Abstract
The development of small molecule modulators of NO/cGMP signaling for use in the CNS has lagged far behind the use of such clinical agents in the periphery, despite the central role played by NO/cGMP in learning and memory, and the substantial evidence that this signaling pathway is perturbed in neurodegenerative disorders, including Alzheimer's disease. The NO-chimeras, NMZ and Nitrosynapsin, have yielded beneficial and disease-modifying responses in multiple preclinical animal models, acting on GABAA and NMDA receptors, respectively, providing additional mechanisms of action relevant to synaptic and neuronal dysfunction. Several inhibitors of cGMP-specific phosphodiesterases (PDE) have replicated some of the actions of these NO-chimeras in the CNS. There is no evidence that nitrate tolerance is a phenomenon relevant to the CNS actions of NO-chimeras, and studies on nitroglycerin in the periphery continue to challenge the dogma of nitrate tolerance mechanisms. Hybrid nitrates have shown much promise in the periphery and CNS, but to date only one treatment has received FDA approval, for glaucoma. The potential for allosteric modulation of soluble guanylate cyclase (sGC) in brain disorders has not yet been fully explored nor exploited; whereas multiple applications of PDE inhibitors have been explored and many have stalled in clinical trials.
Keywords: Neurodegeneration, cGMP, Nitric oxide, NMDA receptor, GABA receptor, Migraine, Alzheimer's disease
1. Introduction
During the past three decades, nitric oxide (NO) has been recognized as one of the most versatile players in maintaining cellular homeostasis. In the CNS, NO is known to activate important physiological cascades involved in regulation of neuronal differentiation and synaptic plasticity [1]. In both neuronal and glial cells, cGMP-dependent protein kinase (PKG) is considered the primary NO effector by which NO mediates its downstream effects, and NO-sensitive soluble guanylyl cyclase (NO-GC or sGC) is the major physiological NO receptor in neurons [2]. The activation of this enzyme is achieved by conformational change upon the binding of NO to the prosthetic heme of sGC, forming a pentacoordinate ferrous-nitrosyl complex. The activated sGC rapidly converts GTP into the second messenger 3‘,5‘-cyclic GMP (cGMP), which, in turn, activates PKG. Through the activation of PKG, NO/cGMP signaling is involved in mediating CREB activation by phosphorylation of Ser133 via the MAPK-ERK cascade [3,4] and possibly in part by the CAMK pathway [3]. In addition, NO is involved in hippocampal and cortical LTP [5-8] via PKG mediated NMDA receptor activation [9,10]. Several lines of evidence also suggest that NO may act as a retrograde messenger in LTP or other forms of synaptic plasticity [11-14], modulating transmitter release under different conditions. Thus, although the major translational interest in NO/cGMP signaling has been in the periphery, there is substantial therapeutic opportunity to modulate NO/cGMP signaling in the CNS and brain. (see Table 1)
Table 1.
Pharmacological modulators of NO/cGMP signaling in the literature that have been utilized in disorders of the CNS, including their pharmacological targets and mode of action.
| Drug | Pharmaceutical Mechanism |
Disease Target | Observed Effect | Model | Clinical | Refs | |
|---|---|---|---|---|---|---|---|
| NO-Donors | Nitrosynapsin | NO-Hybrid drug | AD/Autism | ↓Aβ-induced synapse damage | 3xTg | - | [106] |
| NMZ (GT-1061) | NO-Hybrid drug | AD | LTP restoration in hippocampal slices, ↑ memory, and ↓ Aβ | APP/PS1, 3xTg, and 5xFAD/hAPOE4 | [97,104] | ||
| NCX-116 | NO-NSAID | AD | - | FDA approved for Glaucoma | |||
| HCT-1026 | NO-NSAID | AD | Reversed cognitive deficits induced by scopolamine, ↓ the Aβ1-42-induced glia reaction, iNOS ↑ and p38MAPK activation | APP/PS1 | - | [102,151] [152] | |
| NCX-2216 | NO-NSAID | AD | ↓Aβ1-42-induced glia reaction, ↑ iNOS and ↑ p38MAPK | - | [151] | ||
| CHF-5074 | NO-NSAID | AD | Reversal of contextual memory deficit | Tg2576 | Phase II [176,177] | [171] [172,173] | |
| 9a | Furoxan | AD | ↑ LTP in hippocampal slices treated with oligomeric Aβ | - | [205] | ||
| Sin-1 | NO-Donor | AD | ↓ 7-nitroindazole induced learning deficit, scopolamine-induced amnesia and hypermotility in rats | - | [228-233] | ||
| HNO-Donors | Angeli's salt | HNO-Donor | AD | ↑ cerebral ischemia-reperfusion injury | Experimental stroke model - C57BL6/J | - | [239,242] |
| sGC Stimulators | YC-1 | sGC Stimulator | AD | LTP restoration in hippocampal slices, attenuated scopolamine-induced amnesia | adult Wistar rats | - | [255,256] |
| VL-102 | sGC Stimulator | Migraine | Acute and chronic mechanical cephalic and hind-paw allodynia | C57BL/6 | - | [120] | |
| sGC Inhibitors | ODQ | sGC Inhibitor | PD, Migraine | Improved deficits in forelimb akinesia induced by 6-OHDA and MPTP. ↓ acute and chronic hyperalgesia induced by nitroglycerin | 6-OHDA and MPTP treated rats | - | [266] |
| NOS Inhibitors | L-NMMA | NOS inhibitor | Migraine | - | Phase II [127] | ||
| PDE Inhibitors | Sildenafil | PDE5 Inhibitor | AD | ↑ synaptic function, CREB phosphorylation, and memory. Reversed cognitive impairment of Tg2576 mice | APP/PS1, aging mouse model, J20, Tg2576 | - | [282] [283] |
| Tadalafil | PDE5 Inhibitor | AD | ↑ performance of J20 mice in the Morris water maze test | J20 | - | [284-286] | |
| UK-343664 | PDE5 Inhibitor | AD | Ineffective at preventing MK-801-induced memory disruption, however, ↓ the memory impairment of scopolamine | MK-801 | - | [287] | |
| YF012403 | PDE5 Inhibitor | AD | Rescued the defects in LTP, synaptic, plasticity and memory | APP/PS1 | - | [288] [289] | |
| CM-414 | PDE5 Inhibitor | AD | LTP restoration in hippocampal slices, ↓ brain Aβ and tau phosphorylation, reversed a decrease in dendritic spine density on hippocampal neurons, and reversed cognitive deficits | APP/PS1, Tg2576 | [291] | ||
| BAY 73-6691 | PDE9 Inhibitor | AD | ↑ acquisition, consolidation, and retention of long-term memory (LTM) in a social recognition task ↓ a scoplamine-induced retention deficit in a passive avoidance task, and MK-801-induced short-term memory deficits. | FBNF1 rats | [295] | ||
| PF-04447943 | PDE9 Inhibitor | AD | LTP restoration in hippocampal slices, ↑ indicators of hippocampal synaptic plasticity and improved cognitive function | Tg2576 | Phase II [298] | [296,297] | |
| BI-409306 | PDE9 Inhibitor | AD, Schizophrenia | - | Phase II [299] | |||
| SCH-51866 | PDE1/5 Inhibitor | HD | No effect in the R6/2 mouse model of HD | R6/2 HD | [301] | ||
| BAY 60-7550 | PDE2 Inhibitor | AD | ↑ performance of rats in social and object recognition memory tasks, and reversed MK801-induced deficits | MK-801 | - | [23,302-304] | |
| PF-05180999 | PDE2 Inhibitor | Schizophrenia, Migraine | - | Phase I [306] | [305] | ||
| ND7001 | PDE2 Inhibitor | Various CNS | - | [307] | |||
| Papaverine | PDE10A Inhibitor | Psychosis | ↓ conditioned avoidance responding in rats and mice and ↓PCP induced hyperlocomotion | Male CD rats | - | [308] [309] | |
| PF-02545920 | PDE10A Inhibitor | HD | - | Phase II [310] | |||
| TAK-063 | PDE10A Inhibitor | Schizophrenia | ↓ PCP induced hyperlocomotion | C57BL/6 | - | [311] | |
| “compound 96” | PDE10A Inhibitor | Psychosis | Reversal of MK-801 induced hyperactivity and conditioned avoidance responding | MK-801 | - | [313] |
Activation of CREB by phosphorylation is necessary for memory formation and synaptic strengthening [15-17] and ultimately mediates LTP by acting upon downstream genes involved in synaptic formation and maintenance, and in neuronal plasticity and neurogenesis [18,19]. Mechanistically, it is now recognized that, in coordination with cAMP/PKA signaling, the activation of the cGMP/PKG pathway is a crucial event that contributes to synaptic plasticity and memory acquisition and consolidation through CREB-mediated changes in gene expression [20-24]. In the CSF of patients with Alzheimer's disease (AD), depressed cGMP, but not cAMP levels were observed [25]. Therefore, NO/cGMP has the potential to restore CREB signaling and thereby play a direct role in memory-related synaptic processes relevant to human disease [26-28]. Based on this knowledge, targeting synaptic dysfunction by reactivating the NO/cGMP/CREB pathway may be beneficial in multiple neurodegenerative disorders.
Since NO signaling has important functions in the brain, the etiology and progression of neurodegenerative and cognitive disorders may be associated with: dysfunction in NO production and impaired cGMP signaling [25,29]; and increased phosphodiesterase (PDE) expression levels [30] (reviewed in Ref. [31]). Specifically, aberrant CREB signaling has been linked to Alzheimer's disease (AD) pathology, reflected in mouse models of familial AD (FAD) [32,33]. Dysfunction in CREB signaling has also been implicated in other cognitive disorders such as Huntington's disease (HD) [34,35], suggesting a general role in cognitive dysfunction. Accordingly, targeting NO/cGMP/CREB signaling is now considered as a viable strategy for synaptic repair and neurogenesis, and potentially for disease modification in neurodegenerative disorders.
NO is produced by both neuronal (nNOS) and endothelial NO synthase (eNOS), the interplay between the two isoforms providing an exquisite, temporal, and spatial control of neuronal function [36,37]. In pathological conditions, the inducible isoform (iNOS) provides an important contribution to NO synthesis particularly following pro-inflammatory stimulation [38,39]. Evidence for altered expression of NOS isoforms has been reported in AD, and NO is recognized for its neuroprotective properties [40,41]. Interestingly, the deletion of the inducible NOS2 gene in familial AD transgenic mice exacerbated AD-like pathology, neuronal loss, and behavioral impairments [42-44]. Additionally, chronic loss of endothelial NO in late middle-aged (14–15 month old) eNOS−/− mice increased the amyloidogenic processing, microglial activation, and impaired performance in spatial memory tasks [45]. Therefore, through several mechanisms, chronic loss of endothelial NO, concomitant with downregulation of constitutive NOS and downstream NO/cGMP signaling, is implicated in cognitive decline during aging [45,46] and disease pathogenesis [47-50].
Importantly, activation of the NO/sGC/cGMP/CREB pathway through the application of either a NO donor, sGC potentiator, or cGMP analogue leads to re-establishment of normal levels of LTP and CREB phosphorylation [51]. Different classes of molecules targeting and enhancing components of NO/cGMP/CREB signaling to regulate synaptic plasticity represent promising disease-modifying approaches to treat cognitive dysfunction in neurodegenerative diseases. Although we will discuss nitrates, NO-donors, and alternative pharmacological agents later in this review (Fig. 1 and see Scheme 1 for structures), we begin by comparing two of the most exciting NO mimetic approaches to treatment of brain disorders including AD.
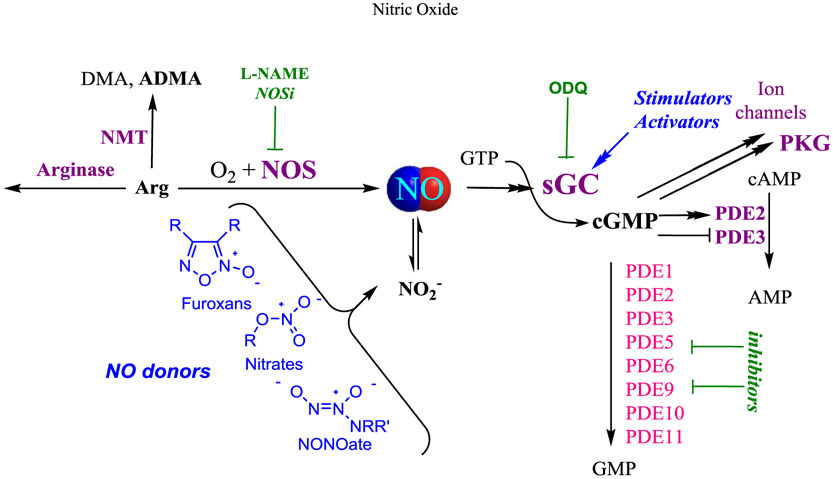
Fig. 1. Opportunities for pharmacological intervention in canonical NO/cGMP signaling.
Under physiological conditions, NO, endogenously synthesized by nitric oxide synthase (NOS), stimulates soluble guanylate cyclase (sGC), increasing cGMP production above basal levels. cGMP binds to and activates cGMP-dependent protein kinases (PKG) and certain ion channels (not shown). cGMP hydrolyzing phosphodiesterases (PDEs) temporally and spatially regulate cGMP levels. Exogenous NO donors spontaneously release NO, or require bioactivation to give NO and nitrite ion (NO2−); nitrite may provide an alternative source of NO after further reductive bioactivation. NOS inhibitors (NOSi), such as L-NAME, have been extensively explored and are not discussed in this review. sGC stimulators directly activate or potentiate the effects NO, enhancing cGMP production by the ferrous-heme enzyme at low levels of bioavailable NO. sGC activators activate the NO-unresponsive, heme-oxidized or heme-free enzyme. 1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) is a heme-dependent sGC inhibitor. ADMA, asymetric dimethyl arginine; ATP, adenosine 5′-triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; DMA, dimethyl arginine; GTP, guanosine 5′-triphosphate; NMT, N-methyl transferase.
Scheme 1.
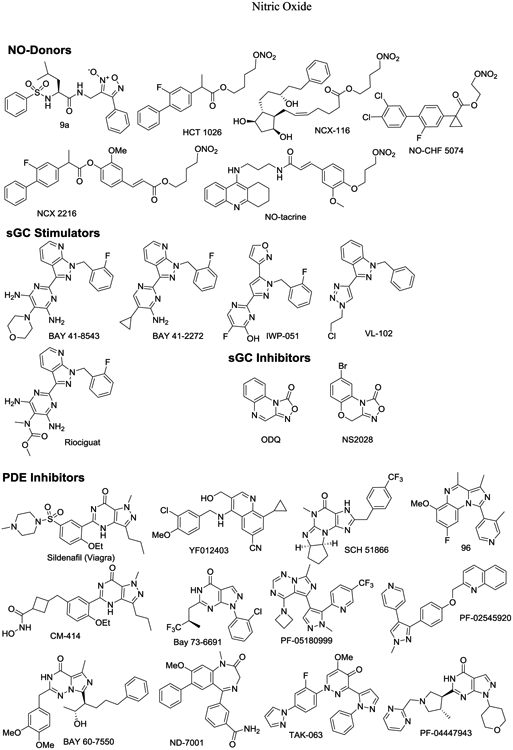
Structures of pharmacological modulators of NO/cGMP signaling.
2. Nomethiazoles and nitromemantines: disease-modifying CNS therapeutics
Excitotoxicity and disrupted Ca2+ homeostasis have long been implicated in neurodegenerative disorders from ischemic stroke to AD [52]. The concept of pharmacologically restoring the balance between excitatory and inhibitory neurotransmission has been central to therapeutic strategies targeted at epilepsy and stroke [53-55]. In this paradigm, excitatory glutamate neurotransmission, primarily mediated at the NMDA receptor; and inhibitory neurotransmission, primarily mediated at the GABAA receptor, are primary targets. Small molecule inhibition of glutamate receptor-mediated currents and potentiation of GABAA receptor-mediated currents have led to a large number of anticonvulsant agents, some of which are used in epilepsy pharmacotherapy, and many of which have been explored as neuroprotective agents, for example, in stroke and AD [56-58].
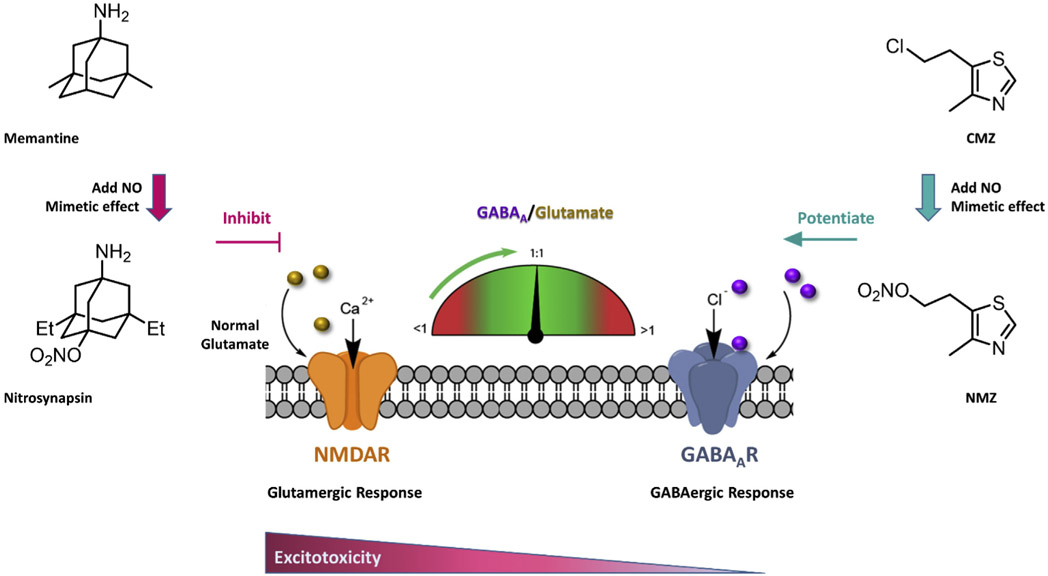
Memantine has activity at a variety of neuroreceptors; however, it is best understood in its pharmacological use as an uncompetitive NMDA receptor antagonist, blocking NMDA receptor currents with IC50 ~2 μM, by binding in the open ion channel proximal to the Mg2+ binding site [59]. The inhibition of extrasynaptic NMDA receptor currents is thought to underlie the efficacy of memantine (Namenda) in moderate-to-severe AD [60]. In contrast to other NMDA receptor channel blockers, memantine does not cause psychotropic effects; however, in common with these channel blockers, memantine is an anticonvulsant [61]. Nitromemantines are nitrate derivatives of memantine [62], one being recently coined Nitrosynapsin (Fig. 2) [63].
Fig. 2.
The complementary mechanisms of NMZ and Nitrosynapsin help restore the balance between excitatory and inhibitory neurotransmission.
Chlomethiazole (CMZ) is a non-benzodiazepine GABAA receptor potentiator and anticonvulsant. CMZ has been used clinically for treatment of seizures, epilepsy, alcoholic dementia and withdrawal, and is prescribed for anxiety and agitation in the elderly [64-70]. Under the brand name Zendra, CMZ was studied in Phase 3 clinical trials as a neuroprotective drug for use in ischemic stroke and spinal cord injury [71-78], and continues to be recommended as a potential component of combination therapies for stroke [79]. CMZ potentiates the function of the inhibitory neurotransmitter GABA in the brain [80-82] and therefore attenuates the glutamate-induced excitotoxic cascade that leads to mitochondrial damage and neuronal loss [83-85]. CMZ is neuroprotective in animal models, attenuating levels of pro-inflammatory cytokines, including TNFα [80,82]. TNFα inhibition is itself a therapeutic goal for treatment of AD [86-88]. Selective pharmacological activation of GABAA receptors has been shown to provide neuroprotection against amyloid-β (Aβ) mediated toxicity [89-92], and a positive allosteric GABAA modulator is predicted to be of clinical utility in AD [92-94]. Nomethiazoles are nitrate analogues of CMZ [95], the most well described being GT-1061(NMZ) (Fig. 2).
Nitrosynapsin and NMZ would appear to be highly complementary in terms of mechanism of action, both adding NO mimetic activity to the complementary activity of the parent drug at NMDA receptors (NMDAR) and GABAA receptors (GABAAR), respectively (Fig. 2). Both NMZ and Nitrosynapsin have generated positive preclinical results that warrant further exploration in clinical trials. NMZ retains the activity of CMZ, both in GABAA receptor potentiation, anticonvulsant, and antiinflammatory properties; and has sedative actions, though less potent than CMZ [96-98]. NMZ and Nitrosynapsin are NO-chimeras, or hybrid nitrates, acting as NO mimetic small molecules; and we have shown that this approach adds procognitive and neuroprotective activity to diverse pharmacophore scaffolds: selective serotonin reuptake inhibitor, SSRI [99]; gamma-secretase modulator, GSM [100-102]; selective estrogen receptor modulator, SERM [103].
NMZ and related nitrates were able to rescue the AD-related impairment of LTP and restore CREB-related synaptic plasticity [96-98]; effects that were blocked by application of the sGC inhibitor 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) [97], indicating a mechanism via NO/cGMP/CREB signaling. NMZ is neuroprotective in vitro in response to various insults including oxygen glucose deprivation (OGD), oligomeric Aβ, and glutamate toxicity; and restores synaptic function in hippocampal slices, in contrast to the parent molecule, CMZ [97,98]. Furthermore, NMZ reversed cholinergic cognitive deficits in rats, and demonstrated improvement of synaptic strengthening and cognition in 4 different mouse models of AD [97,104]. Remarkably, in the three FAD-Tg models (APP/PS1, 3xTg, and 5xFAD/hAPOE4), NMZ treatment attenuated hallmark pathology, and the toxic forms of Aβ and tau [97].
Nitrosynapsin and nitromemantines also retain the properties of the parent drug, memantine, and are proposed to provide dual allosteric modulation of extrasynaptic NMDA receptors [105]. Nitromemantines showed superior neuroprotection and efficacy at lower doses than memantine. In addition, nitromemantines were able to reduce Aβ-induced Ca2+ excitotoxicity, synaptic depression, and tau phosphorylation, and in one FAD-Tg mouse model significantly restored synaptic markers of hippocampal function [106]. In the MEF2C haploinsufficiency mouse model of human autism, Nitrosynapsin displayed promising results when administered b.i.d., improving excitatory and inhibitory neuronal imbalance, synaptic markers, LTP, and autistic-like behavior [63].
Reports on Nitrosynapsin and NMZ show obvious commonalities; however, the NO-mimetic mechanism of action has been interpreted quite differently. The blockade of effects in hippocampal slices by ODQ in studies on NMZ and other nitrates and NO-donors has led to a focus on sGC activation by NMZ; whereas, ODQ has not been used in studies on Nitrosynapsin and nitromemantines, which have been interpreted to function exclusively via S-nitrosylation of specific cysteines located specifically in the extrasynaptic NMDA receptor. Pharmacokinetic data on nitromemantines have not been reported, nevertheless the dose used (2.5 mg/kg/day i.p.) [106] is comparably low to that of NMZ (1 mg/kg/day i.p. + 20 mg/kg/day p.o). An oral dose of NMZ (20 mg/kg delivered continuously over 24 h), representing a procognitive dose, resulted in brain concentrations of NMZ and its metabolite, HMZ, of 0.73 nM and 3.41 nM, respectively (not significantly different from plasma concentrations) in male C57BL/6 mice. These measurements indicate that the brain concentration of NMZ required for memory consolidation after amnestic insult is low or sub-nanomolar. Using the concentration of the HMZ metabolite as a surrogate for the concentration of NO released from NMZ, yields [NO] ≤ 3.4 nM. These data emphasize the high potency and promise of the NO mimetics, NMZ and Nitrosynapsin, and since both deliver disease-modifying effects in animal models after 3 months treatment, the phenomenon of nitrate tolerance is clearly not relevant to the action of these organic nitrates in the CNS.
3. Nitroglycerin and NO/cGMP: migraines are not headaches
The observation that nitroglycerin (glyceryl trinitrate, GTN) exposure and ensuing nitrate tolerance causes headaches was made over a century ago in dynamite factories [107-112]. More recently, GTN was shown to induce specific headaches and other symptoms of migraine attacks in migraineurs, which cannot be directly linked to the very rapid vasodilatory effects induced by GTN [113-118]. Debilitating migraine without aura affects 8% of Americans, with prevalence being strongly linked to age and biased threefold towards females. Migraine symptoms include: allodynia, a central pain sensitization following normally non-painful, often repetitive, stimulation; and hyperalgesia, an increased sensitivity to stimuli normally associated with mild pain. Mechanical allodynia/hyperalgesia caused by cutaneous application of Von Frey filaments to periorbital or plantar surfaces of mice mimics the symptoms of migraine without aura: the pain threshold (mechanical response) decreases as hyperalgesia increases [119,120]. In response to GTN administration, the threshold is lowered: more remarkably, when a single dose of GTN is administered every 2 days for 9 days, there is a chronification of hyperalgesia, which does not rebound to the normal, pretreatment threshold until approximately day 15, many days after GTN has been cleared from the system [121].
GTN must undergo reductive bioactivation in the body to yield NO, and it is commonly believed that depletion of the bioactivation apparatus and concomitant induction of oxidative stress cause the phenomenon of clinical nitrate tolerance, possibly through peroxynitrite formation [122-124]. Recognizing that: (i) both oxidative stress and peroxynitrite have been associated with the development of migraines [125,126]; (ii) doses of GTN used in migraine induction are relatively high; and (iii) there is debate over the relative importance of NO signaling via cGMP versus S-nitrosylation, we chose to define the mechanism of action using pharmacological interventions. We demonstrated that direct activation of sGC by the novel sGC stimulator, VL- 102, replicated the pattern and chronification of migraine-associated hyperalgesia in mice, which was rescued by both acute and preventive clinical migraine treatments [120]. VL-102 treatment also increased the expression of migraine markers such as neuropeptide CGRP in trigeminal ganglia [120]. We also demonstrated that the sGC inhibitor, ODQ, completely blocked GTN induced acute and chronic hyperalgesia, establishing the role of the sGC-cGMP pathway in migraine. ODQ also effectively inhibited the established chronic migraine-associated pain in the absence of GTN or VL-102 [120]. Therefore, blocking this maladaptation by targeting of the sGC-cGMP pathway could represent an attractive approach to treat chronic migraine (Fig. 3). NOS inhibitors have been explored in the last two decades as potential treatment of migraine and headache. Specifically, the non-selective NOS inhibitor, NG-monomethyl-L-arginine hydrochloride (L-NMMA), provided pain relief compared to placebo in chronic tension-type headache in a clinical study [127]. However, selective inhibition of iNOS using GW274150 was ineffective in treating migraine in both prevention [128] and treatment [129] paradigms, suggesting that an upregulation of iNOS in experimental animal models, is unlikely to be a key mediator in migraine pathophysiology [130,131]. The potential of NOS inhibitors in migraine has recently been reviewed [132]. It should also be noted that sildenafil, a PDE5 inhibitor (vide infra), was shown to induce both acute and chronic hyperalgesia in mice [133].
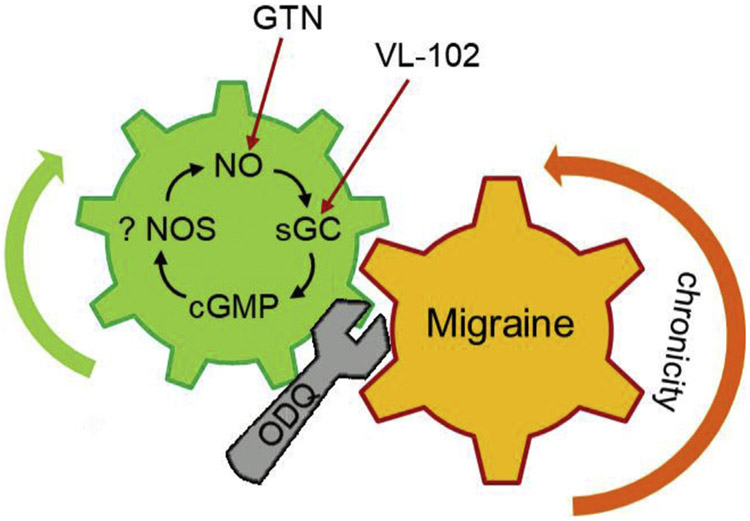
Fig. 3.
Pharmacological activation of NO/cGMP every other day for 9 days causes chronic hyperalgesia that does not revert to baseline until days 13–15[120]. Thus, days after clearance of exogenous NO/cGMP activators, endogenous NO/cGMP signaling is upregulated and potentiates chronicity. Blocking sGC using ODQ restores baseline response on day 10. This migraine model is responsive to various anti-migraine drugs in human use.
Simplistically, the potentiation of allodynia/hyperalgesia by NO/cGMP (Fig. 3) is reminiscent of the potentiation of LTP by NO/cGMP in memory consolidation. However, the patient population susceptible to migraine and the population suffering age-related dementia are quite different, since migraine prevalence decreases after age 50 [134].
4. Hybrid nitrates: enhanced activity?
Nitromemantines and nomethiazoles are examples of hybrid nitrates, in which a parent drug has been modified to incorporate an organic nitrate moiety to deliver NO mimetic activity together with the actions of the parent drug or pharmacophore, thus enhancing activity [135,136]. Considerable research has been conducted over the past 20 years by the biotech industry, notably NitroMed, and NicOx, to develop hybrid nitrates; however, to date only latanoprostene bunod (NCX-116; Scheme 1) has received FDA approval, in this case for topical treatment of glaucoma. NCX-116 undergoes ester hydrolysis to yield hydroxybutyl nitrate, with the evidence for NO release being the measurement of increased ocular cGMP [137].
NCX-116 is typical hybrid nitrates prodrug that links an alkyl or benzyl nitrate to a parent drug by a labile ester linkage. In the majority of literature examples the parent drug is a nonsteroidal anti-inflammatory drug (NSAIDs) such as aspirin (acetylsalicylic acid, ASA) [138-140]. Hybrid NO-donating NSAIDs (NO-NSAIDs) were originally conceived to overcome NSAID gastrotoxicity by releasing NO and overcoming the effects of COX-1 inhibition [136,141-143]. Many preclinical studies focused on the promising spectrum of cancer chemo-preventive and chemotherapeutic activity reported in cell cultures and in animal models. Frequently, the “NO enhanced activity” of NO-NSAIDs was growth inhibition of cancer cell lines, multifold more potent than the parent NSAID [144,145]. However, in many cases, the enhanced activity could be replicated by analogues of NO-NSAIDS lacking any nitrate or NO-donating group [143,146-149]. Rigas, who pioneered the exploration of hybrid nitrates and NO-NSAIDs in cancer chemoprevention, has himself moved away from NO-NSAID nitrate esters to NSAID phosphate esters that have similarly enhanced activity relative to the parent NSAID, which is obviously unrelated to release of NO by the prodrug [150].
With respect to pharmacological use in the brain, two flurbiprofen containing NO-NSAIDs, HCT-1026 and NCX-2216, were compared and shown to have efficacy in a rat AD model [151]; one having been previously shown to attenuate neuroinflammation in vivo [152]. As in the case of NO-NSAIDs in cancer chemoprevention, these studies were stimulated by the epidemiology of NSAIDs associated with AD chemoprevention, and using the NO-NSAID modification to circumvent GI toxicity [153]. Several epidemiological studies have reported that long-term use of NSAIDs reduces AD risk [154], and many neuroin-flammatory contributors to AD pathology exist [155-157], and are considered therapeutic targets for AD [153,158,159]. HCT-1026 was shown to reverse scopolamine induced cognitive deficits in behavioral assays [102], and reduce Aβ load and microglial activation in an APP/PS1 transgenic mouse model [160].
Flurbiprofen is one of a subset of NSAIDs reported to reduce the levels of neurotoxic Aβ42 in cell culture and FAD-Tg mice. Hence, these NSAIDs were referred to as selective amyloid lowering agents (SALAs) [161-164]. The Aβ42 lowering activity of these SALAs required a mechanism of action associated with Aβ42 production or clearance, which was ascribed to γ-secretase modulator (GSM) activity [165,166]. However, the potency of these SALA NSAIDs was an order of magnitude lower than contemporary designer GSMs; and, many alternative mechanisms relevant to Aβ42 lowering have been identified for NSAIDs, including: activation of PPAR-γ and decreased BACE1 gene transcription [167]; inhibition of Rho-kinase activity [168]; and the direct interaction with APP [169].
To explore the SALA activity of NO-flurbiprofens, the mechanism of Aβ1-42 lowering was explored in neuronal cells expressing human Aβ, showing problematic involvement of γ-secretase [101]. A library of flurbiprofen and NSAID analogues was tested for SALA activity and several flurbiprofen analogues were modified and studied as hybrid nitrates [170]. The hybrid nitrates possessed enhanced anti-inflammatory activity and reduced toxicity relative to the parent NSAIDs, and the SALA activity was attributed to the intact hybrid nitrate. A hybrid nitrate based upon CHF-5074/CSP-1103 was an efficacious SALA, which is of interest, because CHF-5074 was reported to reverse contextual memory deficits in an FAD-Tg mouse model, and in clinical trials, to reduce biomarkers of neuroinflammation in patients with mild cognitive impairment (MCI) [171] [172,173]. CHF-5074 continues to be studied in clinical trials [174,175]. That R-flurbiprofen failed to provide any benefit in either cognition or function in a large Phase 3 clinical trial [176,177], and the failure of trials on the related COX-2 inhibitors [178-181], are likely to dampen enthusiasm for pursuit of NSAIDs and NO-NSAIDs in clinical trials for AD.
NSAID NO-donating hybrids have also been reported that incorporate a diazeniumdiolate (NONOate) [182] or a furoxan [183]. Conversely, hybrid nitrates have been designed for brain disorders, which incorporate a parent drug, other than an NSAID. These include hybrids of tacrine, a cholinesterase inhibitor not currently used clinically in AD [184-186], including one containing a ferulic acid linker [186], in simile with the linker incorporated in NCX-2216. Tacrine hybrid nitrates are potent inhibitors of acetylcholinesterase and butyr-ylcholinesterase, and observed to be effective in scopolamine-induced amnesia.
5. Organic nitrates
Glyceryl trinitrate (GTN; nitroglycerin) (Fig. 4) has been used in treatment of angina pectoris for almost 150 years. Nitrates are believed to elicit biological effects via reductive bioactivation to yield NO, stimulating the production of cGMP by sGC [187]. A small number of enzymes have been shown to mediate NO formation from GTN; while a larger number are capable of converting GTN (and organic nitrates in general) to inorganic nitrite (NO2−) (Fig. 4A). This reductive denitration is also mediated by proteins such as deoxyhemoglobin, transition metals, and certain, reactive small molecule thiols [188,189]. In the past decade, the biological activity of NO2− has been recognized to have physiological and potential therapeutic relevance, via reduction to NO in hypoxic tissues [190]. The venodilator activity of organic nitrates is characterized by bioactivation to NO in hypoxic tissues, suggesting that NO2− might be an intermediate in GTN bioactivation. The exact mechanism of nitrate bioactivation is not fully understood and likely involves more than one mechanism [191,192]. Since NO2− is often measured as a metabolite of NO and is used as a surrogate for NO, many studies have mistaken the direct production of NO2− from nitrates as a measure of NO production. Organic nitrates are capable of direct oxidation of thiols such as cysteine, in a reaction that may yield NO or NO2−, and convert the thiol to a disulfide, sulfonate, or sulfinate. This published chemistry is largely overlooked in the biomedical literature, but has been shown to mediate the cGMP-independent activation of PKG1α (Fig. 4A), leading to the intriguing hypothesis that GTN nitrovasodilator activity, and indeed nitrate tolerance, are both mediated by oxidation of PKG1α by GTN, and not by bioactivation of GTN to NO [193].
Fig. 4.
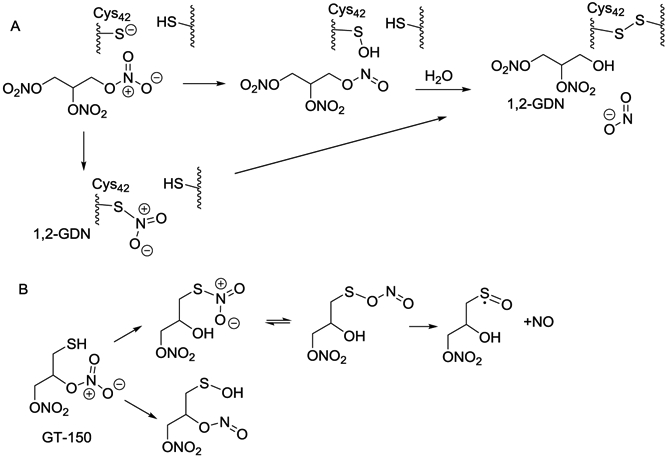
Oxidation of thiols by nitrates: A) Potential mechanism of PKG1α Cys42 oxidation (and NO-independent activation of PKG1α) by GTN. B) Mechanism of spontaneous release of NO from organic nitrate, GT-150.
The concept of using intramolecular reactions to provide models for enzyme-mediated reactions is well proven [194,195] and has been applied to modeling sulfhydryl-dependent nitrate reduction. Cysteine, at physiological pH, has low reactivity towards nitrates [196], therefore incorporating a thiol group adjacent to the nitrate group has been used to facilitate an intramolecular reaction, modeling nitrate bioactivation by cysteine-dependent enzymes/proteins. 1,2-Dinitrooxy-3-mercapto-propane (GT-150), at neutral pH, is a spontaneous NO donor, generating fluxes of NO comparable to NO-donor NONOates. GT-150 acts as an NO donor in activating sGC and inhibiting lipid peroxidation [197,198]. The disulfanyl nitrate (GT-715) is a prodrug of GT-150, and with related aryl disulfanyl dinitrates liberates NO after prodrug activation to give GT-150 [197]. The mechanistic data on GTN, GT-150, GT-715, and model compounds such as tBuSNO2 [199], suggests possible sulfhydryl-dependent mechanisms for NO release (Fig. 4B), and several transition metal facilitated mechanisms. However, the major nitrogen product of organic nitrate metabolism is NO2−.
While the role of GTN and organic nitrates in the cardiovascular system has been extensively investigated, activity in the CNS has been more rarely pursued. Lipton's seminal work on NMDA receptor function showed some nitrovasodilators to be neuroprotective in models of NMDA receptor-mediated excitotoxic neuronal injury [200]. GTN (25 mg/kg), administered i.v. during the 2 h ischemic period, reduced total infarct volume by 20% in a standard rat model of ischemic stroke [201]. Isosorbide dinitrate (ISDN) also provided neuroprotection in the rat stroke model, but required pretreatment before ischemia to demonstrate a neuroprotective effect [202]. The use of potent vasodilators such as GTN is contraindicated in stroke, because of risk of exacerbated hemorrhage, hence the GTN experiment required continuous co-administration of the pressor agent, phenylephrine. GT715, being a weaker vasodilator, with minimal effects on mean arterial pressure in the whole animal compared to GTN, was more potent and more effective as an activator of sGC in the brain, and more effective in elevating cGMP levels in hippocampal brain slices, compared to GTN [203]. GT-715 was shown to reduce infarct volume in the rat MCAO model of ischemic stroke, when administered 4 h after the ischemic event, to be a potent neuroprotective agent in several animal models, and to reverse cognitive deficits induced in animal behavioral models [40,203,204].
6. Furoxans (1,2,5-oxadiazole-N-Oxides)
Compounds containing furoxan (1,2,5-oxadiazole-N-oxide) or benzofuroxan heterocycles are thiol-bioactivated NO-mimetics that demonstrate bioactivation and release of NO [205]. The reactivity of furoxan rings can be manipulated via the incorporation of substituents adjacent to the furoxan ring system, potentially avoiding the cytotoxic effects of high NO concentrations. Lower concentrations of NO in the CNS have been shown to be neuroprotective [205], making furoxans an attractive candidate for CNS drug development, however, in the literature there are few attempts to utilize furoxans in the CNS. The furoxan 9a (Scheme 1) was shown to restore LTP following an Aβ-induced synaptic deficit in mouse hippocampal slices [205], demonstrating the potential of furoxans to restore synaptic function. This neuroprotection could be blocked by the addition of ODQ, indicating that the NO/sGC/cGMP pathway was involved in the restoration of synaptic function [205].
7. Diazeniumdiolates
Diazeniumdiolates (NONOates) decompose spontaneously under physiological conditions to generate NO [206]. Modifications to the structure of NONOates influence the rate of NO generation [206-209]. DEA/NO [51,210,211] and DETA.NO [120,212,213] are amongst the most commonly used NO donating molecules. While NONOates have been studied as potential drug candidates for cardiovascular and oncologic applications, their use in the CNS has been largely associated with neurotoxic effects [211,214,215]. Garthwaite et al., concluded that prolonged exposure to NO was potentially toxic to both axons and glial cells in central white matter, whereas higher NO concentrations, imposed for shorter periods, exclusively damaged axons [216]. Paradoxically, other studies have demonstrated the neuroprotective effects of NONOate derived NO [217]. Lu et al. showed that the NONOate DETA/NO significantly improved the neurological functional outcomes of rats with traumatic brain injury (TBI) [213]. Fernández-Tomé et al. demonstrated DETA/NO-induced stimulation of sGC led to elevation of cGMP, which conferred protection against neuronal cell death induced by H2O2 [212]. SPER/NO was used to demonstrate that elevated extracellular NO levels induced reversible axonal conduction deficits in guinea pig spinal cord neurons [218]. These effects were reversed on washout, at low concentration of SPER/NO (0.5 mM), but were only partially reversed at higher concentrations. PROLI/NO was used to demonstrate the effect of extracellular NO concentration on the permeability of the blood brain barrier (BBB) [219]: PROLI/NO selectively increased intratumoral uptake of radiotracers without significant changes in cerebral and tumor blood flow or arterial blood pressure, an effect blocked by the sGC inhibitor LY83583.
The greatest contribution to pharmacological manipulation of NO has been that of Keefer in his extensive development of diazeniumdiolates, designed to release NO at different rates and in some cases, with specific bioactivation by, for example, glutathione-S-transferase [220], or cytochrome P450 (CYP) [221]. A recent paper from a Merck research team presented a diazeniumdiolate designed to be bioactivated by CYP3A4 and to circumvent the development of tolerance associated with nitrates [222]. The observations in this paper on blood pressure lowering are important, because tolerance developed over 28 days, indicating that NO itself is associated with tolerance in the vascular system.
8. Sydnonimines
The sydnonimine, SIN-1, is often referred to as a source of peroxynitrite [223]. SIN-1 is believed to react with heme proteins and other electron acceptors in biological systems to produce NO. In vivo, SIN-1 will predominantly release NO rather than the superoxide. Molsidomine is a prodrug of SIN-1, metabolized in the liver to SIN-1 to induce slow release of NO [224,225]. Molsidomine crosses the BBB [226], and it has been demonstrated to increase its permeability [227]. Molsidomine (2–4 mg/kg) was found to be effective in restoring memory deficits in several animal models [228-233].
9. Nitroxyl-donors
Nitroxyl (HNO/NO−) is the reduced form of nitric oxide [234]. HNO is more reactive towards thiol groups, leading to the formation of sulfonamides or disulfide bonds [234-236]. HNO donors have been explored in cardiovascular diseases, with one example in Phase 2 clinical trials [237,238]. In contrast to the most commonly used HNO donor, Angeli's salt (AS) [211,235], which spontaneously releases HNO at physiological pH and temperature, the clinical HNO donors are prodrugs with more controlled bioactivation characteristics. AS has been studied both in vitro [239-242] and in vivo [239,242] with regard to neuronal and brain physiology, once again showing neuroprotection [241]. In simile with the proposed mechanism of nitromemantines, a mechanism via HNO reaction with critical thiol groups of the NMDA receptor was proposed to block excessive Ca2+ influx and excitotoxicity [242].
10. S-nitrosothiols
S-Nitrosothiols are stable compounds at 37 °C and pH 7.4, however, in the presence of trace transition metal ions, or photolysis, release of NO can occur [243,244]. S-Nitrosocysteine (Cys-NO) is commonly used as a surrogate for NO in vitro, although since it readily undergoes transnitrosation reactions with protein-thiols, it behaves more as a nitrosonium (NO+) donor than an NO donor [245]. The glutathione S-nitroso adduct, GSNO, is a biologically relevant mediator of NO signaling, and has been proposed as a therapeutic approach to stroke, via stabilization of the HIF-1alpha/VEGF pathway [246]. Transnitrosation of cysteine residues in the NMDAR receptor inhibits the receptor activity [247], which is argued to be central to the mechanism of action of nitromemantines, although direct transnitrosation is not a chemically feasible reaction for nitrates. GSNO reductase (GSNOR), otherwise known as formaldehyde dehydrogenase, is a class III alcohol dehydrogenase that is argued to regulate cellular GSNO levels be degradation of GSNO. Interestingly, GSNOR was reported to be upregulated in the hippocampus of aging humans and mice; and 8–10 week old transgenic mice overexpressing neuronal GSNOR showed a significant deficit in contextual fear and Y-maze tasks [248]. In these studies, focused on protein S-nitrosylation, the involvement of NO/sGC/cGMP signaling was not explored.
11. sGC activators and stimulators
YC-1 was the first reported positive allosteric modulator of sGC, causing a 10–20-fold increase in activity of sGC over basal activity, and a left-shift of the response to NO [196,249,250]. YC-1 and subsequent small molecules derived from this benzylindazole scaffold have been referred to as NO-independent sGC stimulators; differentiated from sGC activators, by the dependence of activators on a reduced heme moiety [249,251]. Stimulators allosterically inhibit dissociation of NO from the heme group of sGC and although described as NO-independent, these agents potentiate activation of sGC by NO, which is the likely mechanism of action [252,253]. In brain slices, YC-1 activates cGMP/PKG signaling to enhance LTP [254]. In mice, YC-1 enhanced both learning and memory in Morris water maze and avoidance tasks in the presence or absence of scopolamine, effects antagonized by NOS and PKG inhibitors [255,256]. YC-1 was also reported to attenuate glutamate-induced excitotocity in a cGMP-dependent manner [257]. However, some activity of YC-1 could be attributed to off-target effects as a PDE inhibitor [258].
Optimization and scaffold-hopping from the benzylindazole lead structure has led to highly potent and selective sGC stimulators [259,260]. BAY 41–2272 and BAY 41–8543 potentiate the effects of NO up to 200-fold [261], and Riociguat (BAY 63–2521) has had success in multiple clinic trials in cardiopulmonary indications [262]. The effect of BAY 63–2521 was studied in atherosclerotic lesions in APOE−/− mice [263], and although ApoE is highly relevant to AD, no CNS studies have been reported. Further structural modifications have led to a new family of selective sGC stimulators with a 5-(isoxazol-3-yl)-1H-pyrazole scaffold, exemplified by IWP-051 [264]. A 5-(isoxazol-3-yl)-1H-pyrazole photoaffinity probe was used to identify the binding site of IWP-051 and displacement of the probe by BAY 41–2272 was used to confirm this as the allosteric site for sGC stimulator binding: a conserved cleft between two subdomains in the sGC heme domain [265]. As yet, no peer-reviewed publications have appeared on the activity of contemporary sGC stimulators/activators in the CNS. As described above, YC-1 and its analogue VL-102 replicate the actions of NO-donors in learning and memory, and in hyperalgesia associated with migraines [120].
12. sGC inhibitors
In contrast to research on sGC stimulators, few sGC inhibitors have been reported [266-268]. The mechanism of action of ODQ and NS2028 in inhibition of sGC requires binding to the ferrous-heme (FeII) in the β-subunit of the enzyme, yielding ferric-heme that cannot bind NO to achieve an activated state [268]. Furthermore, ODQ oxidation of sGC ferrous-heme may lead to conformational change and loss of ferric-heme from the β-subunit [269]. Unsurprisingly, there are examples of sGC inhibition by metal chelators and oxidants. The mechanism of action of ODQ predicts off-target actions at other ferrous-heme proteins and interactions with heme-proteins and enzymes such as hemoglobin and CYPs; however, these have not been extensively nor quantitatively explored. Feelisch et al. implicated CYPs as ODQ targets using the indirect evidence that ODQ inhibited nitrovasodilator bioactivation [270]. Similarly, 300 μM myoglobin attenuated the actions of both NO-donors and ODQ (50 μM) in cardiomyocytes, which might be explained by the ability of myoglobin to trap NO and ODQ [271]. Although the chemistry of NS2028 suggests a mechanism of action identical to ODQ [272], examples of divergent phenotypes exist [273]. Finally, ODQ does not completely replicate the effects of knockout of sGC in vivo, or ex vivo [274].
The universal use of ODQ to define the involvement of sGC in physiology and pathophysiology is demonstrated by over 2000 publications in PubMed. However, ODQ itself has potential therapeutic activity. ODQ has been reported to reverse basal ganglia dysfunction and akinesia in animal models of Parkinson's disease (PD), reversing the increased striatal cGMP levels and neuronal activity in the subthalamic nucleus in the 6-OHDA rat model of PD [266]. ODQ was also effective in improving deficits in forelimb akinesia induced by both 6-OHDA and MPTP [266].
13. cGMP-phosphodiesterase inhibitors
cGMP and cAMP are regulated by phosphodiesterase (PDE) enzymes: cAMP-specific PDE4, PDE7 and PDE8; cGMP-specific PDE5 and PDE9; and dual-substrate PDE1, PDE2 and PDE10 [275,276]. Inhibitors of at least seven PDEs families have been implicated in behavioral changes related to cognition, depression, and anxiety, namely those for PDE 1, 2, 4, 5, 9, 10, and 11 [277] (see Scheme 1). It has been reported that an increase in PDE expression and activity and a decrease in cGMP concentration occurs in the aging brain [278]; therefore brain bioavailable PDE inhibitors activating cGMP signaling are therapeutic targets for AD [23,46].
Research has targeted PDE5 inhibitors to elevate cGMP in the brain [279] [280]. Although the presence of PDE5 in neurons has been a matter of debate [281], aberrant expression of PDE5 in the temporal cortex of AD patients has been reported [25]. The clinical PDE5 inhibitors sildenafil (Viagra), vardenafil (Levitra) and tadalafil (Cialis) have been widely studied. Sildenafil has been shown to rescue cognitive impairment in FAD-Tg mouse models. In an APP/PS1 mouse model, sildenafil activated cGMP/CREB signaling to improve synaptic function and memory, and attenuate Aβ hallmark pathology [282]. In an aging mouse model, sildenafil was neuroprotective and reduced neurotoxic Aβ1-42 [283]. Tadalafil has been proposed as a superior candidate for AD treatment, because of observed brain bioavailability in primates; and both sildenafil and tadalafil were observed to restore behavior in the J20 mouse model (an FAD model with APP KM670/671NL, and APP V717F mutations). In this model, neither altered brain Aβ levels, nor improvement in tau pathology was reported [284-286]. The brain impenetrable PDE5 inhibitor, UK-343,664, improved memory in an object recognition task in rats with cognitive deficits induced by muscarinic or NMDA receptor blockade [287], implying a mechanism via peripheral actions of cGMP, in accord with high expression level of PDE5 in the smooth muscle of the meningeal arteries and blood vessels [284]. Research has been ongoing to optimize PDE5 inhibitors for use in the CNS. The potent PDE5 inhibitor, YF012403, rescued LTP and deficits in contextual memory in the APP/PS1 FAD mouse model [288], and further improvements to this PDE5 inhibitor have been reported and validated in a FAD-Tg (APP/PS1) mouse model [289].
Positive data on the combination of the pan-HDAC (histone deacetylase) inhibitor vorinostat with tadalafil, led to the design and testing of hybrid or chimeric PDE5 inhibitors that incorporate the metal chelating hydroxamate warhead standard to HDAC inhibitors [290]. CM- 414 is a relatively weak inhibitor of Class-I HDACs and of PDE5. In APP/PS1 and Tg2576 FAD-Tg mice treatment led to increased pCREB, rescued synaptic and neuronal function, and amelioration of Aβ and tau hallmark pathology [291]. Hydroxamate HDAC inhibitors have recently been shown to have off-target effects activating cell stress response pathways via the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) and Hypoxia-inducible factor 1 (HIF-1), which can also contribute to efficacy in FAD models [292].
PDE9 has high affinity for cGMP [293], and is insensitive to pan- PDE inhibitors: 3-isobutyl-1-methyl-xanthine (IBMX), vinpocetine, EHNA, enoximone, rolipram, and dipyridamole [294]. Selective PDE9 inhibitors, BAY73–6691 and PF-04447943, improved memory and synaptic plasticity in older rats [295-297]. In the Tg2576 FAD mouse model, PF-04447943 improved memory, LTP, and hippocampal spine density; however, in phase 2 clinical trials, PF-04447943 did not improve cognition over placebo [298]. A novel PDE9 inhibitor, BI-409306, recently completed Phase 1 clinical trials, and entered Phase 2 trials in patients with prodromal or mild to moderate AD [299].
While there are no specific PDE1 inhibitors reported in the literature, many PDE5 inhibitors offer some inhibition of PDE1 [300]. The PDE5/1 dual inhibitor SCH 51866, was unsuccessful in attenuating progression in a R6/2 mouse model of HD [301].
The PDE2 inhibitor, BAY 60–7550, in age impaired rats, produced enhanced learning, memory acquisition, and memory consolidation [23,302-304]. PF-05180999, a pyrazolopyrimidine based PDE2 inhibitor [305], underwent phase I clinical trials for the treatment of schizophrenia and migraine, however, was terminated prematurely due to safety concerns [306]. In 2005 a patent for benzo-1,4-diazepin-2-one based PDE2 inhibitors (i.e. ND7001), for the treatment of various diseases of the central or peripheral nervous system was published [307].
The opium alkaloid, papaverine, a PDE10A inhibitor has been shown to inhibit conditioned avoidance responding in rats and mice and to inhibit PCP–and amphetamine-stimulated locomotor activity in rats [308]. However, chronic administration of papaverine led to motor perturbations, mild cognitive disturbance and anxiety-like behavior [309]. PF-02545920 (Amaryllis) showed promise in HD, but a Phase II clinical trial failed [310]. The PDE10A inhibitor TAK-063 [311], produced dose-dependent antipsychotic-like effects in METH-induced hyperactivity and prepulse inhibition in rodents, in contrast to PF-02545920 [312]. The highly potent PDE10A inhibitor from Pfizer, “compound 96“, reversed MK-801 induced hyperactivity and conditioned avoidance response in rats [313].
14. Conclusions
As outlined in this review, there has been substantial activity over the past decade using selective PDE inhibitors to regulate cGMP in the CNS; however, this has not been matched by efforts to explore alternative therapeutic approaches to regulation of cGMP in the CNS. Striking progress has been made exploring sGC activators and stimulators in the periphery; and, as discussed in this review, extensive studies on hybrid nitrates have led to a single clinical drug for glaucoma.
NO/cGMP signal transduction is important for modulating synaptic transmission, plasticity, and memory in the brain, and this signaling pathway has been shown to be perturbed in many neurodegenerative disorders, making targeting of this pathway an attractive therapeutic strategy. The evidence strongly suggests that NO-donors and sGC modulators effectively regulate NO/cGMP signaling to elicit beneficial effects in many preclinical models of CNS disorders, in particular neurodegenerative diseases. The impressive preclinical data on NMZ and Nitrosynapsin, in particular, should support progress to clinical trials. sGC stimulators in the relatively few studies focused on the CNS have shown promise, often replicating the activity of NO-donors.
Despite significant advances in our understanding of NO and cGMP-dependent signaling mechanisms, important questions remain unsolved. Most importantly, gaps in our knowledge exist with the NO receptor, sGC, notably: the precise mechanism of sGC activation; the role of post-translational modification; modulation by allosteric ligands, such as ATP, GTP and endogenous sGC stimulators; and interactions with protein partners. Therefore, continued progress towards elucidating the structure and mechanism of sGC activation is needed to enable the development of novel drugs that target sGC to treat CNS disorders. For example, targeting the PDZ domain of the sGC α2 subunit using protein-protein interaction inhibitors would yield interesting chemical probes; however, this isoform of sGC, enriched in the brain, is poorly studied.
Modern drug discovery is dominated by development of small organic molecules that bind an individual protein target, with selectivity defined against specific off-target proteins. Increased affinity and potency is best achieved with multiple co-crystal structures of the protein target. The molecule preferably should be stable with a very small number of defined and measurable metabolites. Generally, a single mechanism of action is preferred to polypharmacy. The characteristics of NO-donors and sGC modulators are not compatible with some, or all, of these drug-like characteristics desired in modern drug discovery. NO-donors are by design and definition metabolically labile. In addition, the extensive literature on protein modification caused directly or indirectly by NO, increases the potential targets of any NO-donor. Tolerance to GTN, or “nitrate tolerance” is also perceived to increase risk of development of NO-donors, although no evidence for such a phenomenon in the CNS has been revealed with agents such as NMZ and Nitrosynapsin. Furthermore, based upon doses of NMZ and Nitrosynapsin administered chronically in preclinical animal models, the effective potency of these agents is very high.
In neurodegenerative disorders, but especially in AD, the high rate of Phase 3 clinical trial failures of drugs singularly targeting one protein and one aspect of disease neuropathology has been unprecedented. Targeting NO-sGC signaling in the CNS will inherently modulate more than one aspect of the disease, and multiple preclinical studies with PDE inhibitors and NO-chimeras have demonstrated this approach to be disease-modifying with respect to hallmark neuropathology. The pursuit of these strategies in clinical trials is eagerly awaited.
Supplementary Material
Acknowledgements
NIH is acknowledged for funding of studies on NMZ and related nitrates via grant R42AG044024.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://doi.org/10.1016/j.niox.2018.10.006.
References
- [1].Garthwaite J, Concepts of neural nitric oxide-mediated transmission, Eur. J. Neurosci 27 (11) (2008) 2783–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Garthwaite J, Glutamate, nitric oxide and cell-cell signalling in the nervous system, Trends Neurosci. 14 (2) (1991) 60–67. [DOI] [PubMed] [Google Scholar]
- [3].Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE, The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase, Learn. Mem 15 (10) (2008) 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adams JP, Roberson ED, English JD, Selcher JC, Sweatt JD, MAPK regulation of gene expression in the central nervous system, Acta Neurobiol. Exp. (Wars) 60 (3) (2000) 377–394. [DOI] [PubMed] [Google Scholar]
- [5].Bon CL, Garthwaite J, On the role of nitric oxide in hippocampal long-term potentiation, J. Neurosci. 23 (5) (2003) 1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Izumi Y, Tokuda K, Zorumski CF, Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase, Hippocampus 18 (3) (2008) 258–265. [DOI] [PubMed] [Google Scholar]
- [7].Qiu DL, Knopfel T, An NMDA receptor/nitric oxide cascade in presynaptic parallel fiber-Purkinje neuron long-term potentiation, J. Neurosci 27 (13) (2007) 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tamagnini F, Barker G, Warburton EC, Burattini C, Aicardi G, Bashir ZI, Nitric oxide-dependent long-term depression but not endocannabinoid-mediated long-term potentiation is crucial for visual recognition memory, J. Physiol 591 (16) (2013) 3963–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Costa G, Tozzi A, Siliquini S, Galletti F, Cardaioli G, Tantucci M, Pisani F, Calabresi P, A critical role of NO/cGMP/PKG dependent pathway in hippocampal post-ischemic LTP: modulation by zonisamide, Neurobiol. Dis 44 (2) (2011) 185–191. [DOI] [PubMed] [Google Scholar]
- [10].Ratnayaka A, Marra V, Bush D, Burden JJ, Branco T, Staras K, Recruitment of resting vesicles into recycling pools supports NMDA receptor-dependent synaptic potentiation in cultured hippocampal neurons, J. Physiol 590 (7) (2012) 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Di S, Maxson MM, Franco A, Tasker JG, Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways, J. Neurosci 29 (2) (2009) 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hardingham N, Fox K, The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation, J. Neurosci 26 (28) (2006) 7395–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Volgushev M, Ralaban P, Chistiakova M, Eysel UT, Retrograde signalling with nitric oxide at neocortical synapses, Eur. J. Neurosci 12 (12) (2000) 4255–4267. [DOI] [PubMed] [Google Scholar]
- [14].Szabadits E, Cserep C, Ludanyi A, Katona I, Gracia-Llanes J, Freund TF, Nyiri G, Hippocampal GABAergic synapses possess the molecular machinery for retrograde nitric oxide signaling, J. Neurosci 27 (30) (2007) 8101–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Benito E, Barco A, CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models, Trends Neurosci. 33 (5) (2010) 230–240. [DOI] [PubMed] [Google Scholar]
- [16].Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T, CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning, Behav. Brain Res 133 (2) (2002) 135–141. [DOI] [PubMed] [Google Scholar]
- [17].Viola H, Furman M, Izquierdo LA, Alonso M, Barros DM, de Souza MM, Izquierdo I, Medina JH, Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: effect of novelty, J. Neurosci 20 (23) (2000) RC112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Montminy M, Transcriptional regulation by cyclic AMP, Annu. Rev. Biochem 66 (1997) 807–822. [DOI] [PubMed] [Google Scholar]
- [19].Johannessen M, Delghandi MP, Moens U, What turns CREB on? Cell. Signal 16 (11) (2004) 1211–1227. [DOI] [PubMed] [Google Scholar]
- [20].Rernabeu R, Schmitz P, Faillace MP, Izquierdo I, Medina JH, Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning, Neuroreport 7 (2) (1996) 585–588. [DOI] [PubMed] [Google Scholar]
- [21].Lu YF, Kandel ER, Hawkins RD, Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus, J. Neurosci 19 (23) (1999) 10250–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsumoto Y, Unoki S, Aonuma H, Mizunami M, Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory, Learn. Mem 13 (1) (2006) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rutten K, Prickaerts J, Hendrix M, van der Staay FJ, Sik A, Blokland A, Time-dependent involvement of cAMP and cGMP in consolidation of object memory: studies using selective phosphodiesterase type 2, 4 and 5 inhibitors, Eur. J. Pharmacol 558 (1–3) (2007) 107–112. [DOI] [PubMed] [Google Scholar]
- [24].Bollen E, Puzzo D, Rutten K, Privitera L, De Vry J, Vanmierlo T, Kenis G, Palmeri A, D'Hooge R, Balschun D, et al. , Improved long-term memory via enhancing cGMP-PKG signaling requires cAMP-PKA signaling, Neuropsychopharmacology 39 (11) (2014) 2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ugarte A, Gil-Bea F, Garcia-Barroso C, Cedazo-Minguez A, Ramirez MJ, Franco R, Garcia-Osta A, Oyarzabal J, Cuadrado-Tejedor M, Decreased levels of guanosine 3', 5'-monophosphate (cGMP) in cerebrospinal fluid (CSF) are associated with cognitive decline and amyloid pathology in Alzheimer's disease, Neuropathol. Appl. Neurobiol 41 (4) (2015) 471–482. [DOI] [PubMed] [Google Scholar]
- [26].Paul C, Stratil C, Hofmann F, Kleppisch T, cGMP-dependent protein kinase type I promotes CREB/CRE-mediated gene expression in neurons of the lateral amygdala, Neurosci. Lett 473 (2) (2010) 82–86. [DOI] [PubMed] [Google Scholar]
- [27].Paul C, Schoberl F, Weinmeister P, Micale V, Wotjak CT, Hofmann F, Kleppisch T, Signaling through cGMP-dependent protein kinase I in the amygdala is critical for auditory-cued fear memory and long-term potentiation, J. Neurosci 28 (52) (2008) 14202–14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kleppisch T, Wolfsgruber W, Feil S, Allmann R, Wotjak CT, Goebbels S, Nave KA, Hofmann F, Feil R, Hippocampal cGMP-dependent protein kinase I supports an age- and protein synthesis-dependent component of long-term potentiation but is not essential for spatial reference and contextual memory, J. Neurosci 23 (14) (2003) 6005–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Saavedra A, Giralt A, Arumi H, Alberch J, Perez-Navarro E, Regulation of hippocampal cGMP levels as a candidate to treat cognitive deficits in Huntington's disease, PloS One 8 (9) (2013) e73664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen RW, Williams AJ, Liao Z, Yao C, Tortella FC, Dave JR, Broad spectrum neuroprotection profile of phosphodiesterase inhibitors as related to modulation of cell-cycle elements and caspase-3 activation, Neurosci. Lett 418 (2) (2007) 165–169. [DOI] [PubMed] [Google Scholar]
- [31].Rollen E, Prickaerts J, Phosphodiesterases in neurodegenerative disorders, IUBMB Life 64 (12) (2012) 965–970. [DOI] [PubMed] [Google Scholar]
- [32].Bartolotti N, Segura L, Lazarov O, Diminished CRE-induced plasticity is linked to memory deficits in familial Alzheimer's disease mice, J Alzheimers Dis 50 (2) (2016) 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CR, Downregulation of CREB expression in Alzheimer's brain and in Abeta-treated rat hippocampal neurons, Mol. Neurodegener 6 (2011) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saura CA, Valero J, The role of CREB signaling in Alzheimer's disease and other cognitive disorders, Rev. Neurosci 22 (2) (2011) 153–169. [DOI] [PubMed] [Google Scholar]
- [35].Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D, Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration, Cell 127 (1) (2006) 59–69. [DOI] [PubMed] [Google Scholar]
- [36].Paakkari I, Lindsberg P, Nitric oxide in the central nervous system, Ann. Med 27 (3) (1995) 369–377. [DOI] [PubMed] [Google Scholar]
- [37].Linares D, Taconis M, Mana P, Correcha M, Fordham S, Staykova M, Willenborg DO, Neuronal nitric oxide synthase plays a key role in CNS demyelination, J. Neurosci 26 (49) (2006) 12672–12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heneka MT, Feinstein DL, Expression and function of inducible nitric oxide synthase in neurons, J. Neuroimmunol 114 (1–2) (2001) 8–18. [DOI] [PubMed] [Google Scholar]
- [39].Brown GC, Neher JJ, Inflammatory neurodegeneration and mechanisms of microglial killing of neurons, Mol. Neurobiol 41 (2–3) (2010) 242–247. [DOI] [PubMed] [Google Scholar]
- [40].Reynolds JN, Bennett BM, Boegman RJ, Jhamandas K, Ratz JD, Zavorin SI, Scutaru D, Dumitrascu A, Thatcher GR, Neuroprotection against ischemic brain injury conferred by a novel nitrate ester, Bioorg. Med. Chem. Lett 12 (20) (2002) 2863–2866. [DOI] [PubMed] [Google Scholar]
- [41].Nicolescu AC, Reynolds JN, Barclay LR, Thatcher GR, Organic nitrites and NO: inhibition of lipid peroxidation and radical reactions, Chem. Res. Toxicol 17 (2) (2004) 185–196. [DOI] [PubMed] [Google Scholar]
- [42].Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H, NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer's disease, Proc. Natl. Acad. Sci. U. S. A 103 (34) (2006) 12867–12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Colton CA, Wilcock DM, Wink DA, Davis J, Van Nostrand WE, Vitek MP, The effects of NOS2 gene deletion on mice expressing mutated human AbetaPP, J. Alzheimers Dis 15 (4) (2008) 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA, Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2, J. Neurosci 28 (7) (2008) 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS, Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology, J. Neurochem 127 (5) (2013) 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Domek-Lopacinska KU, Strosznajder JB, Cyclic GMP and nitric oxide synthase in aging and Alzheimer's disease, Mol. Neurobiol 41 (2–3) (2010) 129–137. [DOI] [PubMed] [Google Scholar]
- [47].Gargiulo L, Bermejo M, Liras A, Reduced neuronal nitric oxide synthetase and c-protein kinase levels in Alzheimer's disease, Rev. Neurol 30 (4) (2000) 301–303. [PubMed] [Google Scholar]
- [48].Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T, Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology, Brain Res. 913 (1) (2001) 57–67. [DOI] [PubMed] [Google Scholar]
- [49].Norris PJ, Faull RL, Emson PC, Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer's disease brains, Brain Res. Mol. Brain Res 41 (1–2) (1996) 36–49. [DOI] [PubMed] [Google Scholar]
- [50].Venturini G, Colasanti M, Persichini T, Fioravanti E, Ascenzi P, Palomba L, Cantoni O, Musci G, Beta-amyloid inhibits NOS activity by subtracting NADPH availability, FASEB. J 16 (14) (2002) 1970–1972. [DOI] [PubMed] [Google Scholar]
- [51].Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O, Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity, J. Neurosci 25 (29) (2005) 6887–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD, Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders, Trends Neurosci. 23 (5) (2000) 222–229. [DOI] [PubMed] [Google Scholar]
- [53].Meldrum BS, Update on the mechanism of action of antiepileptic drugs, Epilepsia 37 (Suppl 6) (1996) S4–S11. [DOI] [PubMed] [Google Scholar]
- [54].Meldrum BS, Rogawski MA, Molecular targets for antiepileptic drug development, Neurotherapeutics 4 (1) (2007) 18–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Green AR, Hainsworth AH, Jackson DM, GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke, Neuropharmacology 39 (9) (2000) 1483–1494. [DOI] [PubMed] [Google Scholar]
- [56].Lyden P, Wahlgren NG, Mechanisms of action of neuroprotectants in stroke, J. Stroke Cerebrovasc. Dis 9 (6 Pt 2) (2000) 9–14. [DOI] [PubMed] [Google Scholar]
- [57].Zhang MY, Zheng CY, Zou MM, Zhu JW, Zhang Y, Wang J, Liu CF, Li QF, Xiao ZC, Li S, et al. , Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice, Neurobiol. Aging 35 (12) (2014) 2713–2725. [DOI] [PubMed] [Google Scholar]
- [58].Stepien K, Tomaszewski M, Czuczwar SJ, Profile of anticonvulsant activity and neuroprotective effects of novel and potential antiepileptic drugs-an update, Pharmacol. Rep 57 (6) (2005) 719–733. [PubMed] [Google Scholar]
- [59].Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G, Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents-preclinical studies, Neurosci. Biobehav. Rev 21 (4) (1997) 455–468. [DOI] [PubMed] [Google Scholar]
- [60].Rogawski MA, Wenk GL, The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease, CNS Drug Rev. 9 (3) (2003) 275–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chojnacka-Wojcik E, Tatarczynska E, Maj J, The influence of memantine on the anticonvulsant effects of the antiepileptic drugs, Pol. J. Pharmacol. Pharm 35 (6) (1983) 511–515. [PubMed] [Google Scholar]
- [62].Chen HS, Lipton SA, The chemical biology of clinically tolerated NMDA receptor antagonists, J. Neurochem 97 (6) (2006) 1611–1626. [DOI] [PubMed] [Google Scholar]
- [63].Tu S, Akhtar MW, Escorihuela RM, Amador-Arjona A, Swarup V, Parker J, Zaremba JD, Holland T, Bansal N, Holohan DR, et al. , NitroSynapsin therapy for a mouse MEF2C haploinsufficiency model of human autism, Nat. Commun 8 (1) (2017) 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bregeon C, Renier JC, Cadic AM, Long term development of rhizomelic pseudopolyarthritis. Study of 47 cases after 7 years, Rev. Rhum Mai. Osteoartic 46 (1) (1979) 19–27. [PubMed] [Google Scholar]
- [65].Gardner JM, Kado CI, Polygalacturonic acid trans-eliminase in the osmotic shock fluid of Erwinia rubrifaciens: characterization of the purified enzyme and its effect on plant cells, J. Bacteriol 127 (1) (1976) 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Niiyama M, Deguchi E, Kagota K, Namioka S, Appearance of 15N-labeled intestinal microbial amino acids in the venous blood of the pig colon, Am. J. Vet. Res 40 (5) (1979) 716–718. [PubMed] [Google Scholar]
- [67].East J, Harvey JJ, Tilly R, Transmission of auto-immune haemolytic anaemia and murine leukaemia virus in NZB-BALB/c hybrid mice, Clin. Exp. Immunol 24 (1) (1976) 196–209. [PMC free article] [PubMed] [Google Scholar]
- [68].Karbowski K, Twilight states in epileptic patients (author's transl), Schweiz. Rundsch. Med. Prax 68 (22) (1979) 703–707. [PubMed] [Google Scholar]
- [69].Murphy E, Holland MJ, Cox RP, Adenosine deaminase activity in human diploid skin fibroblasts varies with the age of the donor, J. Med 9 (3) (1978) 237–244. [PubMed] [Google Scholar]
- [70].Kozlova AA, Mikhalev SK, Neurological morbidity based on patient attendance data, Sov. Zdr 8 (4) (1978) 39–41. [PubMed] [Google Scholar]
- [71].Green AR, Clomethiazole (Zendra) in acute ischemic stroke: basic pharmacology and biochemistry and clinical efficacy, Pharmacol. Ther 80 (2) (1998) 123–147. [DOI] [PubMed] [Google Scholar]
- [72].Marshall JW, Cross AJ, Ridley RM, Functional benefit from clomethiazole treatment after focal cerebral ischemia in a nonhuman primate species, Exp. Neurol 156 (1) (1999) 121–129. [DOI] [PubMed] [Google Scholar]
- [73].Farooque M, Isaksson J, Jackson DM, Olsson Y, Clomethiazole (ZENDRA, CMZ) improves hind limb motor function and reduces neuronal damage after severe spinal cord injury in rat, Acta Neuropathol 98 (1) (1999) 22–30. [DOI] [PubMed] [Google Scholar]
- [74].Wahlgren NG, Diez-Tejedor E, Teitelbaum J, Arboix A, Leys D, Ashwood T, Grossman E, Results in 95 hemorrhagic stroke patients included in CLASS, a controlled trial of clomethiazole versus placebo in acute stroke patients, Stroke 31 (1) (2000) 82–85. [DOI] [PubMed] [Google Scholar]
- [75].Wahlgren NG, Ranasinha KW, Rosolacci T, Franke CL, van Erven PM, Ashwood T, Claesson L, Clomethiazole acute stroke study (CLASS): results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients, Stroke 30 (1) (1999) 21–28. [DOI] [PubMed] [Google Scholar]
- [76].Mucke H, Clomethiazole (Astra Arcus AB), Idrugs 2 (2) (1999) 184–193. [PubMed] [Google Scholar]
- [77].Lyden P, Jacoby M, Schim J, Albers G, Mazzeo P, Ashwood T, Nordlund A, Odergren T, The Clomethiazole Acute Stroke Study in tissue-type plasminogen activator-treated stroke (CLASS-T): final results, Neurology 57 (7) (2001) 1199–1205. [DOI] [PubMed] [Google Scholar]
- [78].Lyden P, Shuaib A, Ng K, Levin K, Atkinson RP, Rajput A, Wechsler L, Ashwood T, Claesson L, Odergren T, et al. , Clomethiazole Acute Stroke Study in ischemic stroke (CLASS-I): final results, Stroke 33 (1) (2002) 122–128. [DOI] [PubMed] [Google Scholar]
- [79].Wilby MJ, Hutchinson PJ, The pharmacology of chlormethiazole: a potential neuroprotective agent? CNS Drug Rev. 10 (4) (2004) 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nelson RM, Hainsworth AH, Lambert DG, Jones JA, Murray TK, Richards DA, Gabrielsson J, Cross AJ, Green AR, Neuroprotective efficacy of AR-A008055, a clomethiazole analogue, in a global model of acute ischaemic stroke and its effect on ischaemia-induced glutamate and GABA efflux in vitro, Neuropharmacology 41 (2) (2001) 159–166. [DOI] [PubMed] [Google Scholar]
- [81].Green AR, Hainsworth AH, Misra A, Debens TA, Jackson DM, Murray TK, Nelson RM, Cross AJ, The interaction of AR-A008055 and its enantiomers with the GABA(A) receptor complex and their sedative, muscle relaxant and anticonvulsant activity, Neuropharmacology 41 (2) (2001) 167–174. [DOI] [PubMed] [Google Scholar]
- [82].Colado MI, O'Shea E, Esteban B, Green AR, Studies on the neuroprotective effect of the enantiomers of AR-A008055, a compound structurally related to clomethiazole, on MDMA (“ecstasy”)-induced neurodegeneration in rat brain, Psycho pharmacology (Berlin) 157 (1) (2001) 82–88. [DOI] [PubMed] [Google Scholar]
- [83].Harmon D, Coleman E, Marshall C, Lan W, Shorten G, The effect of clomethiazole on plasma concentrations of interleukin-6, −8, −1beta, tumor necrosis factor-alpha, and neutrophil adhesion molecule expression during experimental extracorporeal circulation, Anesth. Analg 97 (1) (2003) 13–18 (table of contents). [DOI] [PubMed] [Google Scholar]
- [84].Clarkson AN, Liu H, Rahman R, Jackson DM, Appleton I, Kerr DS, Clomethiazole: mechanisms underlying lasting neuroprotection following hypoxia-ischemia, FASEB. J 19 (8) (2005) 1036–1038. [DOI] [PubMed] [Google Scholar]
- [85].Clarkson AN, Clarkson J, Jackson DM, Sammut IA, Mitochondrial involvement in transhemispheric diaschisis following hypoxia-ischemia: clomethiazole-mediated amelioration, Neuroscience 144 (2) (2007) 547–561. [DOI] [PubMed] [Google Scholar]
- [86].Tweedie D, Sambamurti K, Greig NH, TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets, Curr. Alzheimer Res 4 (4) (2007) 378–385. [DOI] [PubMed] [Google Scholar]
- [87].MeAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, et al. , Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology, Neurobiol. Dis 34 (1) (2009) 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tobinick E, Deciphering the physiology underlying the rapid clinical effects of perispinal etanercept in Alzheimer's disease, Curr. Alzheimer Res 9 (1) (2012) 99–109. [DOI] [PubMed] [Google Scholar]
- [89].Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noel F, De Mello FG, Ferreira ST, Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders, FASEB. J 18 (3) (2004) 511–518. [DOI] [PubMed] [Google Scholar]
- [90].Lin X, Jun-Tian Z, Neuroprotection by D-securinine against neurotoxicity induced by beta-amyloid (25–35), Neurol. Res 26 (7) (2004) 792–796. [DOI] [PubMed] [Google Scholar]
- [91].Lee BY, Ban JY, Seong YH, Chronic stimulation of GABAA receptor with muscimol reduces amyloid beta protein (25-35)-induced neurotoxicity in cultured rat cortical cells, Neurosci. Res 52 (4) (2005) 347–356. [DOI] [PubMed] [Google Scholar]
- [92].Marcade M, Bourdin J, Loiseau N, Peillon H, Rayer A, Drouin D, Schweighoffer F, Desire L, Etazolate, a neuroprotective drug linking GABA(A) receptor pharmacology to amyloid precursor protein processing, J. Neurochem 106 (1) (2008) 392–404. [DOI] [PubMed] [Google Scholar]
- [93].Rissman RA, Mobley WC, Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease, J. Neurochem 117 (4) (2011) 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vellas B, Sol O, Snyder PJ, Ousset PJ, Haddad R, Maurin M, Lemarie JC, Desire L, Pando MP, Group EHTs: EHT0202 in Alzheimer's disease: a 3-month, randomized, placebo-controlled, double-blind study, Curr. Alzheimer Res 8 (2) (2011) 203–212. [DOI] [PubMed] [Google Scholar]
- [95].Qin Z, Luo J, VandeVrede L, Tavassoli E, Fa M, Teich AF, Arancio O, Thatcher GR, Design and synthesis of neuroprotective methylthiazoles and modification as NO-chimeras for neurodegenerative therapy, J. Med. Chem 55 (15) (2012) 6784–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Vandevrede L, Tavassoli E, Luo J, Qin Z, Yue L, Pepperberg DR, Thatcher GR, Novel analogues of chlormethiazole are neuroprotective in four cellular models of neurodegeneration by a mechanism with variable dependence on GABA(A) receptor potentiation, Br. J. Pharmacol 171 (2) (2014) 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Luo J, Lee SH, VandeVrede L, Qin Z, Ben Aissa M, Larson J, Teich AF, Arancio O, D’Souza Y, Elharram A, et al. , A multifunctional therapeutic approach to disease modification in multiple familial mouse models and a novel sporadic model of Alzheimer's disease, Mol. Neurodegener 11 (1) (2016) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Luo J, Lee SH, VandeVrede L, Qin Z, Piyankarage S, Tavassoli E, Asghodom RT, Ben Aissa M, Fa M, Arancio O, et al. , Re-engineering a neuroprotective, clinical drug as a procognitive agent with high in vivo potency and with GABAA potentiating activity for use in dementia, BMC Neurosci. 16 (2015) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Abdul-Hay S, Schiefer IT, Chandrasena RE, Li M, Abdelhamid R, Wang YT, Tavassoli E, Michalsen B, Asghodom RT, Luo J, et al. , NO-SSRIs: nitric oxide chimera drugs incorporating a selective serotonin reuptake inhibitor, ACS Med. Chem. Lett 2 (9) (2011) 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schiefer IT, Abdul-Hay S, Wang H, Vanni M, Qin Z, Thatcher GR, Inhibition of amyloidogenesis by nonsteroidal anti-inflammatory drugs and their hybrid nitrates, J. Med. Chem 54 (7) (2011) 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Abdul-Hay SO, Edirisinghe P, Thatcher GR, Selective modulation of amyloid-beta peptide degradation by flurbiprofen, fenofibrate, and related compounds regulates Abeta levels, J. Neurochem 111 (3) (2009) 683–695. [DOI] [PubMed] [Google Scholar]
- [102].Abdul-Hay SO, Luo J, Ashghodom RT, Thatcher GR, NO-flurbiprofen reduces amyloid-beta, is neuroprotective in cell culture, and enhances cognition in response to cholinergic blockade, J. Neurochem 111 (3) (2009) 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].VandeVrede L, Abdelhamid R, Qin Z, Choi J, Piyankarage S, Luo J, Larson J, Bennett BM, Thatcher GR, An NO donor approach to neuroprotective and procognitive estrogen therapy overcomes loss of NO synthase function and potentially thrombotic risk, PloS One 8 (8) (2013) e70740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bennett BM, Reynolds JN, Prusky GT, Douglas RM, Sutherland RJ, Thatcher GR, Cognitive deficits in rats after forebrain cholinergic depletion are reversed by a novel NO mimetic nitrate ester, Neuropsychopharmacology 32 (3) (2007) 505–513. [DOI] [PubMed] [Google Scholar]
- [105].Nakamura T, Lipton SA, Protein S-nitrosylation as a therapeutic target for neurodegenerative diseases, Trends Pharmacol. Sci 37 (1) (2016) 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, et al. , Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss, Proc. Natl. Acad. Sci. U. S. A 110 (27) (2013) E2518–E2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Trainor DC, Jones RC, Headaches in explosive magazine workers, Arch. Environ. Health 12 (2) (1966) 231–234. [DOI] [PubMed] [Google Scholar]
- [108].Schwartz AM, The cause, relief and prevention of headaches arising from contact with dynamite, N. Engl. J. Med 235 (1946) 541–544. [DOI] [PubMed] [Google Scholar]
- [109].Ebright, Effects of nitroglycerin on those engaged in its manufacture, J A M A 62 (1914) 201. [Google Scholar]
- [110].Laws CE, Nitroglycerin head, J. Am. Med. Assoc LIV (10) (1910) 793. [Google Scholar]
- [111].Tassorelli C, Joseph SA, Buzzi MG, Nappi G, The effects on the central nervous system of nitroglycerin—putative mechanisms and mediators, Prog. Neurobiol 57 (6) (1999) 607–624. [DOI] [PubMed] [Google Scholar]
- [112].Di Clemente L, Coppola G, Magis D, Gerardy PY, Fumal A, De Pasqua V, Di Piero V, Schoenen J, Nitroglycerin sensitises in healthy subjects CNS structures involved in migraine pathophysiology: evidence from a study of nociceptive blink reflexes and visual evoked potentials, Pain 144 (1–2) (2009) 156–161. [DOI] [PubMed] [Google Scholar]
- [113].Olesen J, Nitric oxide-related drug targets in headache, Neurotherapeutics 7 (2) (2010) 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ashina M, Hansen JM, BO AD, Olesen J, Human models of migraine - short-term pain for long-term gain, Nat. Rev. Neurol 13 (12) (2017) 713–724. [DOI] [PubMed] [Google Scholar]
- [115].Afridi SK, Kaube H, Goadsby PJ, Glyceryl trinitrate triggers premonitory symptoms in migraineurs, Pain 110 (3) (2004) 675–680. [DOI] [PubMed] [Google Scholar]
- [116].Christiansen I, Daugaard D, Lykke Thomsen L, Olesen J, Glyceryl trinitrate induced headache in migraineurs - relation to attack frequency, Eur. J. Neurol 7 (4) (2000) 405–411. [DOI] [PubMed] [Google Scholar]
- [117].Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J, Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura, Cephalalgia 19 (7) (1999) 660–667 discussion 626. [DOI] [PubMed] [Google Scholar]
- [118].Iversen HK, Olesen J, Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs, Cephalalgia 16 (6) (1996) 412–418. [DOI] [PubMed] [Google Scholar]
- [119].Macolino CM, Daiutolo BV, Albertson BK, Elliott MB, Mechanical alloydnia induced by traumatic brain injury is independent of restraint stress, J. Neurosci. Methods 226 (2014) 139–146. [DOI] [PubMed] [Google Scholar]
- [120].Ben Aissa M, Tipton AF, Bertels Z, Gandhi R, Moye LS, Novack M, Bennett BM, Wang Y, Litosh V, Lee SH, et al. , Soluble guanylyl cyclase is a critical regulator of migraine-associated pain, Cephalalgia 38 (8) (2018) 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Tipton AF, Tar ash I, McGuire B, Charles A, Pradhan AA, The effects of acute and preventive migraine therapies in a mouse model of chronic migraine, Cephalalgia 36 (11) (2016) 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Warnholtz A, Tsilimingas N, Wendt M, Munzel T, Mechanisms underlying nitrate-induced endothelial dysfunction: insight from experimental and clinical studies, Heart Fail. Rev 7 (4) (2002) 335–345. [DOI] [PubMed] [Google Scholar]
- [123].Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V,Mulsch A, Schulz E, Keaney JF Jr.et al. , Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and crosstolerance, J. Clin. Invest 113 (3) (2004) 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].DiFabio J, Ji Y, Vasiliou V, Thatcher GR, Bennett BM, Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance, Mol. Pharmacol 64 (5) (2003) 1109–1116. [DOI] [PubMed] [Google Scholar]
- [125].Borkum JM, Migraine triggers and oxidative stress: a narrative review and synthesis, Headache 56 (1) (2016) 12–35. [DOI] [PubMed] [Google Scholar]
- [126].Bernecker C, Ragginer C, Fauler G, Horejsi R, Moller R, Zelzer S, Lechner A, Wallner-Blazek M, Weiss S, Fazekas F, et al. , Oxidative stress is associated with migraine and migraine-related metabolic risk in females, Eur. J. Neurol 18 (10) (2011) 1233–1239. [DOI] [PubMed] [Google Scholar]
- [127].Ashina M, Lassen LH, Bendtsen L, Jensen R, Olesen J, Effect of inhibition of nitric oxide synthase on chronic tension-type headache: a randomised crossover trial, Lancet 353 (9149) (1999) 287–289. [DOI] [PubMed] [Google Scholar]
- [128].Hoivik HO, Laurijssens BE, Harnisch LO, Twomey CK, Dixon RM,Kirkham AJ, Williams PM, Wentz AL, Lunnon MW, Lack of efficacy of the selective iNOS inhibitor GW274150 in prophylaxis of migraine headache, Cephalalgia 30 (12) (2010) 1458–1467. [DOI] [PubMed] [Google Scholar]
- [129].Palmerini T, Barozzi C, Tomasi L, Sangiorgi D, Marzocchi A, De Servi S, Ortolani P, Reggiani LB, Alessi L, Lauria G, et al. , A randomised study comparing the antiplatelet and antiinflammatory effect of clopidogrel 150 mg/day versus 75 mg/day in patients with ST-segment elevation acute myocardial infarction and poor responsiveness to clopidogrel: results from the DOUBLE study, Thromb. Res 125 (4) (2010) 309–314. [DOI] [PubMed] [Google Scholar]
- [130].Akerman S, Williamson DJ, Kaube H, Goadsby PJ, Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels, Br. J. Pharmacol 137 (1) (2002) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].De Col R, Koulchitsky SV, Messlinger KB, Nitric oxide synthase inhibition lowers activity of neurons with meningeal input in the rat spinal trigeminal nucleus, Neuroreport 14 (2) (2003) 229–232. [DOI] [PubMed] [Google Scholar]
- [132].Pradhan AA, Bertels Z, Akerman S, Targeted nitric oxide synthase inhibitors for migraine, Neurotherapeut.: J. Am. Soc. Exp. NeuroTherapeut 15 (2) (2018) 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A, Characterization of a novel model of chronic migraine, Pain 155 (2) (2014) 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB, Migraine prevalence by age and sex in the United States: a life-span study, Cephalalgia 30 (9) (2010) 1065–1072. [DOI] [PubMed] [Google Scholar]
- [135].Thatcher GR, An introduction to NO-related therapeutic agents, Curr. Top. Med. Chem 5 (7) (2005) 597–601. [DOI] [PubMed] [Google Scholar]
- [136].Bolla M, Almirante N, Benedini F, Therapeutic potential of nitrate esters of commonly used drugs, Curr. Top. Med. Chem 5 (7) (2005) 707–720. [DOI] [PubMed] [Google Scholar]
- [137].Cavet ME, DeCory HH, The role of nitric oxide in the intraocular pressure lowering efficacy of latanoprostene bunod: review of nonclinical studies, J. Ocul. Pharmacol. Therapeut 34 (1–2) (2018) 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Slocum SL, Kensler TW, Nrf2: control of sensitivity to carcinogens, Arch. Toxicol 85 (4) (2011) 273–284. [DOI] [PubMed] [Google Scholar]
- [139].Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, et al. , Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement, Lancet Oncol. 10 (5) (2009) 501–507. [DOI] [PubMed] [Google Scholar]
- [140].Thiagarajan P, Jankowski JA, Aspirin and NSAIDs; benefits and harms for the gut, Best Pract. Res. Clin. Gastroenterol 26 (2) (2012) 197–206. [DOI] [PubMed] [Google Scholar]
- [141].James DS, The multisystem adverse effects of NSAID therapy, J. Am. Osteopath. Assoc 99 (11 Suppl) (1999) S1–S7. [DOI] [PubMed] [Google Scholar]
- [142].Laine L, The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors, Semin. Arthritis Rheum 32 (3 Suppl 1) (2002) 25–32. [DOI] [PubMed] [Google Scholar]
- [143].Dunlap T, Abdul-Hay SO, Chandrasena RE, Hagos GK, Sinha V, Wang Z, Wang H, Thatcher GR, Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: in vitro evidence querying the NO donor functionality, Nitric Oxide 19 (2) (2008) 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P, Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin, Proc. Natl. Acad. Sci. U. S. A 103 (10) (2006) 3914–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Bratasz A, Selvendiran K, Wasowicz T, Bobko A, Khramtsov VV, Ignarro LJ, Kuppusamy P, NCX-4040, a nitric oxide-releasing aspirin, sensitizes drug-resistant human ovarian xenograft tumors to cisplatin by depletion of cellular thiols, J. Transl. Med 6 (2008) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dunlap T, Chandrasena RE, Wang Z, Sinha V, Wang Z, Thatcher GR, Quinone formation as a chemoprevention strategy for hybrid drugs: balancing cytotoxicity and cytoprotection, Chem. Res. Toxicol 20 (12) (2007) 1903–1912. [DOI] [PubMed] [Google Scholar]
- [147].Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, de Esch IJ, Richel D, Wijtmans M, Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin, J. Med. Chem 50 (10) (2007) 2424–2431. [DOI] [PubMed] [Google Scholar]
- [148].Dunlap T, Piyankarage SC, Wijewickrama GT, Abdul-Hay S, Vanni M, Litosh V, Luo J, Thatcher GR, Quinone-induced activation of Keap1/Nrf2 signaling by aspirin prodrugs masquerading as nitric oxide, Chem. Res. Toxicol 25 (12) (2012) 2725–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Pierce EN, Piyankarage SC, Dunlap T, Litosh V, Siklos MI, Wang YT, Thatcher GR, Prodrugs bioactivated to quinones target NF-kappaB and multiple protein networks: identification of the quinonome, Chem. Res. Toxicol 29 (7) (2016) 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Tsioulias GJ, Go MF, Rigas B, NSAIDs and colorectal cancer control: promise and challenges, Curr Pharmacol Rep 1 (5) (2015) 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Prosperi C, Scali C, Barba M, Bellucci A, Giovannini MG, Pepeu G, Casamenti F, Comparison between flurbiprofen and its nitric oxide-releasing derivatives HCT-1026 and NCX-2216 on Abeta(1-42)-induced brain inflammation and neuronal damage in the rat, Int. J. Immunopathol. Pharmacol 17 (3) (2004) 317–330. [DOI] [PubMed] [Google Scholar]
- [152].Prosperi C, Scali C, Pepeu G, Casamenti F, NO-flurbiprofen attenuates excitotoxin-induced brain inflammation, and releases nitric oxide in the brain, Jpn. J. Pharmacol 86 (2) (2001) 230–235. [DOI] [PubMed] [Google Scholar]
- [153].Wallace JL, Muscara MN, de Nucci G, Zamuner S, Cirino G, del Soldato P,Ongini E, Gastric tolerability and prolonged prostaglandin inhibition in the brain with a nitric oxide-releasing flurbiprofen derivative, NCX-2216 [3-[4-(2-fluoro-alpha-methyl-[1,1'-biphenyl]-4-acetyloxy)-3-methoxyphenyl]-2-prop enoic acid 4-nitrooxy butyl ester], J. Pharmacol. Exp. Therapeut 309 (2) (2004) 626–633. [DOI] [PubMed] [Google Scholar]
- [154].Lee YJ, Han SB, Nam SY, Oh KW, Hong JT, Inflammation and Alzheimer's disease, Arch Pharm. Res. (Seoul) 33 (10) (2010) 1539–1556. [DOI] [PubMed] [Google Scholar]
- [155].Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, et al. , APOE-modulated Abeta-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective, J. Neurochem 133 (4) (2015) 465–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Bales KR, Du Y, Holtzman D, Cordell B, Paul SM, Neuroinflammation and Alzheimer's disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E, Neurobiol. Aging 21 (3) (2000) 427–432 discussion 451–423. [DOI] [PubMed] [Google Scholar]
- [157].Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ, The significance of neuroinflammation in understanding Alzheimer's disease, J. Neural. Transm 113 (11) (2006) 1685–1695. [DOI] [PubMed] [Google Scholar]
- [158].Lleo A, Galea E, Sastre M, Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases, Cell. Mol. Life Sci 64 (11) (2007) 1403–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D, Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches, Int. Rev. Neurobiol 82 (2007) 277–296. [DOI] [PubMed] [Google Scholar]
- [160].van Groen T, Kadish I, Transgenic AD model mice, effects of potential anti-AD treatments on inflammation and pathology, Brain Res Brain Res Rev 48 (2) (2005) 370–378. [DOI] [PubMed] [Google Scholar]
- [161].Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, et al. , A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity, Nature 414 (6860) (2001) 212–216. [DOI] [PubMed] [Google Scholar]
- [162].Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, et al. , Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease, J. Neurosci 20 (15) (2000) 5709–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN, Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice, J. Neurosci 22 (6) (2002) 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G, Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease, J. Neurosci 23 (20) (2003) 7504–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS, Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism, J. Biol. Chem 279 (42) (2004) 43419–43426. [DOI] [PubMed] [Google Scholar]
- [166].Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT, Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation, Nat. Med 10 (10) (2004) 1065–1066. [DOI] [PubMed] [Google Scholar]
- [167].Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P,Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, et al. , Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma, Proc. Natl. Acad. Sci. U. S. A 103 (2) (2006) 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, et al. , Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho, Science 302 (5648) (2003) 1215–1217. [DOI] [PubMed] [Google Scholar]
- [169].Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, Healy B, Chapman R, Welzel AT, Price RW, et al. , Substrate-targeting gamma-secretase modulators, Nature 453 (7197) (2008) 925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Schiefer IT, Abdul-Hay S, Wang H, Vanni M, Qin Z, Thatcher GR, Inhibition of amyloidogenesis by nonsteroidal anti-inflammatory drugs and their hybrid nitrates, J. Med. Chem 54 (7) (2011) 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ross J, Sharma S, Winston J, Nunez M, Bottini G, Franceschi M, Scarpini E, Frigerio E, Fiorentini F, Fernandez M, et al. , CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebo-controlled study, Curr. Alzheimer Res 10 (7) (2013) 742–753. [DOI] [PubMed] [Google Scholar]
- [172].Peretto I, Radaelli S, Parini C, Zandi M, Raveglia LF, Dondio G, Fontanella L, Misiano P, Bigogno C, Rizzi A, et al. , Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of beta-amyloid(1)(-)(42) secretion, J. Med. Chem 48 (18) (2005) 5705–5720. [DOI] [PubMed] [Google Scholar]
- [173].Imbimbo BP, Giardino L, Sivilia S, Giuliani A, Gusciglio M, Pietrini V, Del Giudice E, D'Arrigo A, Leon A, Villetti G, et al. , CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer's disease, J Alzheimers Dis 20 (1) (2010) 159–173. [DOI] [PubMed] [Google Scholar]
- [174].Sivilia S, Lorenzini L, Giuliani A, Gusciglio M, Fernandez M, Baldassarro VA, Mangano C, Ferraro L, Pietrini V, Baroc MF, et al. , Multi-target action of the novel anti-Alzheimer compound CHF5074: in vivo study of long term treatment in Tg2576 mice, BMC Neurosci. 14 (2013) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Qiang L, Guan Y, Li X, Liu L, Mu Y, Sugano A, Takaoka Y, Sakaeda T, Imbimbo BP, Yamamura KI, et al. , CSP-1103 (CHF5074) stabilizes human transthyretin in healthy human subjects, Amyloid 24 (1) (2017) 42–51. [DOI] [PubMed] [Google Scholar]
- [176].Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH, Tarenflurbil Phase 3 Study G: effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial, JAMA 302 (23) (2009) 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Marder K, Tarenflurbil in patients with mild Alzheimer's disease, Curr. Neurol. Neurosci. Rep. 10 (5) (2010) 336–337. [DOI] [PubMed] [Google Scholar]
- [178].Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ, Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial, JAMA 289 (21) (2003) 2819–2826. [DOI] [PubMed] [Google Scholar]
- [179].Aisen PS, Thal LJ, Ferris SH, Assaid C, Nessly ML, Giuliani MJ, Lines CR, Norman BA, Potter WZ, Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double-blind, trial, Curr. Alzheimer Res 5 (1) (2008) 73–82. [DOI] [PubMed] [Google Scholar]
- [180].Soininen H, West C, Robbins J, Niculescu L, Long-term efficacy and safety of celecoxib in Alzheimer's disease, Dement. Geriatr. Cognit. Disord 23 (1) (2007) 8–21. [DOI] [PubMed] [Google Scholar]
- [181].Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE. Ladd T, Khan MA, Kache R, Beard J, et al. , Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production, Nat. Med 11 (5) (2005) 545–550. [DOI] [PubMed] [Google Scholar]
- [182].Velazquez CA, Chen QH, Citro ML, Keefer LK, Knaus EE, Second-generation aspirin and indomethacin prodrugs possessing an O(2)-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: design, synthesis, biological evaluation, and nitric oxide release studies, J. Med. Chem 51 (6) (2008) 1954–1961. [DOI] [PubMed] [Google Scholar]
- [183].de Carvalho PS, Marostica M, Gambero A, Pedrazzoli J Jr., Synthesis and pharmacological characterization of a novel nitric oxide-releasing diclofenac derivative containing a benzofuroxan moiety, Eur. J. Med. Chem 45 (6) (2010) 2489–2493. [DOI] [PubMed] [Google Scholar]
- [184].Fang L, Appenroth D, Decker M, Kiehntopf M, Lupp A, Peng S, Fleck C, Zhang Y, Lehmann J, NO-donating tacrine hybrid compounds improve scopolamine-induced cognition impairment and show less hepatotoxicity, J. Med. Chem 51 (24) (2008) 7666–7669. [DOI] [PubMed] [Google Scholar]
- [185].Fang L, Appenroth D, Decker M, Kiehntopf M, Roegler C, Deufel T, Fleck C, Peng S, Zhang Y, Lehmann J, Synthesis and biological evaluation of NO-donortacrine hybrids as hepatoprotective anti-Alzheimer drug candidates, J. Med. Chem 51 (4) (2008) 713–716. [DOI] [PubMed] [Google Scholar]
- [186].Chen Y, Sun J, Fang L, Liu M, Peng S, Liao H, Lehmann J, Zhang Y, Tacrine-ferulic acid-nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl-and butyrylcholinesterase inhibitors, J. Med. Chem 55 (9) (2012) 4309–4321. [DOI] [PubMed] [Google Scholar]
- [187].Thatcher GRJ, Nicolescu AC, Bennett BM, Toader V, Nitrates and no release: contemporary aspects in biological and medicinal chemistry, Free Radical Biol. Med 37 (8) (2004) 1122–1143. [DOI] [PubMed] [Google Scholar]
- [188].Chong S, Fung HL, Biochemical and pharmacological interactions between nitroglycerin and thiols - effects of thiol structure on nitric-oxide generation and tolerance reversal. Biochem. Pharmacol 42 (7) (1991) 1433–1439. [DOI] [PubMed] [Google Scholar]
- [189].Gorren AC, Russwurm M, Kollau A, Koesling D, Schmidt K, Mayer B, Effects of nitroglycerin/L-cysteine on soluble guanylate cyclase: evidence for an activation/inactivation equilibrium controlled by nitric oxide binding and haem oxidation, Biochem. J 390 (Pt 2) (2005) 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO 3rd, Kelm M, Wink DA, Espey MG, et al. , The emerging biology of the nitrite anion, Nat. Chem. Biol 1 (6) (2005) 308–314. [DOI] [PubMed] [Google Scholar]
- [191].Mayer B, Beretta M, The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles, Br. J. Pharmacol 155 (2) (2008) 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Fung HL, Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu. Rev. Pharmacol. Toxicol 44 (2004) 67–85. [DOI] [PubMed] [Google Scholar]
- [193].Rudyk O, Prysyazhna O, Burgoyne JR, Eaton P, Nitroglycerin fails to lower blood pressure in redox-dead Cys42Ser PKG1alpha knock-in mouse, Circulation 126 (3) (2012) 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kirby AJ, Effective molarities of intramolecular reactions, Adv. Phys. Org. Chem 17 (1980) 183. [Google Scholar]
- [195].Page MI, Jencks WP, Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect, Proc. Natl. Acad. Sci. U. S. A 68 (8) (1971) 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Artz JD, Toader V, Zavorin SI, Bennett BM, Thatcher GR, In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics, Biochemistry (Mosc.) 40 (31) (2001) 9256–9264. [DOI] [PubMed] [Google Scholar]
- [197].Zavorin SI, Artz JD, Dumitrascu A, Nicolescu A, Scutaru D, Smith SV. Thatcher GR, Nitrate esters as nitric oxide donors: SS-nitrates, Org. Lett 3 (8) (2001) 1113–1116. [DOI] [PubMed] [Google Scholar]
- [198].Nicolescu AC, Zavorin SI, Turro NJ, Reynolds JN, Thatcher GR, Inhibition of lipid peroxidation in synaptosomes and liposomes by nitrates and nitrites, Chem. Res. Toxicol 15 (7) (2002) 985–998. [DOI] [PubMed] [Google Scholar]
- [199].Jellinger K, Slowik F, [Affection of the nervous system in leucoses and malignant lymphomas (author's transl)], Zentralbl Allg Pathol 122 (5) (1978) 439–461. [PubMed] [Google Scholar]
- [200].Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS, A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds [see comments], Nature 364 (6438) (1993) 626–632. [DOI] [PubMed] [Google Scholar]
- [201].Lipton SA, Singel DJ, Stamler JS, Neuroprotective and neurodestructive effects of nitric oxide and redox congeners, Ann. N. Y. Acad. Sci 738 (1994) 382–387. [DOI] [PubMed] [Google Scholar]
- [202].Ramos-Zuniga R, Velazquez-Santana H, Mercado-Pimentel R, Cerda-Camacho F, Neuroprotection in selective focal ischemia in rats by nitrates, an alternative redox manipulation on nitric oxide: experimental model, Minim. Invasive Neurosurg 41 (3) (1998) 152–160. [DOI] [PubMed] [Google Scholar]
- [203].Smith S, Dringenberg HC, Bennett BM, Thatcher GR, Reynolds JN, A novel nitrate ester reverses the cognitive impairment caused by scopolamine in the Morris water maze, Neuroreport 11 (17) (2000) 3883–3886. [DOI] [PubMed] [Google Scholar]
- [204].Thatcher GR, Bennett BM, Dringenberg HC, Reynolds JN, Novel nitrates as NO mimetics directed at Alzheimer's disease, J Alzheimers Dis 6 (6 Suppl) (2004) S75–S84. [DOI] [PubMed] [Google Scholar]
- [205].Schiefer IT, VandeVrede L, Fa M, Arancio O, Thatcher GR, Furoxans (1,2,5-oxadiazole-N-oxides) as novel NO mimetic neuroprotective and procognitive agents, J. Med. Chem 55 (7) (2012) 3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Keefer LK, Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates, Annu. Rev. Pharmacol. Toxicol 43 (1) (2003) 585–607. [DOI] [PubMed] [Google Scholar]
- [207].Miller MR, Megson IL, Recent developments in nitric oxide donor drugs, Br. J. Pharmacol 151 (3) (2007) 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK, Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver, J. Med. Chem 40 (13) (1997) 1947–1954. [DOI] [PubMed] [Google Scholar]
- [209].Burch GE, Harb JM, Lesions induced by encephalomyocarditis virus and coxsackievirus B in newborn mice, Arch. Pathol. Lab Med 103 (7) (1979) 348–354. [PubMed] [Google Scholar]
- [210].Xu Z-QD, De Vente J, Steinbusch H, Grillner S, Hokfelt T, The NO-cGMP pathway in the rat locus coeruleus: electrophysiological, immunohistochemical andin situhybridization studies, Eur. J. Neurosci 10 (11) (1998) 3508–3516. [DOI] [PubMed] [Google Scholar]
- [211].Boje KM, Lakhman SS, Nitric oxide redox species exert differential permeability effects on the blood-brain barrier, J. Pharmacol. Exp. Therapeut 293 (2) (2000) 545–550. [PubMed] [Google Scholar]
- [212].Fernandez-Tome P, Lizasoain I, Leza JC, Lorenzo P, Moro MA, Neuroprotective effects of DETA-NONOate, a nitric oxide donor, on hydrogen peroxide-induced neurotoxicity in cortical neurones, Neuropharmacology 38 (9) (1999) 1307–1315. [DOI] [PubMed] [Google Scholar]
- [213].Lu D, Mahmood A, Zhang R, Copp M, Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats, J. Neurosurg 99 (2) (2003) 351–361. [DOI] [PubMed] [Google Scholar]
- [214].Thompson AJ, Mander PK, Brown GC, The NO donor DETA-NONOate reversibly activates an inward current in neurones and is not mediated by the released nitric oxide, Br. J. Pharmacol 158 (5) (2009) 1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM, Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity, Nat. Rev. Neurosci 8 (10) (2007) 766–775. [DOI] [PubMed] [Google Scholar]
- [216].Garthwaite G, Goodwin DA, Batchelor AM, Leeming K, Garthwaite J, Nitric oxide toxicity in CNS white matter: an in vitro study using rat optic nerve, Neuroscience 109 (1) (2002) 145–155. [DOI] [PubMed] [Google Scholar]
- [217].Chiueh CC, Neuroprotective properties of nitric oxide, Ann. N. Y. Acad. Sci 890 (1999) 301–311. [DOI] [PubMed] [Google Scholar]
- [218].Ashki N, Hayes KC, Shi R, Nitric oxide reversibly impairs axonal conduction in Guinea pig spinal cord, J. Neurotrauma 23 (12) (2006) 1779–1793. [DOI] [PubMed] [Google Scholar]
- [219].Weyerbrock A, Walbridge S, Saavedra JE, Keefer LK, Oldfield EH, Differential effects of nitric oxide on blood-brain barrier integrity and cerebral blood flow in intracerebral C6 gliomas, Neuro Oncol. 13 (2) (2011) 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Kitagaki J, Yang Y, Saavedra JE, Colburn NH, Keefer LK, Perantoni AO, Nitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53, Oncogene 28 (4) (2009) 619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Inami K, Nims RW, Srinivasan A, Citro ML, Saavedra JE, Cederbaum AI, Keefer LK, Metabolism of a liver-selective nitric oxide-releasing agent, V-PYRRO/NO, by human microsomal cytochromes P450, Nitric Oxide 14 (4) (2006) 309–315. [DOI] [PubMed] [Google Scholar]
- [222].Knox CD, de Kam PJ, Azer K, Wong P, Ederveen AG, Shevell D, Morabito C, Meehan AG, Liu W, Reynders T, et al. , Discovery and clinical evaluation of MK-8150, a novel nitric oxide donor with a unique mechanism of nitric oxide release, J Am Heart Assoc 5 (9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Singh RJ, Hogg N, Joseph J, Konorev E, Kalyanaraman B, The peroxynitrite generator SIN-1, becomes a nitric oxide donor in the presence of electron acceptors, Arch. Biochem. Biophys 361 (2) (1999) 331–339. [DOI] [PubMed] [Google Scholar]
- [224].Boger RH, Bodeboger SM, Gerecke U, Frolich JC, Long-term administration of L-arginine, L-name, and the exogenous No donor molsidomine modulates urinary nitrate and cgmp excretion in rats, Cardiovasc. Res 28 (4) (1994) 494–499. [DOI] [PubMed] [Google Scholar]
- [225].Lorenc-Koci E, Czarnecka A, Lenda T, Kaminska K, Konieczny J, Molsidomine, a nitric oxide donor, modulates rotational behavior and monoamine metabolism in 6-OHDA lesioned rats treated chronically with L-DOPA, Neurochem. Int 63 (8) (2013) 790–804. [DOI] [PubMed] [Google Scholar]
- [226].Maccario M, Oleandri SE, Procopio M, Grottoli S, Avogadri E, Camanni F, Ghigo E, Comparison among the effects of arginine, a nitric oxide precursor, isosorbide dinitrate and molsidomine, two nitric oxide donors, on hormonal secretions and blood pressure in man, J. Endocrinol. Invest 20 (8) (1997) 488–492. [DOI] [PubMed] [Google Scholar]
- [227].Mayhan WG, Nitric oxide donor-induced increase in permeability of the blood-brain barrier, Brain Res. 866 (1–2) (2000) 101–108. [DOI] [PubMed] [Google Scholar]
- [228].Meyer RC, Spangler EL, Patel N, London ED, Ingram DK, Impaired learning in rats in a 14-unit T-maze by 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, is attenuated by the nitric oxide donor, molsidomine, Eur. J. Pharmacol 341 (1) (1998) 17–22. [DOI] [PubMed] [Google Scholar]
- [229].Pitsikas N, Rigamonti AE, Cella SG, Locatelli V, Sala M, Muller EE, Effects of molsidomine on scopolamine-induced amnesia and hypermotility in the rat, Eur. J. Pharmacol 426 (3) (2001) 193–200. [DOI] [PubMed] [Google Scholar]
- [230].Pitsikas N, Rigamonti AE, Cella SG, Muller EE, Effects of the nitric oxide donor molsidomine on different memory components as assessed in the object-recognition task in the rat, Psycho pharmacology (Berl) 162 (3) (2002) 239–245. [DOI] [PubMed] [Google Scholar]
- [231].Pitsikas N, Rigamonti AE, Celia SG, Muller EE, The GABAB receptor and recognition memory: possible modulation of its behavioral effects by the nitrergic system, Neuroscience 118 (4) (2003) 1121–1127. [DOI] [PubMed] [Google Scholar]
- [232].Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE, The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat, Neurobiol. Aging 26 (2) (2005) 259–264. [DOI] [PubMed] [Google Scholar]
- [233].Pitsikas N, Zisopoulou S, Sakellaridis N, Nitric oxide donor molsidomine attenuates psychotomimetic effects of the NMDA receptor antagonist MK-801, J. Neurosci. Res 84 (2) (2006) 299–305. [DOI] [PubMed] [Google Scholar]
- [234].Choe CU, Lewerenz J, Gerloff C, Magnus T, Donzelli S, Nitroxyl in the central nervous system, Antioxidants Redox Signal. 14 (9) (2011) 1699–1711. [DOI] [PubMed] [Google Scholar]
- [235].Kemp-Harper BK, Nitroxyl (HNO): a novel redox signaling molecule, Antioxidants Redox Signal. 14 (9) (2011) 1609–1613. [DOI] [PubMed] [Google Scholar]
- [236].DuMond JF, King SB, The chemistry of nitroxyl-releasing compounds, Antioxidants Redox Signal. 14 (9) (2011) 1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Tita C, Gilbert EM, Van Bakel AB, Grzybowski J, Haas GJ, Jarrah M, Dunlap SH, Gottlieb SS, Klapholz M, Patel PC, et al. , A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction, Eur. J. Heart Fail 19 (10) (2017) 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Roof SR, Ueyama Y, Mazhari R, Hamlin RL, Hartman JC, Ziolo MT, Reardon JE, Del Rio CL, CXL-1020, a novel nitroxyl (HNO) prodrug, is more effective than milrinone in models of diastolic dysfunction-A cardiovascular therapeutic: an efficacy and safety study in the rat, Front. Physiol 8 (2017) 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Choe CU, Lewerenz J, Fischer G, Uliasz TF, Espey MG, Hummel FC, King SB, Schwedhelm E, Boger RH, Gerloff C, et al. , Nitroxyl exacerbates ischemic cerebral injury and oxidative neurotoxicity, J. Neurochem 110 (6) (2009) 1766–1773. [DOI] [PubMed] [Google Scholar]
- [240].Hewett SJ, Espey MG, Uliasz TF, Wink DA, Neurotoxicity of nitroxyl: insights into HNO and NO biochemical imbalance, Free Radic. Biol. Med 39 (11) (2005) 1478–1488. [DOI] [PubMed] [Google Scholar]
- [241].Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS, Lipton SA, Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO, Neuron 24 (2) (1999) 461–469. [DOI] [PubMed] [Google Scholar]
- [242].Vaananen AJ, Moed M, Tuominen RK, Helkamaa TH, Wiksten M, Liesi P, Chiueh CC, Rauhala P, Angeli's salt induces neurotoxicity in dopaminergic neurons in vivo and in vitro, Free Radic. Res 37 (4) (2003) 381–389. [DOI] [PubMed] [Google Scholar]
- [243].Singh RJ, Hogg N, Joseph J, Kalyanaraman B, Mechanism of nitric oxide release from S-nitrosothiols, J. Biol. Chem 271 (31) (1996) 18596–18603. [DOI] [PubMed] [Google Scholar]
- [244].Singh RJ, Hogg N, Joseph J, Kalyanaraman B, Photosensitized decomposition of S-nitrosothiols and 2-methyl-2-nitrosopropane possible use for site-directed nitric-oxide production. FEBS Lett. 360 (1) (1995) 47–51. [DOI] [PubMed] [Google Scholar]
- [245].Hickok JR, Vasudevan D, Thatcher GR, Thomas DD, Is S-nitrosocysteine a true surrogate for nitric oxide? Antioxidants Redox Signal. 17 (7) (2012) 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Khan M, Dhammu TS, Dhaindsa TS, Khan H, Singh AK, Singh I, An NO/GSNO-based neuroregeneration strategy for stroke therapy, J. Neurol. Neurosci 6 (4) (2015). [PMC free article] [PubMed] [Google Scholar]
- [247].Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS, A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds, Nature 364 (6438) (1993) 626–632. [DOI] [PubMed] [Google Scholar]
- [248].Zhang Y, Wu K, Su W, Zhang DF, Wang P, Qiao X, Yao Q, Yuan Z, Yao YG, Liu G, et al. , Increased GSNOR expression during aging impairs cognitive function and decreases S-nitrosation of CaMKIIalpha, J. Neurosci 37 (40) (2017) 9741–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Hoenicka M, Becker EM, Apeler H, Sirichoke T, Schroder H, Gerzer R, Stasch JP, Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide, J. Mol. Med. (Berl.) 77 (1) (1999) 14–23. [DOI] [PubMed] [Google Scholar]
- [250].Friebe A, Koesling D, Mechanism of YC-1-induced activation of soluble guanylyl cyclase, Mol. Pharmacol 53 (1) (1998) 123–127. [DOI] [PubMed] [Google Scholar]
- [251].Mulsch A, Bauersachs J, Schafer A, Stasch JP, Kast R, Busse R, Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators, Br. J. Pharmacol 120 (4) (1997) 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Russwurm M, Mergia E, Mullershausen F, Koesling D, Inhibition of deactivation of NO-sensitive guanylyl cyclase accounts for the sensitizing effect of YC-1, J. Biol. Chem 277 (28) (2002) 24883–24888. [DOI] [PubMed] [Google Scholar]
- [253].Friebe A, Mullershausen F, Smolensk A, Walter U, Schultz G, Koesling D, YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets, Mol. Pharmacol 54 (6) (1998) 962–967. [DOI] [PubMed] [Google Scholar]
- [254].Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM, Enhancement of long-term potentiation by a potent nitric oxide-guanylyl cyclase activator, 3-(5-hydroxymethyl-2-furyl)-1-benzyl-indazole, Mol. Pharmacol. 63 (6) (2003) 1322–1328. [DOI] [PubMed] [Google Scholar]
- [255].Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM, Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1, Eur. J. Neurosci 21 (6) (2005) 1679–1688. [DOI] [PubMed] [Google Scholar]
- [256].Chien WL, Liang KC, Fu WM, Enhancement of active shuttle avoidance response by the NO-cGMP-PKG activator YC-1, Eur. J. Pharmacol 590 (1–3) (2008) 233–240. [DOI] [PubMed] [Google Scholar]
- [257].Yang X, Wang Y, Luo J, Liu S, Yang Z, Protective effects of YC-1 against glutamate induced PC12 cell apoptosis, Cell. Mol. Neurobiol 31 (2) (2011) 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Galle J, Zabel U, Hubner U, Hatzelmann A, Wagner B, Wanner C, Schmidt HH, Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity, Br. J. Pharmacol 127 (1) (1999) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Bischoff E, Stasch JP, Effects of the sGC stimulator BAY 41–2272 are not mediated by phosphodiesterase 5 inhibition, Circulation 110 (12) (2004) e320–321 author reply e320–321. [DOI] [PubMed] [Google Scholar]
- [260].Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP, NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential, Nat. Rev. Drug Discov 5 (9) (2006) 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Schmidt P, Schramm M, Schroder H, Stasch JP, Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase, Eur. J. Pharmacol 468 (3) (2003) 167–174. [DOI] [PubMed] [Google Scholar]
- [262].Follmann M, Griebenow N, Hahn MG, Hartung I, Mais FJ, Mittendorf J, Schafer M, Schirok H, Stasch JP, Stoll F, et al. , The chemistry and biology of soluble guanylate cyclase stimulators and activators, Angew Chem. Int. Ed. Engl 52 (36) (2013) 9442–9462. [DOI] [PubMed] [Google Scholar]
- [263].Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Muller D, Schluter KD, Dingendorf A, Hackemack S, Kolosionek E, Kaulen C, et al. , Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension, Eur. Respir. J 32 (4) (2008) 881–891. [DOI] [PubMed] [Google Scholar]
- [264].Nakai T, Perl NR, Barden TC, Carvalho A, Fretzen A, Germano P, Im GY, Jin H, Kim C, Lee TW, et al. , Discovery of IWP-051, a novel orally bioavailable sGC stimulator with once-daily dosing potential in humans, ACS Med. Chem. Lett 7 (5) (2016) 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Wales JA, Chen CY, Breci L, Weichsel A, Bernier SG, Sheppeck JE 2nd, Solinga R, Nakai T, Renhowe PA, Jung J, et al. , Discovery of stimulator binding to a conserved pocket in the heme domain of soluble guanylyl cyclase, J. Biol. Chem 293 (5) (2018) 1850–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Tseng KY, Caballero A, Dec A, Cass DK, Simak N, Sunu E, Park MJ, Blume SR, Sammut S, Park DJ, et al. , Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism, PloS One 6 (11) (2011) e27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B, Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, Mol. Pharmacol 48 (2) (1995) 184–188. [PubMed] [Google Scholar]
- [268].Zhao Y, Brandish PE, Di Valentin M, Schelvis JP, Babcock GT, Marietta MA, Inhibition of soluble guanylate cyclase by ODQ, Biochemistry (Mosc.) 39 (35) (2000) 10848–10854. [DOI] [PubMed] [Google Scholar]
- [269].Pan J, Zhang X, Yuan H, Xu Q, Zhang H, Zhou Y, Huang ZX, Tan X, The molecular mechanism of heme loss from oxidized soluble guanylate cyclase induced by conformational change, Biochim. Biophys. Acta 1864 (5) (2016) 488–500. [DOI] [PubMed] [Google Scholar]
- [270].Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HHHW, The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation, Mol. Pharmacol 56 (2) (1999) 243–253. [DOI] [PubMed] [Google Scholar]
- [271].Wegener JW, Closs EI, Forstermann U, Nawrath H, Failure of 1H-[1,2,4]ox-adiazolo[4,3-a]quinoxalin-1-one (ODQ) to inhibit soluble guanylyl cyclase in rat ventricular cardiomyocytes, Br. J. Pharmacol 127 (3) (1999) 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Olesen SP, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, Busse R, Mulsch A, Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br. J. Pharmacol 123 (2) (1998) 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Haramis G, Zhou Z, Pyriochou A, Koutsilieris M, Roussos C, Papapetropoulos A, cGMP-independent anti-tumour actions of the inhibitor of soluble guanylyl cyclase, ODQ, in prostate cancer cell lines, Br. J. Pharmacol 155 (6) (2008) 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Lies B, Groneberg D, Gambaryan S, Friebe A, Lack of effect of ODQ does not exclude cGMP signalling via NO-sensitive guanylyl cyclase, Br. J. Pharmacol 170 (2) (2013) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J, Selective phosphodiesterase inhibitors: a promising target for cognition enhancement, Psychopharmacology (Berl) 202 (1–3) (2009) 419–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Bales KR, Plath N, Svenstrup N, Menniti FS, Phosphodiesterase inhibition to target the synaptic dysfunction in Alzheimer's disease, Top. Med. Chem 6 (2010) 57–59. [Google Scholar]
- [277].Xu Y, Zhang HT, O'Donnell JM, Phosphodiesterases in the central nervous system: implications in mood and cognitive disorders, Handb. Exp. Pharmacol (204) (2011) 447–485. [DOI] [PubMed] [Google Scholar]
- [278].Domek-Lopacinska KU, Strosznajder JB, Cyclic GMP and nitric oxide synthase in aging and Alzheimer's disease, Mol. Neurobiol 41 (2–3) (2010) 129–137. [DOI] [PubMed] [Google Scholar]
- [279].Francis SH, Corbin JD, Bischoff E, Cyclic GMP-hydrolyzing phosphodiesterases, Handb. Exp. Pharmacol 191 (2009) 367–408. [DOI] [PubMed] [Google Scholar]
- [280].Peixoto CA, Nunes AK, Garcia-Osta A, Phosphodiesterase-5 inhibitors: action on the signaling pathways of neuroinflammation, neurodegeneration, and cognition, Mediat. Inflamm 2015 (2015) 940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Teich AF, Sakurai M, Patel M, Holman C, Saeed F, Fiorito J, Arancio O, PDE5 exists in human neurons and is a viable therapeutic target for neurologic disease, J Alzheimers Dis 52 (1) (2016) 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, et al. , Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model, J. Neurosci 29 (25) (2009) 8075–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Puzzo D, Loreto C, Giunta S, Musumeci G, Frasca G, Podda MV, Arancio O, Palmeri A, Effect of phosphodiesterase-5 inhibition on apoptosis and beta amyloid load in aged mice, Neurobiol. Aging 35 (3) (2014) 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Perez-Roldan JM, Garcia-Barroso C, Franco R, Aguirre N, Garcia-Osta A, Sildenafil restores cognitive function without affecting beta-amyloid burden in a mouse model of Alzheimer's disease, Br. J. Pharmacol 164 (8) (2011) 2029–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Gomez-Vallejo V, Ugarte A, Garcia-Barroso C, Cuadrado-Tejedor M, Szczupak B, Dopeso-Reyes IG, Lanciego JL, Garcia-Osta A, Llop J, Oyarzabal J, et al. , Pharmacokinetic investigation of sildenafil using positron emission tomography and determination of its effect on cerebrospinal fluid cGMP levels, J. Neurochem 136 (2) (2016) 403–415. [DOI] [PubMed] [Google Scholar]
- [286].Garcia-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA, Salles J, Lanciego JL, Oyarzabal J, Franco R, et al. , Tadalafil crosses the blood-brain barrier and reverses cognitive dysfunction in a mouse model of AD, Neuropharmacology 64 (2013) 114–123. [DOI] [PubMed] [Google Scholar]
- [287].Reneerkens OA, Rutten K, Akkerman S, Blokland A, Shaffer CL, Menniti FS, Steinbusch HW, Prickaerts J, Phosphodiesterase type 5 (PDE5) inhibition improves object recognition memory: indications for central and peripheral mechanisms, Neurobiol. Learn. Mem 97 (4) (2012) 370–379. [DOI] [PubMed] [Google Scholar]
- [288].Fiorito J, Saeed F, Zhang H, Staniszewski A, Feng Y, Francis YI, Rao S, Thakkar DM, Deng SX, Landry DW, et al. , Synthesis of quinoline derivatives: discovery of a potent and selective phosphodiesterase 5 inhibitor for the treatment of Alzheimer's disease, Eur. J. Med. Chem 60 (2013) 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Fiorito J, Vendome J, Saeed F, Staniszewski A, Zhang H, Yan S, Deng SX, Arancio O, Landry DW, Identification of a novel 1,2,3,4-Tetrahydrobenzo[b] [1,6]naphthyridine analogue as a potent phosphodiesterase 5 inhibitor with improved Aqueous solubility for the treatment of Alzheimer's disease, J. Med. Chem 60 (21) (2017) 8858–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Rabal O, Sanchez-Arias JA, Cuadrado-Tejedor M, de Miguel I, Perez- Gonzalez M, Garcia-Barroso C, Ugarte A, Estella-Hermoso de Mendoza A, Saez E, Espelosin M, et al. , Design, synthesis, and biological evaluation of first-in-class dual acting histone deacetylases (HDACs) and phosphodiesterase 5 (PDE5) inhibitors for the treatment of Alzheimer's disease, J. Med. Chem 59 (19) (2016) 8967–9004. [DOI] [PubMed] [Google Scholar]
- [291].Cuadrado-Tejedor M, Garcia-Barroso C, Sanchez-Arias JA, Rabal O, Perez-Gonzalez M, Mederos S, Ugarte A, Franco R, Segura V, Perea G, et al. , A first-in-class small-molecule that acts as a dual inhibitor of HDAC and PDE5 and that rescues hippocampal synaptic impairment in Alzheimer's disease mice, Neuropsychopharmacology 42 (2) (2017) 524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Gaisina IN, Lee SH, Kaidery NA, Ben Aissa M, Ahuja M, Smirnova NN, Wakade S, Gaisin A, Bourassa MW, Ratan RR, et al. , Activation of Nrf2 and hypoxic Adaptive response contribute to neuroprotection elicited by phenylhy-droxamic acid selective HDAC6 inhibitors, ACS Chem. Neurosci 9 (5) (2018) 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB, Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase, J. Biol. Chem 273 (25) (1998) 15559–15564. [DOI] [PubMed] [Google Scholar]
- [294].Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M, Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line, Mol. Pharmacol 68 (6) (2005) 1775–1781. [DOI] [PubMed] [Google Scholar]
- [295].van der Staay FJ, Rutten K, Barfacker L, Devry J, Erb C, Heckroth H, Karthaus D, Tersteegen A, van Kampen M, Blokland A, et al. , The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents, Neuropharmacology 55 (5) (2008) 908–918. [DOI] [PubMed] [Google Scholar]
- [296].Hutson PH, Finger EN, Magliaro BC, Smith SM. Converso A, Sanderson PE, Mullins D, Hyde LA, Eschle BK, Turnbull Z, et al. , The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-py ran-4-yl)-1,5-dihydro-4H-pyrazolo [3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents, Neuropharmacology 61 (4) (2011) 665–676. [DOI] [PubMed] [Google Scholar]
- [297].Vardigan JD, Converso A, Hutson PH, Uslaner JM, The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 attenuates a scopolamine-induced deficit in a novel rodent attention task, J. Neurogenet 25 (4) (2011) 120–126. [DOI] [PubMed] [Google Scholar]
- [298].Schwam EM, Nicholas T, Chew R, Billing CB, Davidson W, Ambrose D, Altstiel LD, A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer's disease, Curr. Alzheimer Res 11 (5) (2014) 413–421. [DOI] [PubMed] [Google Scholar]
- [299].Mason VL, Alzheimer's association international conference on Alzheimer's disease 2015 (AAIC; 2015) (July 18-23, 2015 - Washington, D.C., USA), Drugs Today (Bare) 51 (7) (2015) 447–452. [DOI] [PubMed] [Google Scholar]
- [300].Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM, Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development, Cell. Mol. Life Sci 62 (11) (2005) 1198–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Beaumont V, Park L, Rassoulpour A, Dijkman U, Heikkinen T, Lehtimaki K, Kontkanen O, Al Nackkash R, Bates GP, Gleyzes M, et al. , The PDE1/5 inhibitor SCH-51866 does not modify disease progression in the R6/2 mouse model of Huntington's disease, PLoS Curr 6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G, Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance, Neuropharmacology 47 (7) (2004) 1081–1092. [DOI] [PubMed] [Google Scholar]
- [303].Masood A, Nadeem A, Mustafa SJ, O'Donnell JM, Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice, J. Pharmacol. Exp. Therapeut 326 (2) (2008) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Domek-Lopacinska K, Strosznajder JB, The effect of selective inhibition of cyclic GMP hydrolyzing phosphodiesterases 2 and 5 on learning and memory processes and nitric oxide synthase activity in brain during aging, Brain Res. 1216 (2008) 68–77. [DOI] [PubMed] [Google Scholar]
- [305].Helal CJM, CT, US), Chappie Thomas Allen (Carlisle, MA, US), Humphrey John Michael (Mystic, CT, US): Pyrazolo[3,4-d]pyrimidine Compounds and Their Use as PDE2 Inhibitors And/or CYP3A4 Inhibitors In. United States: Pfizer Inc. (New York, NY, US: ); 2014. [Google Scholar]
- [306].A Study of the Safety, Tolerability, Pharmacokinetics, and Effects on Histamine-induced Wheal of PF-05180999 in Healthy Adults.
- [307].Abarghaz M, Biondi S, Duranton J, Limanton E, Mondadori C, Wagner P, Benzo'1,4 ! diazepin-2-one derivatives as phosphodiesterase pde2 inhibitors, preparation and therapeutic use thereof, Neuro3D, 2005. [Google Scholar]
- [308].Siuciak JA, Chapin DS, Harms JF, Lebel LA, McCarthy SA, Chambers L, Shrikhande A, Wong S, Menniti FS, Schmidt CJ, Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis, Neuropharmacology 51 (2) (2006) 386–396. [DOI] [PubMed] [Google Scholar]
- [309].Hebb AL, Robertson HA, Denovan-Wright EM, Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice, Eur. Neuropsychopharmacol 18 (5) (2008) 339–363. [DOI] [PubMed] [Google Scholar]
- [310].Randomized, Placebo Controlled Study of the Efficacy and Safety of PF-02545920 in Subjects with Huntington’s Disease.
- [311].Kunitomo J, Yoshikawa M, Fushimi M, Kawada A, Quinn JF, Oki H, Kokubo H, Kondo M, Nakashima K, Kamiguchi N, et al. , Discovery of 1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy-3-(1-phenyl-1H-pyrazol-5-yl)pyri dazin-4(1H)-one (TAK-063), a highly potent, selective, and orally active phosphodiesterase 10A (PDE10A) inhibitor, J. Med. Chem 57 (22) (2014) 9627–9643. [DOI] [PubMed] [Google Scholar]
- [312].Suzuki K, Harada A, Suzuki H, Miyamoto M, Kimura H, TAK-063, a PDE10A inhibitor with balanced activation of direct and indirect pathways, provides potent antipsychotic-like effects in multiple paradigms, Neuropsychopharmacology 41 (9) (2016) 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Malamas MS, Ni Y, Erdei J, Stange H, Schindler R, Lankau HJ, Grunwald C, Fan KY, Parris K, Langen B, et al. , Highly potent, selective, and orally active phosphodiesterase 10A inhibitors, J. Med. Chem 54 (21) (2011) 7621–7638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.