher risk of suffering from the secondary outcome are shown in Table S4. As expected, multivariate analysis was similar to the previous one and showed that respiratory insufficiency during hospitalization (HR 6.14, 95% CI 2.22‐16.93), SIRS during hospitalization (HR 2.18, 95% CI 1.29‐3.71) and the Short‐Form CCI (HR 1.23, 95% CI 1.04‐1.46) were the main risk factors for the secondary endpoint.
3.2. Subanalysis of the primary outcome in AF patients
Since AF was the most common indication for OAC in the cohort of patients with OAC therapy prior to admission, we also performed an analysis of the primary outcome in this specific population. The median CHA2DS2‐VASc score in AF patients was 4 (IQR 3‐5), and 95.6% of patients had a CHA2DS2‐VASc ≥ 2. No association between the CHA2DS2‐VASc score and mortality in AF patients was found (HR 1.08, 95% CI 0.90‐1.30).
Full results of the univariate and multivariate Cox regression analyses are shown in Table S5. As for the results in the overall cohort of patients on OAC therapy, respiratory insufficiency during hospitalization (HR 6.03, 95% CI 2.17‐16.62), SIRS during hospitalization (HR 2.28, 95% CI 1.34‐3.88) and the Short‐Form CCI (HR 1.23, 95% CI 1.03‐1.45) were independently associated with higher risk of all‐cause mortality.
4. DISCUSSION
In this study, we found that patients under OAC admitted for COVID‐19 had a particularly high risk of mortality. Almost 70% of patients died, which represented a much higher in‐hospital mortality rate than that reported for the general population, 23 and higher risk of mortality compared to patients without prior OAC at hospital admission in this study. In these patients on OAC therapy at admission, we did not found differences in terms of mortality between VKA or DOACs users, but the study is not enough powered to assess these differences. However, we identified that respiratory insufficiency and SIRS during hospitalization, as well as higher comorbidity, are associated with poor outcomes in patients taking anticoagulation. A previous systematic review and meta‐analysis showed that several comorbidities are associated with SARS‐CoV‐2 severity. 13 Notwithstanding, to the best of our knowledge, this is the first study exploring prognosis specifically in patients under OAC previously.
Of note, despite being an elderly population, the Short‐Form CCI was relatively low, suggesting that patients requiring anticoagulation (mostly by AF and VTE) are already at increased risk for other reasons, probably due to a pro‐inflammatory state that makes them more vulnerable. Thus, anticoagulated patients may improve their prognosis by blocking inflammation‐hypercoagulability activation in the setting of COVID‐19. 24 , 25 In addition, COVID‐19 has been shown to produce abnormal coagulation markers, 26 and in its severe form, it may predispose to thrombotic disease due to excessive inflammation, platelet activation, endothelial dysfunction and stasis. 27 , 28 In these cases, SARS‐CoV‐2 also induces a cytokine storm that leads to the activation of the coagulation cascade, causing thrombotic phenomena. 29 Thus, this could be a pathophysiological explanation for the observed VTE events in different series of COVID‐19 patients. 30 , 31 , 32 Indeed, the prevalence of VTE in these patients admitted is ~25%. 33 , 34 In Asian patients, anticoagulation with LMWH appears to be associated with better prognosis, 35 but maybe the COVID‐19 does not manifest in the same way in the Western world. In fact, a study in France showed that a high number of patients developed life‐threatening thrombotic complications despite anticoagulation with LMWH, even though 30% of them received therapeutic doses. 32 Given these factors, it would be expected that the presence of SARS‐CoV‐2 infection may increase the already‐high thrombotic risk of AF and VTE patients. It is therefore plausible that these patients have an inflammatory and hypercoagulable state that is enhanced by the infection. The results shown in this study seem to reinforce this hypothesis since COVID‐19 patients on OAC therapy at admission showed lower survival and higher mortality risk compared to patients without prior OAC.
On the other hand, it is well described that there is a higher risk in the ageing population. Recent reports demonstrated that, in proportion, mortality of elderly patients with COVID‐19 is higher than that of young or middle‐aged patients, with a high rate of severe to critical cases in the elderly. 36 , 37 , 38 In this way, AF patients who are usually older with multiple comorbidities are more susceptible to COVID‐19, particularly in its most aggressive form. Furthermore, it is recognized that AF increases the risk of stroke and thromboembolism and the same applies to patients with mechanical heart valves. 8 Although limited, most of the evidence showed an increased risk of VTE in patients with COVID‐19, but it has been described cases of arterial thrombosis such as stroke and ST‐elevation myocardial infarction. 27 , 39 , 40 , 41 For this reason, the association between AF (and/or mechanical heart valve) and severe COVID‐19 forms (with respiratory insufficiency and SIRS) could be a 'lethal cocktail'. Indeed, despite the high rate of mortality, there was an extremely low rate of thromboembolic or thrombotic events in our patients with even similar rate of the primary outcome in VKA and DOAC users, which suggest that anticoagulation may play an important role in these patients. Notwithstanding, most patients with VTE, AF and mechanical heart valves are treated with OAC, which have shown several drug‐drug interactions with some antiviral treatments. For example, atazanavir, lopinavir/ritonavir, chloroquine and hydroxychloroquine may increase the levels of some DOACs, and even VKA therapy may require caution and potential dose adjustment. Tocilizumab presents an interaction of weak intensity with VKA, and methylprednisolone can interact with the VKA, so its joint use is not recommended. 42 , 43
Given these data, LMWH (or even unfractionated heparin) is likely to be preferred in acutely ill hospitalized patients. 44 , 45 However, to date no study has shown what should be the proper management in patients who presented an indication for anticoagulation prior to SARS‐CoV‐2 infection. Recently, Testa and colleagues showed that DOAC‐treated patients with SARS‐CoV‐2 infection and antiviral drug therapy have increased plasma levels of DOACs. Therefore, they suggested withholding them and replacing with parenteral anticoagulants as long as antiviral agents are deemed necessary and until discharge. 46 Nevertheless, some societies consider that OAC could be maintained based on clinical conditions and assessment for drug‐drug interactions. Therefore, potential interactions should be carefully evaluated, and if switch to LMWH is decided, the appropriate algorithm needs to be followed in order to minimize thromboembolic and bleeding risks of an incorrect LMWH bridging therapy. 12 , 27 , 47
4.1. Limitations
There are some limitations regarding this article. First, it is limited by its observational design. Second, the sample size is limited, since it only includes those patients with prior anticoagulation therapy in the HOPE Registry. Third, in this analysis, the majority of patients derived from the initial phase of the registry, and therefore, most were from Spanish centres. However, this fact serves to homogenize national practice patterns, resources, common drugs and point in the pandemic curve. Subsequently, the internalization of the HOPE Registry was expanded. The limitations to the use of DOACs in Spain according to the therapeutic positioning report of the Ministry of Health for AF hinder the comparison of VKA users and DOACs. At the same time, since DOACs are not reimbursed for VTE, many patients with this pathology do not use DOACs.
The high age of the included patients may contribute to a worse prognosis and high mortality, even though it is not associated with a significant risk in Cox regression analyses. We also recognize that including several different indications for OAC and therefore different risk profiles in the analysis of the primary outcome can hinder and dissipate the specific effect that each indication has.
Regarding switch to LMWH, it cannot be determined in all patients where it was done and the dose (prophylactic or anticoagulant). Similarly, the type of DOAC in some users of this drug was unknown. As such, we decided not to include this information.
Finally, although this cohort was collected in a prospective manner, the results reported in this study are based on a post hoc analysis and should be regarded as hypothesis‐generating. However, this is the first study that analyses a previously anticoagulated population with COVID‐19.
5. CONCLUSION
In this study, patients on OAC therapy admitted for COVID‐19 showed lower survival and higher mortality risk compared to COVID‐19 patients without prior OAC. The exact reasons remain uncertain but may be related to an inherent pro‐inflammatory state. The presence of SARS‐CoV‐2 infection may increase the already‐high thrombotic risk of such patients, thus increasing a baseline inflammatory and hypercoagulable state. Moreover, we found that the prevalence of several comorbidities in COVID‐19 patients on OAC therapy at admission is high. Respiratory insufficiency, SIRS during hospitalization and higher comorbidity pointed out those anticoagulated patients with increased risk of mortality. Thus, patients with prior OAC therapy are more vulnerable, and require special attention and even more careful management to avoid worse clinical outcomes.
CONFLICT OF INTEREST
None declared.
Supporting information
Tables S1‐S5
ACKNOWLEDGEMENTS
The authors thank Cardiovascular Excellence SL, for their essential support in the database and registry webpage, and all HOPE COVID‐19 researchers.
Rivera‐Caravaca JM, Núñez‐Gil IJ, Vivas D, et al; on behalf of HOPE COVID‐19 Investigators . Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID‐19. Eur J Clin Invest. 2021;51:e13436. 10.1111/eci.13436
Trial Numbers: NCT04334291/ EUPAS34399.
Funding informationThis study was supported by the nonconditioned grant (Fundación Interhospitalaria para la investigación Cardiovascular, FIC. Madrid, Spain). This nonprofit institution had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
REFERENCES
- 1. World Health Organization . WHO announces COVID‐19 outbreak a pandemic [updated 12–03‐2020; cited 17–04‐2020]. Available from: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic. Accessed on April 17, 2020.
- 2. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation. 2020;141(20):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Society of Cardiology . ESC Guidance for the Diagnosis and Management of CV Disease during the COVID‐19 Pandemic [updated 21–04‐2020; cited 2020 10–05‐2020]. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance#p00. Accessed on May 10, 2020.
- 5. Malaty M, Kayes T, Amarasekera AT, Kodsi M, MacIntyre CR, Tan TC. Incidence and treatment of arrhythmias secondary to coronavirus infection in humans: a systematic review. Eur J Clin Invest. 2020:e13428. Epub Ahead Of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision‐making. Thromb Haemost. 2017;117(7):1230‐1239. [DOI] [PubMed] [Google Scholar]
- 9. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lippi G, Favaloro EJ. D‐dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131‐E134. [DOI] [PubMed] [Google Scholar]
- 12. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID‐19: a systematic review and meta‐analysis. Eur J Clin Invest. 2020;50(10):e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(6):949‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thachil J. The versatile heparin in COVID‐19. J Thromb Haemost. 2020;18:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International COVID19 Clinical Evaluation Registry, (HOPE COVID 19) (ClinicalTrials.gov Identifier: NCT04334291) 2020 [updated 6 April 2020]. Available at: https://clinicaltrials.gov/ct2/show/NCT04334291. Accessed: April 17, 2020
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 20. Berkman LF, Leo‐Summers L, Horwitz RI. Emotional support and survival after myocardial infarction. A prospective, population‐based study of the elderly. Ann Intern Med. 1992;117(12):1003‐1009. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost. 2020;18(7):1559‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(1):122‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carbone F, Montecucco F, Twickler M. SARS‐CoV‐2: what is known and what there is to know‐Focus on coagulation and lipids. Eur J Clin Invest. 2020;50(7):e13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7(6):e438‐e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421‐1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4:1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID‐19 in elderly patients: a comparison with young and middle‐aged patients. J Infect. 2020;80(6):e14‐e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Figliozzi S, Masci PG, Ahmadi N, et al. Predictors of adverse prognosis in COVID‐19: a systematic review and meta‐analysis. Eur J Clin Invest. 2020;50(10):e13362. [DOI] [PubMed] [Google Scholar]
- 39. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bangalore S, Sharma A, Slotwiner A, et al. ST‐Segment elevation in patients with covid‐19—a case series. N Engl J Med. 2020;382(25):2478‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liverpool Drug Interaction Group . Detailed recommendations for interactions with experimental COVID‐19 therapies 2020 [updated 09 April 2020]. Available from: https://www.covid19-druginteractions.org/. Accessed June 10, 2020
- 43. Mueck W, Kubitza D, Becka M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Testa S, Paoletti O, Giorgi‐Pierfranceschi M, Pan A. Switch from oral anticoagulants to parenteral heparin in SARS‐CoV‐2 hospitalized patients. Intern Emerg Med. 2020;15(5):751‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moores LK, Tritschler T, Brosnahan S, , et al. Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID‐19: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vivas D, Roldán V, Esteve‐Pastor MA, et al. Recommendations on antithrombotic treatment during the COVID‐19 pandemic: positioning of the Cardiovascular Thrombosis Working Group of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73(9):749‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S5


