Summary
Apart from posing various mechanical and medical issues compromising general health, obesity is a major factor for respiratory tract infections, due to specific inflammation and immunological compromise. The burden of obesity on morbidity and mortality of SARS‐CoV‐2 infection/COVID‐19 is considerable. Herein, we aimed to search the literature and present to the readers pathophysiologic pathways that may associate obesity and COVID‐19. We present potential mechanisms, which might partly explain why patients with obesity are more prone to suffer from respiratory infections in the context of COVID‐19. Better understanding of these pathways could eventually guide management strategies and therapies for COVID‐19 in the future.
1. INTRODUCTION
The world is struggling to fight the novel COVID‐19 pandemic caused by SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2), previously known as “2019 novel coronavirus”. 1 Per the World Health Organization (WHO), data converge that the infection fatality rate of COVID‐19 is 0.5‐1.0% 2 ; seasonal influenza has a rate of 0.1. 2 In an attempt to identify the risk factors that might aggravate the prognosis of outpatients or hospitalized patients, obesity might be a potential suspect, since it seems to interfere with the presence or worsening of respiratory tract infections (RTIs), a major component of the mortality of COVID‐19. 1
Obesity is defined as having a body mass index equal to or higher than 30 kg/m2 for all populations worldwide, with the exception of China, where according to the World Health Organization obesity is defined as having a BMI equal to or higher than 27.5 kg/m2. 3 Obesity rates have almost tripled during the last 40 years, establishing it as an epidemic. Statistics from WHO in 2016, showed that 13% of the world population suffer from obesity (over 650 million subjects in total) and that 39% (more than 1.9 billion subjects) are overweight. 4 In industrialized countries, roughly half of the population is overweight or obese. In USA, the National Health and Nutrition Examination Survey (NHANES) report reported that 34% of inhabitants have obesity. 5
In the past, from the “Spanish” influenza of 1918 onwards, in all influenza epidemics, including the 2009 Influenza A virus (IAV) H1N1 pandemic, malnutrition and obesity were associated with severe disease, complications, hospitalization, need for treatment in intensive care units (ICU) and mortality. 6 This pattern seems to be replicated with COVID‐19 and the World Obesity Federation has stated that “obesity‐related conditions seem to worsen the effect of COVID‐19 1 ; indeed, the Center for Disease Control and Prevention (CDC) reported that subjects with heart disease and diabetes are at higher risk of COVID‐19 complications and that severe obesity (body mass index [BMI] of 40 kg/m2or higher) poses a higher risk for severe illness 7 ”.
Many pathophysiological changes occurring with increased adiposity may contribute to poor prognosis of COVID‐19 patients. 8 , 9 In the present review, we aimed to search the scientific literature for pathophysiologic pathways that associate obesity and COVID‐19. We discuss published data regarding the association of obesity with the incidence and severity of respiratory tract infections (RTIs), along with RTI caused by COVID‐19, focusing on molecular or hormonal pathways that may be possibly involved and drawing parallels to previous similar outbreaks of RTIs in previous epidemics.
2. LITERATURE SEARCH
The PubMed, Google Scholar, MedRxiv and BioRxiv databases were accessed by all the co‐authors to identify relevant English‐language articles published up to September 15, 2020. The search terms included “human, coronavirus, COVID‐19, SARS‐CoV‐2, cytokine, adipokine, obesity, complications, human, humoral immunity, cellular immunity”. Twenty‐eight articles were initially selected and additional publications of relevance to the present article were identified by reviewing the references of the eligible articles.
3. EPIDEMIOLOGICAL DATA
Due to the very recent and not yet well‐studied outbreak of COVID‐19, epidemiological data and risk factors in the normal‐weight and/or populations with obesity are still lacking. While age and male sex are regarded as significant risk factors, accumulating evidence suggests a strong association with an impaired cardiometabolic profile. Initial reports from Wuhan, China, where the COVID‐19 first occurred, indicated a higher prevalence of hypertension and diabetes among patients with severe compared to non‐severe illness. 10 Both entities are associated with obesity, forming part of the criteria of the cluster of the metabolic syndrome. Furthermore, hypertension and diabetes, as well as cardiovascular disease were identified as risk factors associated with fatal outcomes. 11 However, most patients recruited in Chinese studies had normal BMI or a BMI compatible with overweight classification, but not obesity (please see above; in China obesity is defined by the World health Organization as having a BMI over 27.5 kg/m2 3). Thus, increased BMI was not listed among the risk factors for COVID‐19; however, this may reflect lower prevalence of obesity in the region due to a differential body fat distribution in individuals of Asian origin. 12 Nevertheless, it was reported that the percentage of patients with overweight BMI is markedly higher in non‐survivors compared to survivors from COVID‐19, 13 while most recent data have shown that in patients presenting with metabolic fatty liver disease, the presence of obesity correlated with a 6‐fold increased risk for severe illness from COVID‐19. 14 Results from a population‐based surveillance program across 14 states representing approximately 10% of US population, have shown that among 1482 hospitalized patients in March 2020 for confirmed COVID‐19, obesity was the second most common underlying condition in the general population, closely following hypertension, and first among younger individuals aged 18‐49. 15 In this age group, 59% of COVID‐19 patients that required hospitalization suffered from obesity and this proportion was substantially higher than any other underlying condition, suggesting that obesity might be one of the main risk factors for severe COVID‐19 in young or middle‐aged adults. 15 The same finding has been reported from Spain, 16 although regional data have not been officially published yet. Selected data on obesity and outcome are shown in Table 1. 17 , 18 , 19 , 20 , 21 , 22 , 23
TABLE 1.
Selected reports on the outcome of subjects with obesity and COVID‐19 vs subjects without obesity
| Country of origin | N (subjects with obesity /total study subjects with COVID‐19) | Outcome | Odds ratio for outcome (95% confidence interval) |
|---|---|---|---|
| United States 17 | 402/3615 | Severe disease | 1.80‐3.60 (1.20‐5.30) |
| United States 18 | 56/102 | Mortality in intensive care unit | 0.79 (0.41‐1.53) a |
| China b 19 | 37/96 | Admission to intensive care unit | 1.26 (NA) |
| China c 20 | 36/95 | Mortality | 8.62 (0.40‐184.9) |
| France 23 | 895/5795 | Mortality | 1.89‐2.55 (1.45‐3.97) |
| Spain 21 | 119/1000 | Mortality | 2.53 (1.47‐4.36) |
| Mexico 22 | 10 708/51633 | Mortality | 1.26 (1.11‐1.43) |
Calculated from the data provided in the article.
In this study results were noted for BMI > 24 kg/m2.
In this study results were noted for BMI > 24.9 kg/m2.
In western countries, although data from Italy have confirmed earlier Chinese reports, 24 a French study showed that patients with BMI > 35 kg/m2 were at significantly higher risk for the requirement of intensive mechanical ventilation compared to normal‐weight individuals (with BMI lower than 25 kg/m2), even after adjusting for age, diabetes and hypertension. 25 These results were later replicated in a different hospital in France 26 and have generated more interest in the association between adipose tissue excess and disease pathophysiology. In a meta‐analysis, patients with obesity and COVID‐19 had a significantly elevated Odds Ratio of 1.20‐7.36 to be critically ill and to be mechanically ventilated vs subjects with normal BMI. Being overweight (BMI > 25 kg/m 2 ) or having obesity with COVID‐19 entailed an Odds Ratio of 1.22‐3.68 for death compared with normal weight subjects. 27 , 28
Emerging data from the UK National Intensive Care National Audit and Research Centre indicated that 7 out of 10 patients admitted to ICU were overweight or had obesity and increased body fat accumulation correlated with serious or fatal complications, 29 while in New York hospitals, patients that required mechanical ventilation were again predominantly males with obesity. 30
It has been established that obesity may impair outcomes in patients hospitalized for respiratory infections. In a study of 1455 individuals with obesity, the latter was correlated significantly with lower RTIs (adjusted OR = 2.02, 95%CI = 1.36‐3.00) and upper RTIs (adjusted OR = 1.55, 95%CI = 1.22‐1.96), with a stronger association for women. 31 Influenza‐like illness, bronchitis and pneumonia, pharyngitis, laryngitis but also rhinitis and sinusitis proved to occur more frequently in subjects with obesity. 31 Interestingly, in a counter‐intuitive fashion, a quasi‐protective effect of obesity on acute lung infections has also been noted. Researchers have hypothesized that the chronic pro‐inflammatory state of subjects with obesity may act towards the preconditioning of these subjects to better withstand severe lung injury or sepsis. However, this purported preconditioning may not apply for COVID‐19, although experts are still debating this issue. 32 , 33 , 34 , 35 , 36 Of note, reports indicate that the adipose tissue may be a viral reservoir in patients with COVID‐19. 37
Since reports regarding COVID‐19 are limited due to the ongoing pandemic, data can be mirrored with previous similar infectious spreads, such as the 2012 Middle East respiratory syndrome coronavirus (MERS‐CoV) outbreak. In that outbreak, age, male gender and cardio‐metabolic conditions including hypertension, diabetes and obesity proved to be independent risk factors for severe illness, 38 similarly to the current pandemic. This distinct pattern may suggest a shared molecular pathway between coronaviruses and pathophysiological changes occurring in metabolic syndrome related diseases. In addition, high BMI was among the most frequently identified underlying conditions in patients with pandemic 2009 influenza A (H1N1) 39 and obesity was a significant risk factor for severe illness and mortality, 40 with one third of the individuals requiring ICU being patients with obesity. 41
4. GENERAL CONSIDERATIONS IN RESPIRATORY TRACT INFECTIONS IN PERSONS WITH OBESITY
Patients with obesity may need a bariatric hospital bed, which is rarely found, or if so, certainly in small numbers not in all primary care hospitals/institutions. Given that COVID‐19 poses a great challenge for hospitals, facilities for individuals with obesity may be in shortage. 42 They also pose a serious challenge in terms of intubations, since the excess adipose tissue on the larynx makes it technically more difficult to intubate. In patients with obesity it may be challenging to obtain a proper imaging diagnosis (there are weight limits on imaging equipment), making it less straightforward to diagnose an infection in the lung parenchyma. Moreover, patients with obesity are definitely more difficult to position and transport by nursing staff and at the same time suffer from poorer mobility; these patients may not be able to self‐care and may have poor skin integrity and skin deterioration (thus becoming vulnerable to infection). 43 , 44 , 45 Of note, Sattar et al suggested that the higher prevalence of severe COVID‐19 in the elderly population may not necessarily be associated with obesity: the relative increase in fat mass may be due to loss of muscle mass and sarcopenia (the same authors advised the need for clear public health advice regarding increased physical activity and healthy diet to reduce the burden of obesity on COVID‐19, especially during lockdown measures). 46 Regarding pure respiratory characteristics of these patients, the lungs of patients with obesity exert altered mechanics, leading to compromised lung ventilation and gas perfusion, thereby lowering oxygen supply due to trunk pressure on the lung parenchyma. 47 Central adiposity limits thoracic expansion and as a result, the pulmonary parenchyma at the lung bases is suppressed, along with a potential weakness of the thoracic muscles. 47
Obesity is often complicated by type 2 diabetes, which seems to be a risk factor for infections per se due to poor skin healing and higher vulnerability to infection. 48 , 49 Fat deposition in the liver may often lead to liver steatosis and possible functional impairment, thus affecting the pharmacokinetics of medications, too, while co‐existence of chronic renal insufficiency, a common comorbidity in obesity, may complicate the excretion of metabolites or toxic byproducts. 50 , 51 In technical terms, individuals with obesity sometimes face difficulties in swallowing of medication and very often exhibit hiatus hernia or gastro‐oesophageal reflux disease (GORD), which might impair proper absorption of a chemical substance. 6 Another aspect that might worsen the prognosis of an infection in a patient with obesity is an exaggerated pro‐thrombotic response and increased thrombogenic risk along with increased C‐Reactive Protein (CRP) and fibrinogen levels. 52 , 53
5. MOLECULAR PATHOPHYSIOLOGY OF RESPIRATORY TRACT INFECTIONS IN PERSONS WITH OBESITY
Obesity is a state of low‐grade inflammation and defective innate immunity, resulting from the release of adipokines (leptin, adiponectin, visfatin, resistin), Plasminogen Activator Inhibitor‐1 (PAI‐1), angiotensinogen and vascular endothelial growth factor (VEGF), inflammatory cytokines [Tumour Necrosis Factor‐alpha (TNF‐α), Interleukin‐1 (IL‐1), IL‐6, IL‐10], Transforming Growth Factor‐ β (TGF‐β) and Monocyte Chemoattractive Protein‐1 (MCP‐1) 54 , 55 (Figure 1). COVID‐19 may be complicated by acute respiratory distress syndrome, caused by systemic hyper‐inflammation and cytokine release, in response to increased production of CD14+ and CD16+ inflammatory monocytes (which lead to excessive synthesis of IL‐6). Circulating IL‐6 levels are associated with serum viral load, 56 and a meta‐analysis has confirmed that increased IL‐6 concentrations are associated with disease severity. 57 Monoclonal antibodies against the IL‐6 receptor could be used against COVID‐19 complications but large‐scale relevant studies are lacking. 58 , 59
FIGURE 1.
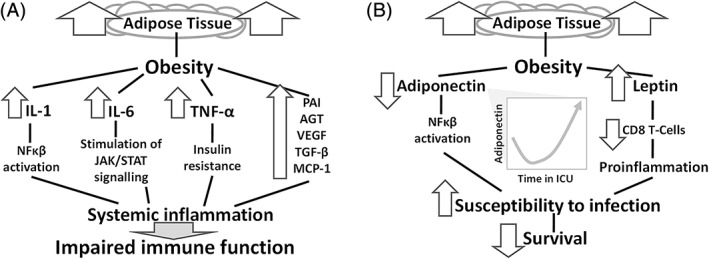
A, Cytokines and inflammation in subjects with obesity. Release of inflammatory molecules represents the cornerstone of obesity‐induced inflammation. NF‐κB: Nuclear Factor kappa‐light chain‐enhancer of activated B‐cells. B, Adipose tissue and adipokines in subjects with obesity. Healthy adipose tissue secretes less leptin and more adiponectin, preventing inflammation. In obesity, this is inversed and reduced levels of the anti‐inflammatory adiponectin favour resistance to leptin and subsequent susceptibility to infections. This is notable for lung infections and critical illness that requires treatment in an intensive care unit (ICU). AGT, angiotensinogen; MCP‐1, monocyte chemoattractive protein‐1; PAI‐1, plasminogen activator inhibitor‐1; TGF‐β, transforming growth factor‐ β; VEGF, vascular endothelial growth factor
Interferons (IFNs) are released in response to viral infections. Smith et al showed that diet‐induced obese mice had worse clinical presentation following influenza infection, with increased mortality (42% vs 5% in lean mice). 60 In the same report, reduced lung type I IFNα/β mRNA expression, reduced natural killer cell potential and diminished lung pro‐inflammatory cytokine (IL‐6, TNF‐α and IL‐1β) and chemokine mRNA expression 60 were noted. In COVID‐19 patients, expression of IFNγ was lower in patients with moderate compared to mild disease. 61
Macrophage activation vs an antigen, along with B‐ and T‐ cell responses, are reduced in obesity. In studies of obese mice, respiratory infection disease showed increased severity and increased secondary bacterial infections, along with a delayed and barely blunted immune response and increased morbidity. 62 Obesity minimizes the response of CD8 + T cells to viruses. 6 Post‐vaccination for influenza, subjects with obesity show lower antibodies' levels compared to lean subjects, and a weaker influenza‐specific CD8 + T cell function; these subjects with obesity show a two‐ to 3fold higher incidence of influenza despite being vaccinated. 63
The cytokine leptin is released from excess adipose tissue, signalling to the brain the amount of this tissue, albeit with no tangible effect on adiposity per se. 51 Increased leptin (caused by leptin resistance) is often seen in obesity ‐ similarly to insulin resistance ‐ and may predispose to a proinflammatory status. Leptin resistance compromises defence to infection and increases susceptibility to respiratory infections. 64 Zhang et al suggested that leptin resistance could aggravate outcome with 2009 A (H1N1) influenza, by exerting effects in B cell maturation, development and function. 65 Leptin binds the Ob‐Rb receptor, stimulating the Janus kinase‐signal transducer and activator of transcription JAK‐STAT pathway, promoting the translocation of phosphorylated STAT proteins to the nucleus and subsequent gene transcription. Ob/ob and db/db mice (mice deficient in leptin signalling) present with increased rates of bacterial infections and pneumonia. Leptin has been found to be implicated in asthma, chronic obstructive pulmonary disease (COPD) and obstructive sleep apnoea. 66 IFN signalling uses the same JAK‐STAT pathway, thus offering a potential correlation of leptin, IFN and host defence vs viruses. 66 , 67
Adipose tissue (mainly white) secretes the adipokine adiponectin. The latter promotes insulin sensitivity and is known to display anti‐inflammatory effects, by reducing the inflammatory cascade induced by adipose cells, mainly via activation of NFkB. 68 Adiponectin exerts immunomodulatory actions; patients with lower adiponectin levels at admission to the ICU have lower survival rates. 69 In general, healthy (ie, non‐excessive) adipose tissue secretes more adiponectin and less leptin, thus reducing inflammation. In obesity the adiponection/leptin ratio is inversed, favouring inflammation. 70 Increased TNF‐α following inflammatory processes leads to the downregulation of the latter’s receptors (attenuating adiponectin's inflammatory signalling and insulin sensitizing actions). Opposing roles have been attributed to adiponectin and IL‐6 vis‐a‐vis the modulation of insulin sensitivity. Inhibition of adiponectin production by IL‐6 and TNF‐a, lowers adiponectin levels and perpetuates the low‐grade chronic inflammation of metabolic disorders. 71 , 72 It would be reasonable to study levels of these or other adipokines in patients with COVID‐19 and assess their clinical utility as biomarkers of disease severity and progression.
6. OBESITY AND CORONOVIRUSES: SPECIFIC MOLECULAR TARGETS AND SHARED PATHOPHYSIOLOGY
Gattinoni et al suggested that COVID‐19 pneumonia presents with two time‐related main phenotypes identified based on CT findings: Type L, characterized by low elastance (ie, high compliance), low ventilation‐to‐perfusion ratio, low lung weight and low recruitability and Type H, characterized by high elastance, high right‐to‐left shunt, high lung weight and High recruitability. 73 AMong many hypotheses proposed to explain the robust epidemiological findings of severe illness from coronaviruses strains, such as SARS‐CoV‐2 and MERS‐CoV, in patients with deteriorated metabolic profile, the role of angiotensin converting enzyme 2 (ACE2) and DPP4 receptor has gathered some attention. In specific, SARS‐CoV‐2 utilizes ACE2 as cell entry receptor via a serine protease termed transmembrane serine protease 2 (TMPRSS2) and TMPRSS2 blocking agents have shown to prevent SARS‐CoV‐2 entry. 74 ACE2 is widely expressed in many organs and particularly in the lungs, pancreas, enterocytes, blood vessel endothelium and membrane of fat cells. 75 This may explain the systemic, multi‐organ damage noticed in COVID‐19 patients with preferential mortality in patients with diabetes and hypertension. Angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) upregulate ACE2 and their role in managing hypertension or diabetes complications in patients with COVID‐19 has been debated; however, in the absence of clinical evidence of any adverse effects of these agents, continuation of treatment has been advised. 76 Regarding DPP4, this receptor was shown to be a functional MERS‐CoV target. 77 After binding to DPP4, MERS‐CoV induces immune response by activating T‐cells via NFkB pathway. Therefore, DPP4 inhibitors (DPP4i) have been suggested as potentially useful agents in the fight against coronaviruses. 78 These drugs, together with glucagon‐like peptide −1 receptor agonists (GLP‐1RA) exert immunomodulatory and anti‐inflammatory effects and may represent tempting therapeutic targets in individuals with diabetes with respiratory or other systemic inflammation. However, SARS‐CoV‐2 does not bind to DPP4 and currently there is no evidence to support their use in COVID‐19. 79
Regarding COVID‐19, there may be a possible direct effect of the SARS‐CoV‐2 virus on pancreatic insulin secretion. 80 , 81 In severe COVID‐19 infection, cells from the lung epithelium, as well as from endothelial cells, secrete abundantly plasminogen activator inhibitor 1 (PAI‐1), leading to hypofibrinolysis 82 , 83 , 84 and hypercoagulability and adding to the disease's morbidity. This should be more severe in patients who have obesity and suffer from COVID‐19, who already are in a hypercoagulable state via the same mechanisms. 85 , 86 , 87
7. ETHNIC BACKGROUND AND THE ROLE OF VITAMIN D
Accumulating data show that ethnic and socioeconomic differences may be listed among risk factors for severe COVID‐19 infection. Subjects with increased BMI are prone to be vitamin D deficient (with an odds ratio of 1.35 compared to subjects with BMI less than 25 kg/m2). 88 , 89 , 90 Vitamin D insufficiency has been associated with compromised immune responses and may have an effect on ICU mortality; this is currently being evaluated in COVID‐19 patients. 91 , 92
Results from the UK Biobank have identified individuals of Black, Asian, Minority Ethnic (BAME) origin, and primarily Pakistani ethnicity, are at higher risk for being admitted to hospital compared to white British subjects. 93 Another implication of COVID‐19 outbreak is that it occurred in late winter, with different fatality rates among countries and lower hospitalization and mortality in countries close to the Equator and southern latitudes. 94 These epidemiological data, with higher prevalence of severe COVID‐19 AMong individuals with darker skin, together with genetic and social factors may contribute to this phenomenon, many investigators have suggested that vitamin D deficiency may play a role. Vitamin D receptors are found in various tissues and are present in immune cells, such as B and T‐ cells, suggesting an involvement in immune response. 95 Low vitamin D levels are associated with presence of several chronic diseases and impaired immunity, thereby leading to higher risk for recurrent respiratory infections. 96 A comprehensive meta‐analysis has shown that vitamin D replacement can reduce the risk for acute RTIs and its protective effects were even more evident in vitamin D deficient individuals. 97 , 98 It is well established that individuals with obesity are more often vitamin D deficient 89 due to the soluble nature of vitamin D in fat, and therefore vitamin D supplementation, as an adjunct therapeutic approach aiming to prevent cytokine storm, 98 may be of importance in the population who suffer from obesity. This is why, at least 10 trials examining the association between vitamin D supplementation and COVID‐19 outcomes are now registered with the ClinicalTrials.gov website. 99 Data collected during the early phase of the pandemic showed that lower serum 25‐hydroxyvitamin D levels were associated with a higher risk for admission to ICUs due to COVID‐19‐related illness. 100 A thorough analysis of the UK Biobank data, using historic vitamin D levels that were collected many years before the COVID‐19 outbreak, has also shown an association between vitamin D levels and COVID‐19 infection. 101 , 102 These findings were corroborated by a recent large study from Israel, though it is still unclear whether vitamin D replacement reduces the risk of severe illness from COVID‐19. 103 Thus, more studies evaluating vitamin D levels in COVID‐19 are needed to examine any associations more in depth.
8. CHARACTERISTICS OF THE CONTAGIOUS PATIENT WITH OBESITY
It seems that patients with high BMI ‐ apart from being in danger to show a worse RTI clinical prognosis ‐ are in fact more contagious in terms of spreading a virus or an infection, in general. As mentioned before, the immune response of an individual with obesity is compromised, partly because of reduced or delayed interferon production. This delay allows the virus to produce new potentially mutant strains, via RNA replication, thus making it more difficult for mounting a potent immune response. Moreover, adipose tissue may act as a reservoir for many viruses, and potentially for COVID‐19, 104 while patients with obesity may host viruses for a slightly longer time in their body, thus extending the period of contagion. 105 Additionally, studies have shown that the viral load in the exhalation of subjects with obesity and influenza may be higher, due to increased ventilation volume, thus suggesting that these subjects can spread pathogens more easily. 106
9. CONCLUSIONS
Obesity favours mechanisms that apply both to innate immunity and in the development of infection, which might partly explain why patients with obesity are more prone to suffer from respiratory infections in the context of COVID‐19. Future studies evaluating the role of obesity‐related cytokines and adipokines, including leptin and adiponectin, are needed, to identify potentially involved shared pathophysiological pathways that can explain the already established epidemiological associations, at a molecular level. Better understanding of these pathways could eventually guide management strategies and therapies for COVID‐19. Subjects with obesity are prone to be vitamin D deficient; encouraging vitamin D replacement in subjects with obesity during winter, may be a proactive, yet reasonable, harmless and inexpensive approach against COVID‐19.
CONFLICT OF INTEREST
The authors declare no financial or other relationships leading to a conflict of interest.
AUTHOR CONTRIBUTIONS
Both Kalliopi Pazaitou‐Panayiotou and Grigorios Panagiotou conceived the idea for this review.
Konstantinos Michalakis, Grigorios Panagiotou, Ioannis Ilias and Kalliopi Pazaitou‐Panayiotou searched the available literature and wrote the draft and final form of this article.
Kalliopi Pazaitou‐Panayiotou was responsible for the co‐ordination of the project.
Michalakis K, Panagiotou G, Ilias I, Pazaitou‐Panayiotou K. Obesity and COVID‐19: A jigsaw puzzle with still missing pieces. Clin Obes. 2021;11:e12420. 10.1111/cob.12420
REFERENCES
- 1. World Health Organisation (WHO) . Coronavirus disease (Covid‐19) Pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Anonymous. Estimating mortality from COVID‐19: Scientific Brief World Health Organization; 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1
- 3. de Siqueira JVV, Almeida LG, Zica BO, Brum IB, Barceló A, de Siqueira Galil AG. Impact of obesity on hospitalizations and mortality, due to COVID‐19: a systematic review. Obes Res Clin Pract. Jul 23 2020;doi: 10.1016/j.orcp.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anonymous. World Health Organization ‐ Global Health Observatory (GHO) data ‐ Overweight and obesity. 2016. Accessed on September 17, 2020, https://www.who.int/gho/ncd/risk_factors/overweight_text/en/
- 5. Centres for Disease Control and Prevention NCfHS . National Health and Nutrition Examination Survey (NHANES) 2020, April 24, 2020. https://www.cdc.gov/nchs/nhanes/index.htm
- 6. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID‐19 pandemic. Acta Diabetol. 2020;57:759–764. 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centres for Disease Control and Prevention . Information for Healthcare Professionals about Coronavirus (COVID‐19). 2020. Accessed September 17, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html
- 8. Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID‐19. Obesity (Silver Spring). 2020;28:1178‐1179. 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrakis D, Margina D, Tsarouhas K, et al. Obesity a risk factor for increased COVID19 prevalence, severity and lethality (review). Mol Med Rep. 2020;22(1):9‐19. 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158:97‐105. 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the patient with obesity ‐ the editors speak out. Obesity (Silver Spring). 2020;28(5):847. 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019‐nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 14. Zheng KI, Gao F, Wang XB, et al. Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 ‐ COVID‐NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puig‐Domingo M, Marazuela M, Giustina A. COVID‐19 and endocrine diseases. A statement from the European society of endocrinology. Endocrine. 2020;68(1):2‐5. 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis. 2020;71(15):896‐897. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capone S, Abramyan S, Ross B, et al. Characterization of critically ill COVID‐19 patients at a Brooklyn safety‐net hospital. Cureus. 2020;12(8):e9809. 10.7759/cureus.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai SH, Liao W, Chen SW, Liu LL, Liu SY, Zheng ZD. Association between obesity and clinical prognosis in patients infected with SARS‐CoV‐2. Infect Dis Poverty. 2020;9(1):80. 10.1186/s40249-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang Z, Luo S, Gui Y, et al. Obesity is a potential risk factor contributing to clinical manifestations of COVID‐19. Int J Obes (London). 2020. 10.1038/s41366-020-00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gil‐Rodrigo A, Miró Ò, Piñera P, Burillo‐Putze G, Jiménez S, Martín A, Martín‐Sánchez FJ, Jacob J, Guardiola JM, García‐Lamberechts EJ, Espinosa B, Martín Mojarro E, González Tejera M, Serrano L, Agüera C, Soy E, Llauger L, Juan MÁ, Palau A, del Arco C, Rodríguez Miranda B, Maza Vera MT, Martín Quirós A, Tejada de Los Santos L, Ruiz de Lobera N, Iglesias Vela M, Torres Garate R, Alquézar‐Arbé A, González del Castillo J, Llorens P, en representación de la red de investigación SIESTA Analysis of clinical characteristics and outcomes in patients with COVID‐19 based on a series of 1000 patients treated in Spanish emergency departments [Evaluación de las características clínicas y evolución de pacientes con COVID‐19 a partir de una serie de 1000 pacientes atendidos en servicios de urgencias españoles]. Emergencias 2020;32(4):233–241. [PubMed] [Google Scholar]
- 22. Ortiz‐Brizuela E, Villanueva‐Reza M, González‐Lara MF, et al. Clinical and epidemiological characteristics of patients diagnosed with COVID‐19 in a tertiary care center in Mexico City: a prospective cohort study. Rev Invest Clin. 2020;72(3):165‐177. 10.24875/ric.20000211. [DOI] [PubMed] [Google Scholar]
- 23. Czernichow S, Beeker N, Rives‐Lange C, et al. Obesity doubles mortality in patients hospitalized for SARS‐CoV‐2 in Paris hospitals, France: a cohort study on 5795 patients. Obesity (Silver Spring). 2020. 10.1002/oby.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775‐1776. 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 25. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28:1195‐1199. 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID‐19. Obesity (Silver Spring). 2020;28:1175. 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obesity Research & Clinical Practice. 2020;14(4):295‐300. 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obesity Rev. 2020;21:e13128. 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. UK National Intensive Care National Audit and Research Centre . ICNARC Report on COVID‐19 in Critical Care; 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports
- 30. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maccioni L, Weber S, Elgizouli M, et al. Obesity and risk of respiratory tract infections: results of an infection‐diary based cohort study. BMC Public Health. 2018;18(1):271. 10.1186/s12889-018-5172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amundson DE, Djurkovic S, Matwiyoff GN. The obesity paradox. Crit Care Clin. 2010;26(4):583‐596. 10.1016/j.ccc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 33. Cho WH, Oh JY, Yeo HJ, et al. Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: analysis of the national registry. J Crit Care. 2018;48:453‐457. 10.1016/j.jcrc.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 34. Jose RJ, Manuel A. Does coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? Obesity (Silver Spring). 2020;28(6):1007. 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng PY, Eikermann M. The obesity conundrum in sepsis. BMC Anesthesiol. 2017;17(1):147. 10.1186/s12871-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rios‐Diaz AJ, Lin E, Williams K, et al. The obesity paradox in patients with severe soft tissue infections. Am J Surg. 2017;214(3):385‐389. 10.1016/j.amjsurg.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 37. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring). 2020;28(7):1191‐1194. 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS‐CoV): a systematic review and meta‐analysis. Int J Infect Dis. 2016;49:129‐133. 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kusznierz G, Uboldi A, Sosa G, et al. Clinical features of the hospitalized patients with 2009 pandemic influenza a (H1N1) in Santa Fe, Argentina. Influenza Other Respir Viruses. 2013;7(3):410‐417. 10.1111/j.1750-2659.2012.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta‐analysis. BMJ. 2013;347:f5061. 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2009 H1N1 influenza. Mayo Clin Proc. 2010;85(1):64‐76. 10.4065/mcp.2009.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finer N, Garnett SP, Bruun JM. COVID‐19 and obesity. Clin Obes. 2020;10:e12365. 10.1111/cob.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Markoff B, Amsterdam A. Impact of obesity on hospitalized patients. Mt Sinai J Med. 2008;75(5):454‐459. 10.1002/msj.20072. [DOI] [PubMed] [Google Scholar]
- 44. Gallagher S. The challenges of obesity and skin integrity. Nurs Clin North Am. 2005;40(2):325‐335. 10.1016/j.cnur.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45. Lowe JR. Skin integrity in critically ill obese patients. Crit Care Nurs Clin North Am. 2009;21(3):311‐v. 10.1016/j.ccell.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID‐19 infection: multiple potential mechanisms. Circulation. 2020;142:4‐6. 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 47. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755‐767. 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphree RW. Impairments in skin integrity. Nurs Clin North Am. 2017;52(3):405‐417. 10.1016/j.cnur.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 49. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81‐94. 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 50. Buechler C, Weiss TS. Does hepatic steatosis affect drug metabolizing enzymes in the liver? Curr Drug Metab. 2011;12(1):24‐34. 10.2174/138920011794520035. [DOI] [PubMed] [Google Scholar]
- 51. Poggesi I, Benedetti MS, Whomsley R, Le Lamer S, Molimard M, Watelet JB. Pharmacokinetics in special populations. Drug Metab Rev. 2009;41(3):422‐454. 10.1080/10837450902891527. [DOI] [PubMed] [Google Scholar]
- 52. Lowe GD. Venous and arterial thrombosis: epidemiology and risk factors at various ages. Maturitas. 2004;47(4):259‐263. 10.1016/j.maturitas.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 53. Hunt BJ. Hemostasis at extremes of body weight. Semin Thromb Hemost. 2018;44(7):632‐639. 10.1055/s-0038-1661385. [DOI] [PubMed] [Google Scholar]
- 54. Michalakis K, Venihaki M, Mantzoros C, et al. In prostate cancer, low adiponectin levels are not associated with insulin resistance. Eur J Clin Invest. 2015;45(6):572‐578. 10.1111/eci.12445. [DOI] [PubMed] [Google Scholar]
- 55. Alipoor E, Mohammad Hosseinzadeh F, Hosseinzadeh‐Attar MJ. Adipokines in critical illness: a review of the evidence and knowledge gaps. Biomed Pharmacother. 2018;108:1739‐1750. 10.1016/j.biopha.2018.09.165. [DOI] [PubMed] [Google Scholar]
- 56. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS‐CoV‐2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL‐6) level in critically ill COVID‐19 patients. Clin Infect Dis. 2020;ciaa449. 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ulhaq ZS, Soraya GV. Interleukin‐6 as a potential biomarker of COVID‐19 progression. Med mal Infect. 2020;50:382‐383. 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;2020;92:814–818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):164. 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith AG, Sheridan PA, Harp JB, Beck MA. Diet‐induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137(5):1236‐1243. 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 61. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Brien KB, Vogel P, Duan S, et al. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis. 2012;205(2):252‐261. 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Neidich SD, Green WD, Rebeles J, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond). 2017;41(9):1324‐1330. 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and inflammation. Curr Immunol Rev. 2008;4(2):70‐79. 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang AJ, To KK, Li C, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza a(H1N1) infection associated with cytokine dysregulation in mice with diet‐induced obesity. J Infect Dis. 2013;207(8):1270‐1280. 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 66. Almond MH, Edwards MR, Barclay WS, Johnston SL. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax. 2013;68(7):684‐686. 10.1136/thoraxjnl-2012-203009. [DOI] [PubMed] [Google Scholar]
- 67. Mullen M, Gonzalez‐Perez RR. Leptin‐induced JAK/STAT signaling and cancer growth. Vaccines (Basel). 2016;4(3):26. 10.3390/vaccines4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031‐1063. 10.1002/cphy.c170046. [DOI] [PubMed] [Google Scholar]
- 69. Koch A, Sanson E, Voigt S, Helm A, Trautwein C, Tacke F. Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J Crit Care. 2011;26(2):166‐174. 10.1016/j.jcrc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 70. Michalakis K. Adiponectin: the "unusual suspect" between insulin resistance and cancer? Prostate Cancer Prostatic Dis. 2019;22(4):636‐637. 10.1038/s41391-019-0142-5. [DOI] [PubMed] [Google Scholar]
- 71. Robinson K, Prins J, Venkatesh B. Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15(2):221. 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue‐derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285(3):E527‐E533. 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 73. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099‐1102. 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280 e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. European Society of Cardiology . Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers; 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 77. Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus‐EMC. Nature. 2013;495(7440):251‐254. 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iacobellis G. COVID‐19 and diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125. 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41:bnaa011. 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ilias I, Jahaj E, Kokkoris S, et al. Clinical study of hyperglycemia and SARS‐CoV‐2 infection in intensive care unit patients. In Vivo. 2020;34:3029‐3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ilias I, Zabuliene L. Hyperglycemia and the novel Covid‐19 infection: Possible pathophysiologic mechanisms. Med Hypotheses. 2020;139:109699. 10.1016/j.mehy.2020.109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang H, Yuan Z, Pavel MA, Hansen SB. The role of high cholesterol in age‐related COVID19 lethality. bioRxiv. 2020. 10.1101/2020.05.09.086249. [DOI] [Google Scholar]
- 83. Nougier C, Benoit R, Simon M, et al. Hypofibrinolytic state and high thrombin generation may play a major role in sars‐cov2 associated thrombosis. J Thromb Haemost. 2020;18:2215‐2219. 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. J Thromb Haemost. 2020;18(7):1548‐1555. 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Barnard SA, Pieters M, De Lange Z. The contribution of different adipose tissue depots to plasma plasminogen activator inhibitor‐1 (PAI‐1) levels. Blood Rev. 2016;30(6):421‐429. 10.1016/j.blre.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 86. Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345‐379. 10.1007/978-3-319-48382-5_15. [DOI] [PubMed] [Google Scholar]
- 87. Madhusudhan T, Ruf W. Coagulation Signalling and metabolic disorders: lessons learned from animal models. Hamostaseologie. 2019;39(2):164‐172. 10.1055/s-0039-1688800. [DOI] [PubMed] [Google Scholar]
- 88. Mallard SR, Howe AS, Houghton LA. Vitamin D status and weight loss: a systematic review and meta‐analysis of randomized and nonrandomized controlled weight‐loss trials. Am J Clin Nutr. 2016;104(4):1151‐1159. 10.3945/ajcn.116.136879. [DOI] [PubMed] [Google Scholar]
- 89. Pereira‐Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta‐analysis. Obes Rev. 2015;16(4):341‐349. 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 90. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of Vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10(11):e0141770. 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marazuela M, Giustina A, Puig‐Domingo M. Endocrine and metabolic aspects of the COVID‐19 pandemic. Rev Endocr Metab Disord. 2020;1‐13. 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mitchell F. Vitamin‐D and COVID‐19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. 10.1016/s2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Niedzwiedz CL, O'Donnell CA, Jani BD, et al. Ethnic and socioeconomic differences in SARS‐CoV‐2 infection: prospective cohort study using UKbiobank. BMC Med. 2020;18(1):160. 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Panarese A, Shahini E. Letter: Covid‐19, and vitamin D. Aliment Pharmacol Ther. 2020;51:993‐995. 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gruber‐Bzura BM, Vitamin D. Influenza‐prevention or therapy? Int J Mol Sci. 2018;19(8):2419. 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community‐acquired pneumonia: a meta‐analysis of observational studies. Medicine (Baltimore). 2019;98(38):e17252. 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grant WB, Lahore H, McDonnell SL, et al. Evidence that Vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12(4):988. 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. U.S. National Library of Medicine . Vitamin D supplementation trials in COVID‐19 patients. ClinicalTrialsgov website. 2020, Accessed May 29, 2020. https://clinicaltrials.gov/ct2/results?cond=vitamin+D+covid&term=&cntry=&state=&city=&dist=
- 100. Panagiotou G, Tee SA, Ihsan Y, et al. Low serum 25‐hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID‐19 are associated with greater disease severity. Clinical Endocrinology. 2020;93:508‐511. 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID‐19 infection in UKbiobank. Diabetes Metab Syndr. 2020;14(4):561‐565. 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hastie CE, Mackay DF, Ho F, et al. Corrigendum to "Vitamin D concentrations and COVID‐19 infection in UKbiobank" [Diabetes Metabol Syndr: Clin Res Rev 2020 14 (4) 561–5]. Diabetes Metab Syndr. 2020;14(5):1315‐1316. 10.1016/j.dsx.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. FEBS J. 2020;287:3693‐3702. 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kassir R. Risk of COVID‐19 for patients with obesity. Obes Rev. 2020;21:e13034. 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218(9):1378‐1382. 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci. 2018;115(5):1081‐1086. 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]


