Summary
The coronavirus disease 2019 (COVID‐19) pandemic is straining the healthcare system, particularly for patients with severe outcomes requiring admittance to the intensive care unit (ICU). This study investigated the potential associations of obesity and diabetes with COVID‐19 severe outcomes, assessed as ICU admittance. Medical history, demographic and patient characteristics of a retrospective cohort (1158 patients) hospitalized with COVID‐19 were analysed at a single centre in Kuwait. Univariate and multivariate analyses were performed to explore the associations between different variables and ICU admittance. Of 1158 hospitalized patients, 271 had diabetes, 236 had hypertension and 104 required admittance into the ICU. From patients with available measurements, 157 had body mass index (BMI) ≥25 kg/m2. Univariate analysis showed that overweight, obesity class I and morbid obesity were associated with ICU admittance. Patients with diabetes were more likely to be admitted to the ICU. Two models for multivariate regression analysis assessed either BMI or diabetes on ICU outcomes. In the BMI model, class I and morbid obesities were associated with ICU admittance. In the diabetes model, diabetes was associated with increased ICU admittance, whereas hypertension had a protective effect on ICU admittance. In our cohort, overweight, obesity and diabetes in patients with COVID‐19 were associated with ICU admittance, increasing the risk of poor outcomes.
Keywords: Covid19, diabetes, intensive care, obesity
What is already known about this subject?
Obesity and diabetes are two major risk factors for COVID‐19 hospitalization
People with Obesity and Diabetes are at higher risk for severe illness
What this study adds
Our findings indicate that more patients with obesity and diabetes are likely to be admitted to the ICU as the pandemic continues. Hence, patients with COVID‐19 with underlying obesity or diabetes must be categorized as a high‐risk group.
1. INTRODUCTION
The outbreak and unprecedented spread of SARS‐CoV‐2, responsible for COVID‐19, has taken the world by storm since December 2019. On March 11, 2020, the World Health Organization labelled the COVID‐19 outbreak as a pandemic. 1
The novel coronavirus SARS‐CoV‐2 appears to affect certain individuals more severely than others. Most patients with SARS‐CoV‐2 infection have only mild or no symptoms; thus, they are considered to have a mild form of the disease. However, some patients have developed life‐threatening acute respiratory distress syndrome, septic shock and multiorgan failure, including acute renal failure and cardiac injury caused by a cytokine storm, which increases the risk of mortality by 15% to 20%. 2
Various risk factors have been linked to the progression of COVID‐19. Recognition of such factors can help to highlight a high‐risk population and determine prevention strategies. Old age, chronic disease, respiratory disease, and cardiovascular disease have been studied intensively and found to have a significant association with the severity of COVID‐19. 3 Patients with obesity and diabetes are also at risk of severe COVID‐19 outcomes, which is particularly important to healthcare workers considering the high prevalence of these conditions in the Middle East, Europe, and the United States. 4 , 5
In this study, we explored the potential association of obesity and diabetes with severe outcomes in patients hospitalized with SARS‐CoV‐2 infection in Kuwait.
2. MATERIALS AND METHODS
2.1. Study design and data collection
This retrospective cohort study included 1158 patients previously diagnosed with COVID‐19, who were admitted to the Jaber Al‐Ahmad Al‐Sabah hospital in Kuwait from 24 February to April 7, 2020. This study is approved by Ministry of health Kuwait, ethical approval committee; Approval number: 1402/2020; Approval Date: March 29, 2020. Testing for COVID‐19 was conducted via real‐time reverse‐transcription polymerase chain reaction assays of nasal swab specimens. Only patients with positive results were included in the study; patients with negative or equivocal results were excluded.
Demographic data, including age, sex, body mass index (BMI), fasting blood glucose level, systolic blood pressure, diastolic blood pressure and past medical history, were extracted from the Jaber Al‐Ahmed Al‐Sabah Hospital's electronic medical records. To maintain confidentiality, these data were de‐identified.
The subjects were classified according to their BMI as follows: normal weight (BMI of 18.5‐24.9 kg/m2), overweight (BMI of 25.0‐29.9 kg/m2) and obese (BMI ≥30 kg/m2). The subjects with obesity were further stratified into classes: class I obesity was defined as a BMI of 30‐34.9 kg/m2; class II obesity, by a BMI of 35‐39.9 kg/m2; and morbid obesity, by a BMI ≥40 kg/m2. 6 Hypertension was defined as systolic blood pressure of ≥130 mmHg or diastolic blood pressure of ≥85 mmHg. 7 Prediabetes was defined as a fasting blood glucose level of 100 to 126 mg/dL, and diabetes was defined as a fasting blood glucose level of ≥126 mg/dL. 8
COVID‐19 progression was defined as a patient's admission to the intensive care unit (ICU) for more active systemic treatment, such as systemic glucocorticoids or intravenous immunoglobulin, or for further advanced respiratory support, such as mechanical ventilation or extracorporeal membrane oxygenation.
2.2. Statistical analysis
Categorical variables were summarized as percentages and analysed with the chi‐square test. Continuous variables were summarized as medians with interquartile ranges (IQRs) and compared in Student's t test or the nonparametric Mann‐Whitney U test. Each promising variable with a plausible biological reason for inclusion in further analysis was verified for normal distribution using the Shapiro‐Wilk and Kolmogorov‐Smirnov tests. Continuous score variables were set to 0 or 1 to represent the values below or above a predefined threshold based on the literature; binary variables were also set at 0 or 1 (absent vs present, respectively).
Potential predictors for admission into the ICU were assessed using univariate and multivariate logistic regression analyses, and logistic regression models were adjusted for age and sex. Two multivariate models were constructed to consider the high association between obesity and diabetes. The associations between the exposure and outcomes were expressed in terms of the odds ratio (OR), along with 95% confidence intervals (CIs). Goodness‐of‐fit analyses of the models were performed using the C‐statistic (area under the receiver operating characteristic [ROC] curve) and 95% CI. A 5% level was considered statistically significant for all the statistical tests. All statistical analyses were performed with R software (R Project for Statistical Computing, Vienna, Austria; R Core Team, 2019).
3. RESULTS
3.1. Characteristics of hospitalized patients
From 24 February to April 7, 2020, 1158 consecutive patients with COVID‐19 were treated at Jaber Al‐Ahmed Al‐Sabah Hospital. Of these, 945 (81.6%) were male (mean age ± SD [SD]: 41.5 ± 13.5 years), and 213 (18.4%) were female (mean age ± SD: 44.8 ± 18.9 years). The majority of the patients were Indians (n = 550, 47.5%), 301 (26%) were Kuwaiti, and the remaining 307 patients were of a different nationality. The most common symptoms of COVID‐19 were cough (n = 344, 29.7%), chills (n = 327, 28.2%) and sore throat (n = 135, 11.7%).
The baseline characteristics of the study patients are listed in Table 1 of patients with BMI measured at baseline (n = 727), 461 patients (39.8%) had BMI ≥25 kg/m2: 304 were overweight, 89 had class I obesity, 40 had class II obesity and 19. had morbid obesity. Blood glucose measurements revealed that 314 (27.1%) of the patients had pre‐diabetes and 271 (23.4%) had diabetes. Hypertension was diagnosed in 236 patients (20.4%). By the time of the analysis, 104 patients (9%) had been admitted to the ICU for treatment of COVID‐19, and a total of 40 deaths had occurred (Table 1).
TABLE 1.
Characteristics of patients hospitalized with COVID‐19
| Characteristic | Value a |
|---|---|
| Total | 1158 (100%) |
| Age, years | 40.5 (31.5‐52.1) |
| Sex – Male | 945 (81.6%) |
| BMI | |
| Normal weight | 266 (36.6%) |
| Overweight | 304 (41.8%) |
| Class I obesity | 98 (13.5%) |
| Class II obesity | 40 (5.5%) |
| Morbid obesity | 19 (2.6%) |
| Fasting plasma glucose | |
| Normal | 573 (49.5%) |
| Pre‐diabetes | 314 (27.1%) |
| Diabetes | 271 (23.4%) |
| Hypertension | 236 (20.4%) |
| Systolic BP | 127 (118‐137) |
| Diastolic BP | 80 (72‐85) |
| ICU admission | 104 (9%) |
| Death | 40 (3.5%) |
Abbreviations: BMI, body mass index; BP, blood pressure; COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
Values are expressed as medians (interquartile ranges) or as numbers (%).
3.2. Characteristics of patients admitted to ICU
The characteristics of patients admitted to ICU were compared with those who did not require ICU care (Table 2). Among patients admitted to the ICU, the median (IQR) blood glucose level was 147 (108‐208) mg/dL. In contrast, the remaining 1054 patients who did not require ICU care had significantly lower blood glucose levels than those who required ICU care (median [IQR]: 98.6 [86.2‐118] mg/dL; P < .001). The patients admitted to the ICU were also significantly older (median age [IQR]: 54 [46.4‐63.4] years) than those who did not require ICU care (median age [IQR]: 39.3 [30.7‐50.4] years; P < .001). The median BMI of patients admitted to ICU was also statistically significantly higher than those who did not require ICU care (median [IQR]: 27.5 [25.3‐31.4] kg/m2 vs 26 [23‐29] kg/m2, respectively; P < .001). The number of patients with hypertension among those admitted into ICU was significantly higher than those who were not (16% vs 8.1%; P = .004).
TABLE 2.
Comparison of vitals and laboratory measurements between patients with COVID‐19 with and without ICU admittance
| Characteristic | ICU admittance a | P value | |
|---|---|---|---|
| No (N = 1054) | Yes (N = 104) | ||
| Age, years | 39.3 (30.7–50.4) | 54.0 (46.4‐63.4) | <.001 b |
| Sex ‐ Male | 274 (26%) | 66 (63.5%) | |
| Body Mass Index, kg/m2 | 26 (23‐29) | 27.5 (25.3‐31.4) | <.001 d |
| Normal weight | 253 (95.1%) | 13 (4.9%) | .006 c |
| Overweight | 270 (88.8%) | 34 (11.2%) | .006 c |
| Class I obesity | 83 (84.7%) | 15 (15.3%) | .006 c |
| Class II obesity | 35 (87.5%) | 5 (12.5%) | .006 c |
| Morbid obesity | 15 (78.9%) | 4 (21.1%) | .006 c |
| Fasting Plasma Glucose, mg/dL | 98.6 (86.2–118) | 147 (108‐208) | <.001 d |
| Normal | 554 (96.7%) | 19 (3.3%) | <.001 c |
| Pre‐diabetes | 295 (93.9%) | 19 (6.1%) | <.001 c |
| Diabetes | 205 (75.6%) | 66 (24.4%) | <.001 c |
| Hypertension | 47 (8.14%) | 24 (16%) | .004 d |
| Systolic BP, mm Hg | 127 (118‐136) | 126 (116‐142) | |
| Diastolic BP, mm Hg | 80 (72–85) | 73.5 (65.6‐82) | <.001 c |
Abbreviations: BP, blood pressure, COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
Values are expressed as medians (interquartile ranges) or as numbers (%).
Student's t test.
Chi‐squared test.
Mann‐Whitney U test.
All classifications of BMI that were higher than normal were associated with admission to the ICU (P‐value = .006). The association between obesity, diabetes and hypertension was tested with the chi‐square test, which revealed a relationship between obesity and diabetes (P‐value <.001); we therefore decided to construct two predictive models for ICU progression.
3.3. Univariate analysis for ICU admission
Potential predictors for admission into ICU, including age, BMI and diabetes status, were analysed using univariate logistic regression (Table 3). Using BMI as the only predictor of ICU admission, the likelihood of admission was approximately 2.5‐fold higher in patients who were overweight than in those with normal weight (OR = 2.45; 95% CI: 1.26‐4.74; P = .008). Patients with obesity were 3.5 and 5.2 times more likely to be admitted to ICU than patients with normal weight, depending on whether they had class I or morbid obesity, respectively (OR: 3.51 [95% CI: 1.60‐7.69] for class I obesity; and OR: 5.18 [95% CI: 1.50‐17.85] for morbid obesity). Diabetes was also an independent predictor for admission into the ICU (OR = 9.38; 95% CI: 5.49‐16.02; P<.001). Other variables, including age, sex, hypertension, pre‐diabetes and class II obesity were not significant predictors for ICU admission.
TABLE 3.
Univariable and multivariable analyses for obesity and other comorbidities, odds ratio adjusted by age and gender
| Characteristic | Univariate | Multivariate a | Multivarite b | |||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value | |
| Age (years) | 1.06 (1.05‐1.08) | <.001 | 1.07 (1.04‐1.09) | <.001 | 1.06 (1.04‐1.08) | <.001 |
| Sex – Male | 0.73 (0.41‐1.28) | .275 | 3.58 (1.45–8.8) | .005 | 2.23 (1.18‐4.22) | .007 |
| Body mass index | ||||||
|---|---|---|---|---|---|---|
| Normal weight | Reference | Reference | Reference | Reference | Reference | |
| Overweight | 2.45 (1.26–4.74) | .008 | 1.91 (0.94‐3.84) | .07 | ||
| Class I obesity | 3.51 (1.60–7.69) | .002 | 2.7 (1.17–6.20) | .019 | ||
| Class II obesity | 2.78 (0.93‐8.27) | .066 | 1.61 (0.50‐5.15) | .423 | ||
| Morbid obesity | 5.18 (1.50–17.85) | .009 | 3.95 (1.00‐15.20) | .046 | ||
| Diabetes | ||||||
|---|---|---|---|---|---|---|
| Normal | Reference | Reference | Reference | Reference | Reference | |
| Pre‐diabetes | 1.87 (0.97‐3.60) | .058 | 1.4 (0.71–2.45) | .324 | ||
| Diabetes | 9.38 (5.49‐16.02) | <.001 | 5.49 (3.13‐9.65) | <.001 | ||
| Hypertension | 0.73 (0.42‐1.26) | .265 | 0.63 (0.32–1.26) | .025 | 0.51 (0.28‐0.91) | .197 |
Obesity multivariate model.
Diabetes multivariate model.
3.4. Multivariate analysis for ICU admission
Considering the association between obesity and diabetes and excluding the potential confounding effect of these two variables on the outcome, we constructed two multivariate logistic regression models of prediction to accurately assess the associations of each condition with ICU admittance (Figure 1). In the first multivariate analysis model, where obesity was assessed, BMI (adjusted for age and sex) was significantly associated with ICU admittance. In patients who were overweight with class I or morbid obesities, the adjusted OR (AOR) were 1.91 (95% CI: 0.94‐3.84), 2.7 (95% CI: 1.17‐6.20) and 3.95 (95% CI: 1.00‐15.2), respectively (Figure 1). Sex was also identified as a risk factor for ICU admission (AOR: 3.58 [95% CI: 1.45‐8.8]). On the other hand, hypertension reduced the risk of ICU admission by approximately 40% (AOR: 0.63; 95% CI: 0.32‐1.26; P = .025).
FIGURE 1.
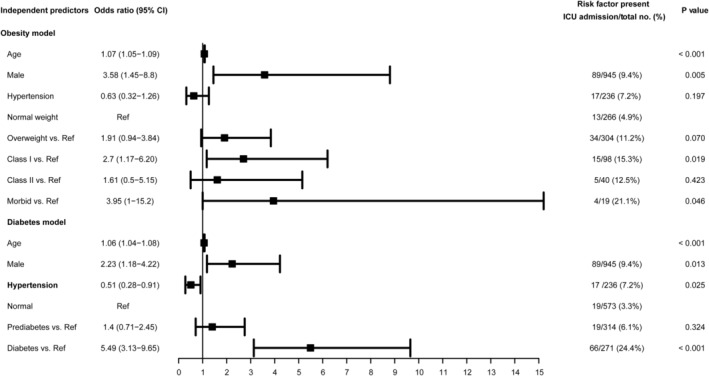
Independent predictors of ICU admission from multivariate logistic regression analysis. CI, confidence interval; ICU, intensive care unit; Ref, reference value
In the second multivariate analysis model of prediction, diabetes (adjusted for age and sex) was associated with an increased the risk of ICU admittance (AOR = 5.49; 95% CI: 3.13‐9.25, P≤.001); however, patients with pre‐diabetes did not have an increased risk of ICU care (AOR: 1.4 [95% CI: 0.71‐2.45]; Figure 1). Both multivariate models were validated by calculating the area under the ROC curve, which were > 0.800 for both multivariable models, indicating a good model discrimination (Figures 2 and 3).
FIGURE 2.
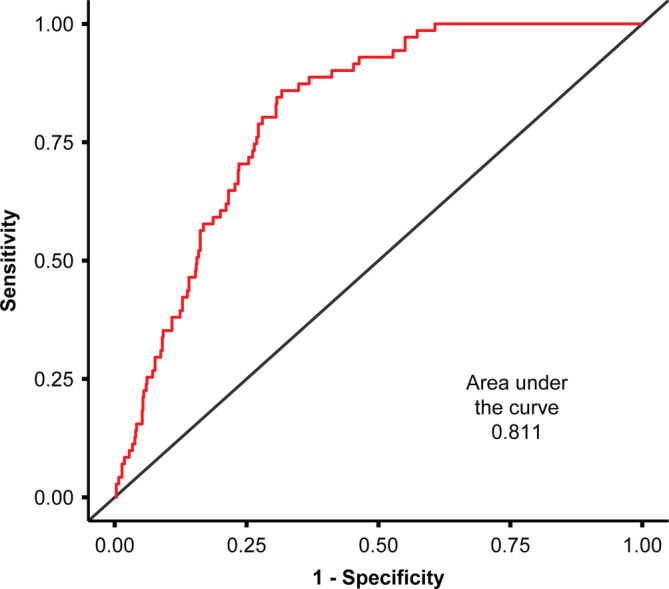
Receiver operating characteristic curve for the BMI multivariate model of prediction. BMI, body mass index
FIGURE 3.
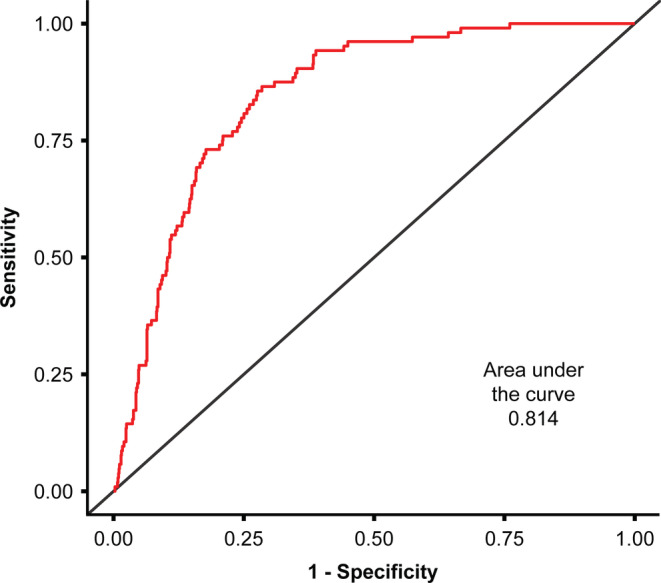
Receiver operating characteristic curve for the diabetes multivariate model of prediction
4. DISCUSSION
This was the first retrospective cohort study in Kuwait and the Middle East which analysed the potential association of obesity and diabetes with COVID‐19 disease severity. All patients in our cohort were admitted to a single centre, where they underwent standardized sets of investigations and received standardized treatment protocols.
In this study, univariate logistic regression analysis revealed that BMI and diabetes were independent factors associated with ICU admission. After adjusting for confounding factors, further multivariate analysis confirmed these results, revealing that in our population, diabetes and higher BMI were associated with ICU admittance. In concordance with our findings, a study from a New York Hospital by Lighter et al. demonstrated that patients with COVID‐19 and BMI between 30 and 34.9 were two times more likely to be admitted to the acute critical care unit than patients without obesity. 9 In the same vein, in a retrospective cohort of 124 patients in France, Simonnet et al found a high prevalence of obesity among patients admitted to the ICU. 10 Although obesity is not as prevalent in China as in the Middle East, Peng et al. obtained similar results in a retrospective analysis of 112 patients with COVID‐19, who were admitted to Wuhan Union Hospital in Wuhan, China. 11 When patients were divided into two groups according to the severity of the disease (critical and general), researchers observed that the BMI of the critical group was significantly higher than that of the general group (25.5 [23.0‐27.5] kg/m2 vs 22.0 [20.0‐24.0] kg/m2, respectively; P = .003). 11 A recent meta‐analysis by Husain et al. also corroborated these observations. 12
In our study sample, class II obesity was not significantly associated with admittance to the ICU, though this could be attributed to the small sample size and the fact that a large proportion of patients in this class had no symptoms.
The association between diabetes and ICU admission seen in our cohort using multivariate prediction analysis was not entirely surprising. Patients with diabetes have a greater risk of severe COVID‐19, as reported in several studies; the higher risk of respiratory infections has been attributed to the compromised immune system, especially the innate immunity, of patients with diabetes. 13 Even transient hyperglycaemia may temporarily affect the innate immune response to infection. 14
The exact mechanism underlying obesity and diabetes contributing to severe outcomes among patients with COVID‐19 is still unclear. However, one explanation could be related to the fact that expression of angiotensin‐converting enzyme 2, the functional receptor for SARS‐CoV, is up‐regulated in patients with obesity and diabetes. 15 Furthermore, obesity and diabetes are linked with dysregulated lipid synthesis and clearance, which can initiate or aggravate pulmonary inflammation and injury. 16 Mechanistic studies on this matter are required to elucidate the mechanism by which diabetes and obesity contribute to disease severity and poor outcomes among patients with COVID‐19.
5. CONCLUSION
The novel COVID‐19 pandemic has created an unprecedented challenge in health care, exacerbating the unavailability of medical resources throughout the world. Bariatric surgeons have witnessed the rise of obesity and diabetes in epidemic proportion and have come to realize the effect of this rise on the medical care system. In our study, diabetes and BMI were associated with severe COVID‐19 outcomes, as assessed by the ICU admittance of hospitalized patients. We acknowledge that our study had some limitations. Given its retrospective nature, the unavailability of data as a result of omission or inadequate recording was a major limitation of this study. Another limitation of this study was the relatively higher ratio of male to female patients, which may limit the generalisability of the results to the population. Another factor that may impact the applicability of these findings to other populations, is the fact that obesity is quite different in the Middle East and Asia, compared to the Western part of the world, both from an epidemiological and physiological perspective. 17 , 18 The relatively small size of our sample population was another limitation. Thus, larger, multicentre studies are required to confirm our findings, which will provide more robust scientific evidence. Nevertheless, our findings indicate that more patients with obesity and diabetes are likely to be admitted to the ICU as the pandemic continues. Hence, patients with COVID‐19 with underlying obesity or diabetes must be categorized as a high‐risk group.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
We would like to thank Dr Diana Marouco, PhD, for the editorial support provided. We would also like to thank Bruno Siegel Guerra for assistance with statistical analysis.
Al‐Sabah S, Al‐Haddad M, Al‐Youha S, Jamal M, Almazeedi S. COVID‐19: Impact of obesity and diabetes on disease severity. Clin Obes. 2020;10:e12414. 10.1111/cob.12414
Salman Al‐Sabah and Mohannad Al‐Haddad contributed equally
Funding information Kuwait Foundation for the Advancement of Sciences, Grant/Award Number: Corona prop 35
REFERENCES
- 1. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity (Silver Spring, md). 2020.28(7):1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan RE, Adab P, Cheng KK. Covid‐19: risk factors for severe disease and death. BMJ (Clinical Research Ed). 2020;368‐m1198. 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 4. Abbas AM, Fathy SK, Fawzy AT, Salem AS, Shawky MS. The mutual effects of COVID‐19 and obesity. Obes Med. 2020;100250 19 100250. 10.1016/j.obmed.2020.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abbas AM, Sayed R, Omar F, Ahmed L. Birdirectional relationship between COVID‐19 and diabetes. AJBSR. 001442 2020;9(6): 10.34297/AJBSR.2020.09.001442. [DOI] [Google Scholar]
- 6.WHO. WHO Mean Body Mass Index (BMI). 2015.
- 7. Whelton PK, Carey RM, Wilbert SA, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 2. Classification and diagnosis of diabetes . Standards of medical care in diabetes‐ 2019. Diabetes Care. 2019;42(Suppl 1):S13‐S28. [DOI] [PubMed] [Google Scholar]
- 9. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60years is a risk factor for Covid‐19 hospital admission. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020;71(15):896–897. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring, md). 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. [DOI] [PubMed] [Google Scholar]
- 12. Hussain AH, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020.14(4):295–300. 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Aggarwal G, Lippi G, Lavie CJ, Brandon MH, Sachis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. J Diabetes. 2020;12(11):851–855. 10.1111/1753-0407.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes & Metabolic Syndrome. 2020;14(4):395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring, md). 2020;28:1187‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heialy SA, Hachim M, Senok A, et al. Regulation of angiotensin converting enzyme 2 (ACE2) in obesity: implications for COVID‐19. bioRxiv. 2020;046938. 10.1101/2020.04.17.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanchis‐Gomar F, Lavie CJ, Mehra MR, Brandon MH, Obesity LG. Outcomes in COVID‐19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157‐163. [DOI] [PubMed] [Google Scholar]


