Abstract
Background;
Previous studies have demonstrated that the A1A2A3 domains of VWF play a key role in regulating macrophage-mediated clearance in-vivo. In particular, the A1-domain has been shown to modulate interaction with macrophage LRP1 clearance receptor. Furthermore, N-linked glycans within the A2-domain have been shown to protect VWF against premature LRP1-mediated clearance. Importantly however, the specific regions within A1A2A3 that enable macrophage binding have not been defined.
Objective and Methods;
To address this, we utilised site-directed PEGylation and introduced novel targeted N-linked glycosylation within A1A2A3-VWF and subsequently examined VWF clearance.
Results;
Conjugation with a 40-kDa PEG moiety significantly extended the half-life of A1A2A3-VWF in VWF−/− mice in a site-specific manner. For example, PEGylation at specific sites within the A1-domain (S1286) and A3-domain (V1803, S1807) attenuated VWF clearance in-vivo, compared to wild-type A1A2A3-VWF. Furthermore, PEGylation at these specific sites ablated binding to differentiated THP-1 macrophages and LRP1 cluster II and cluster IV in-vitro. Conversely, PEGylation at other positions (Q1353-A1-domain and M1545-A2-domain) had limited effects on VWF clearance or binding to LRP1.Novel N-linked glycan chains were introduced at N1803 and N1807 in the A3-domain. In contrast to PEGylation at these sites, no significant extension in half-life was observed with these N-glycan variants.
Conclusions;
These novel data support the hypothesis that the A1A2A3 domains regulate macrophage-mediated clearance of VWF and highlight that specific regions within the A1- and A3-domains may be key in facilitating interaction with LRP1. These results also provide insights into how macrophage-LRP1 mediated VWF clearance may be attenuated using site-directed PEGylation.
Keywords: von Willebrand factor, Clearance, PEGylation, Glycosylation, Low density lipoprotein receptor-related protein
Introduction
Von Willebrand factor (VWF) is a large plasma glycoprotein that plays essential roles in normal haemostasis. First, VWF mediates platelet adhesion to exposed subendothelial collagen at sites of vascular injury and thus facilitates platelet plug formation.1 Second, VWF acts as a carrier molecule for procoagulant factor VIII (FVIII), thereby extending its circulatory half-life.2 Consequently, inherited VWF deficiency is associated with a significant bleeding phenotype in patients with von Willebrand disease (VWD).3,4 Conversely, elevated plasma levels of the VWF-FVIII complex constitute an independent risk factor for venous thrombosis.5,6 In recent years, significant advances have been made in our understanding of VWF biosynthesis, its structure, and its various functional properties.7,8 In contrast, the biological mechanisms regulating VWF clearance from the plasma remains unclear.9
Hepatic and splenic macrophages have been identified as important cellular mediators of VWF clearance.10,11 Studies have shown that radiolabelled VWF co-localises with CD68+ macrophage cells in the murine liver, and that macrophage depletion significantly prolongs the survival of infused VWF in vivo.12 Furthermore, dose-dependent binding of VWF to primary human macrophages and differentiated THP-1 macrophages has been observed.10,13 A number of specific macrophage surface receptors have also been implicated in modulating VWF binding and endocytosis.14 These include macrophage scavenger receptors (e.g. low-density lipoprotein receptor-related protein-1 (LRP1), scavenger receptor A1 (SR-A1) and C-type lectin receptor macrophage galactose lectin (MGL).15–17 Increased plasma VWF levels have been reported in a number of genetically-engineered mice including MGL1−/− and in macrophage-specific, LRP1-deficient mice.15,17 In addition, reduced VWF propeptide (VWFpp) to VWF antigen (VWF;Ag) ratios have been reported in SR-A1−/− mice, suggesting decreased VWF clearance in vivo.16 Critically however, the molecular mechanisms through which these receptors interact with VWF, and their relative importance in regulating physiological and/or pathological VWF clearance have not been elucidated.
Although VWF clearance in vivo appears to be independent of multimer size, preliminary data suggest that specific VWF domains may regulate macrophage-mediated clearance. In particular, a number of lines of evidence suggest that the A-domains of VWF may be important in this context. First, previous studies have shown that a monomeric A1A2A3-VWF fragment binds to macrophages and is cleared at a similar rate to full length multimeric VWF.11,13 Second, in-vitro binding studies have demonstrated that an isolated A1-domain can bind to LRP1 and SR-A1.16 Third, we recently demonstrated that the N-linked glycans expressed at N1515 and N1574 within the A2-domain play a role in protecting VWF against premature in-vivo clearance via the macrophage LRP1 receptor.13,18 This putative role for the A-domains in modulating macrophage-mediated clearance is further supported by the observation that the binding of both A1A2A3 and full length VWF to macrophages (and specifically to macrophage LRP1) were significantly enhanced in the presence of ristocetin.15,19 In addition, many VWF mutations associated with increased clearance in patients, with VWD, are clustered within the A1A2A3 region.20 Cumulatively, these data suggest that important receptor-recognition site(s) for VWF clearance are located within the A-domains.
In this study, we aimed to further investigate the role of the A-domains in regulating macrophage-mediated clearance of VWF. To study the importance of defined regions within A1A2A3, a series of engineered variants were generated that contained either (i) site-directed surface PEGylation or (ii) additional novel surface N-linked glycan determinants. Our data demonstrate that PEGylation at specific sites within the A1-domain and A3-domain of A1A2A3-VWF served to ablate binding to THP-1 macrophages and LRP1 in vitro. This resulted in markedly reduced clearance of these PEGylated A1A2A3-VWF variants in vivo. Conversely however, insertion of novel N-linked glycans at these same sites failed to extend the plasma half-life of A1A2A3-VWF. Collectively, these findings suggest that these specific regions within the A1- and A3-domains may be important in regulating LRP1-mediated macrophage clearance of VWF. Moreover, these LRP1 interactive sites within VWF may be shielded using a site-directed PEGylation strategy to extend the plasma half-life of VWF.
Materials and Methods
PEGylation and N-glycosylation of A1A2A3-VWF
Following assessment of the crystal structures of the A1-, A2- and A3-domains, sites were selected for cysteine mutagenesis. These sites were surface-exposed, non-conserved amino acids which were not involved in known functional motifs (GPIbα binding, ADAMTS13 proteolysis or collagen binding sites) and were not known VWD mutations. Site-directed mutagenesis was used to introduce novel surface cysteine residues at these selected sites within A1A2A3-VWF as before.19 His-tagged A1A2A3-VWF variants were expressed and purified from Human embryonic kidney (HEK)293F cells using nickel affinity chromatography. To facilitate polyethylene glycol (PEG) conjugation, A1A2A3-VWF cysteine variants were treated with 100-150 eq of tris 2-carboxyethylphosphine (TCEP) for 90 min at room temperature, after which the TCEP was removed and the reaction allowed to reoxidize at 4 °C for 3 h. The variants were then incubated with 7.5-10eq of branched 40-kDa PEG maleimide at 4 °C overnight. The PEGylated variants were then purified using size exchange chromatography to > 90% purity.
At selected sites of interest within A1A2A3-VWF, novel N-linked glycans were engineered by insertion of the consensus N-glycan sequence NXT. These N-linked glycan (NLG) variants were expressed and purified from HEK239F cells using nickel affinity chromatography. Analytical size exclusion chromatography was performed on all variants to remove any high molecular weights aggregates and ensure a homogenous monomeric population of protein. NLG site occupancy was confirmed using reducing capillary gel electrophoresis.
VWF clearance studies in VWF−/− mice
VWF−/− mice on a C57BL/6J background were obtained from the Jackson Laboratory (Sacramento, Ca, USA). All in vivo clearance experiments were performed on 6-8 week-old mice, in accordance with the Health Product Regulatory Authority, Ireland as previously described.19 Mice were infused with 30 nM A1A2A3-VWF or PEGylated variants thereof via tail-vein injection and anesthetised with 2.5% tribromoethanol (0.2 ml per 10 g body weight). Blood was collected via sub-clavicle incision into lithium-heparin coated microtainers. Three to five mice per time point were used. Residual plasma VWF:Ag levels were determined at specific time points up to 24 h by VWF:Ag ELISA, using polyclonal rabbit anti-human VWF (Dako, Denmark). As previous a highly purified A1A2A3-VWF protein was first used to create a standard curve this for this ELISA.19
In vitro GPIbα and collagen binding
In order to examine some of the biological functions of A1A2A3-VWF, binding of cysteine and PEGylated variants to GPIbα and collagens type III and IV were assessed using immunosorbant assays. Briefly, 5µg/ml of either GPIbα (R&D Systems), collagen type III or collagen IV (BioVision) were coated on 96-well microtiter plate overnight in 50mM carbonate buffer (pH 9.6). Following blocking, in 1% Polyvinylpyrrolidone, 3% BSA solution, TBS with 0.1% Tween (TBS-T), A1A2A3-VWF samples were incubated in 1% BSA, TBS-T buffer for 2 h at 37 °C. Binding of A1A2A3 variants were equally detected using polyclonal sheep anti-VWF-A2 (R&D Systems) and anti-sheep-HRP (R&D Systems) or biotinylated anti-histidine (Abcam) and HRP-streptavidin conjuguated (R&D Systems).
In vitro VWF-LRP1 binding studies
Recombinant LRP1 cluster II (2 µg/ml) or cluster IV (1 µg/ml) (R&D Systems) were immobilised onto a 96-well microtiter plate in 50 mM sodium carbonate buffer (pH 9.6) overnight at 4 °C. The wells were blocked for 2 h using 1% Polyvinylpyrrolidone, 5% BSA solution in TBS with 0.1% Tween (TBS-T) containing 2.5 mM calcium chloride. A1A2A3-VWF variants (0-50 nM) were incubated for 2 h at 37 °C in the TBS-T buffer. Bound A1A2A3-VWF variants were detected using a panel of antibodies. All A1A2A3 cysteine variants were equally detected using polyclonal sheep anti-VWF-A2 (R&D systems) and corresponding anti-sheep-HRP antibody (supplementary Fig. 1A). Following PEGylation within the A2-domain, this anti-A2 antibody displayed significantly reduced binding affinity for A2-PEGylated variants (supplemental Fig. 1B). Consequently, a polyclonal rabbit biotinylated anti-His tag antibody (Abcam) and Strep-HRP (R&D Systems) were used to ensure equal detection of the A2-PEGylated variants (M1545C-PEG, L1591C-PEG and Q1652C-PEG) (supplementary Fig. 1C and 1D).
THP-1 Macrophage binding studies
Human macrophages were obtained by differentiating THP-1 monocytes using 100 nM phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich, Ireland), 10 ng/ml of human macrophage colony stimulating factor and 1 ng/ml of human granulocyte macrophage colony stimulating Factor (R&D systems). THP-1 macrophages were then maintained in RPMI medium, supplemented with 10% fetal bovine serum, 1% sodium pyruvate, 1% penicillin/streptomycin, 10 mM HEPES Buffer and 0.02 mM β-mercaptoethanol. THP-1 macrophages were serum starved for 1 h before washing and detachment using 0.02% EDTA. A1A2A3-VWF variants (1 µg/ml) were incubated with THP-1 macrophage suspensions of 2 × 106 cells/ml in HEPES buffered saline supplemented with 2.5 mM calcium and 1 mg/ml ristocetin for 1 h at room temperature. Cells were washed using PBS and blocked using 1% BSA supplemented with human Fc-blocking agent (eBiosciences, Ireland) for 15 min at 4°C. VWF binding was detected using a panel of specific primary antibodies, including polyclonal sheep anti-A2-vWF (R&D systems) or polyclonal mouse biotinylated anti-His (Abcam, UK), followed by fluorescent tagged secondary antibody Alexa488-labeled streptavidin-Alexa488 (Biolegend, UK). Cells were fixed with 1% paraformaldehyde and fluorescence intensity was measured using FITC-filter with CyAn™ ADP Analyzer (Beckman Coulter, USA). Each assay was performed in triplicate. Results were analysed using FlowJo software.
Data presentation and statistical analysis
Experimental data were analysed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, USA). Data were expressed as mean values ± standard error of the mean. Data were analyzed with Student’s unpaired two-tailed t-test and P-values of <0.05 were considered to be significant. Clearance studies data were fitted to a monoexponential equation. Pharmacokinetic parameters including Mean Residence Time (MRT = 1/K) and half-life (t1/2 = ln2/K) were calculated.
Results
The effect of cysteine mutagenesis on the clearance of A1A2A3-VWF
To investigate the role of specific regions within the A1A2A3 domains of VWF in modulating VWF clearance, the A-domain crystal structures were examined to select specific surface sites for targeted PEG conjugation.21–23 In keeping with previous studies, a cysteine-mediated conjugation strategy using PEG-maleimide was then utilized (Fig. 1A).24 There are only six cysteine resides within native A1A2A3-VWF, all of which are involved in disulphide bonds (Fig. 1A). Given the number of free cysteines in full-length VWF, a cysteine-mediated PEGylation approach is unlikely to be useful in attempting to develop an extended half-life (EHL) VWF therapy. However, its use in this truncated VWF fragment may provide insight into the mechanisms through which A1A2A3 clearance is regulated. Thus, site-directed mutagenesis was first used to engineer novel surface exposed cysteine residues at selected sites within A1A2A3-VWF. A total of 8 sites were selected, including two in the A1-domain (S1286C and Q1353C; Fig. 1B), four in the A2-domain, (M1545C, L1591C, V1636C and Q1652C; Fig. 1C) and two in the A3-domain, (V1803C and S1807C; Fig. 1D). The effect of these novel A1A2A3 cysteine variants on VWF clearance was then studied in VWF-deficient mice. (Fig. 1E).
Figure 1. Identification of sites within A1A2A3-VWF for cysteine-mediated PEGylation.
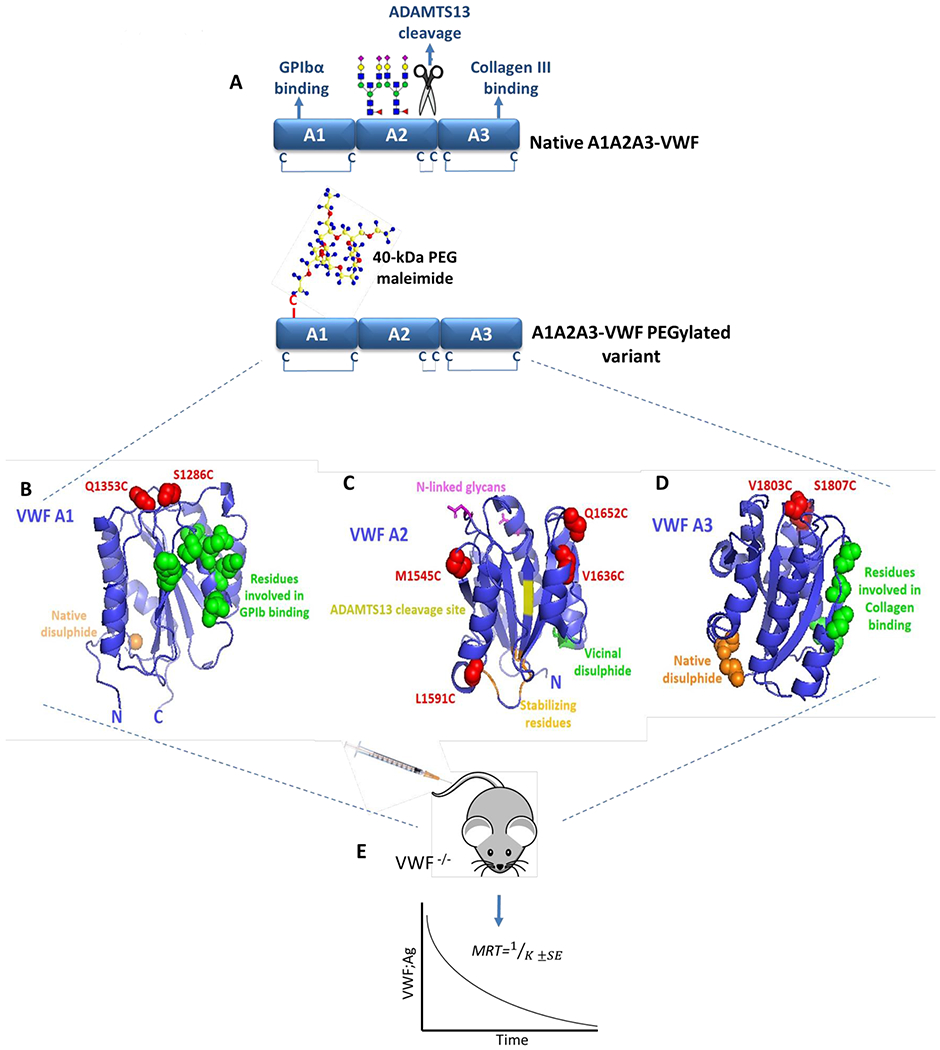
(A) Illustration of the tri-domain structure of native A1A2A3-VWF. GPIbα binding and type III collagen binding are regulated by specific sites within the A1- and A3-domains, respectively. In addition, the ADAMTS13 cleavage site and two N-linked glycan structures are located within the A2-domain. No free cysteine residues are present within A1A2A3-VWF, however a long-range disulphide bond exits within both the A1- and A3-domains while a vicinal disulphide bond is found within the C-terminal of the A2-domain. Specific sites within A1A2A3-VWF were selected to engineer novel free cysteine residues to subsequently facilitate covalent attachment of a 40-kDa PEG maleimide molecule. The crystal structure of A1 (B), A2 (C) and A3 (D) (Protein Data Bank ID, 1auq, 3zqk, 1ao3) are depicted with positions of novel engineered cysteine residues highlighted in red (S1286C and Q1353C in A1, M1545C, L1591C, V1636C and Q1652C in A2 and V1803C, S1807C in A3). (E) All novel A1A2A3-VWF variants were infused into VWF-deficient mice and the mean resident times (MRT) were calculated from the clearance rates.
A1A2A3-VWF cysteine variants were expressed and purified from HEK293F cells (Fig. 2A). Interestingly, the introduction of novel cysteine residues into the A1 and A3 domain of VWF had no significant effect on A1A2A3-VWF clearance compared to wild-type A1A2A3 (Figs. 2B, 2D and 2E). In contrast however, enhanced clearance was observed for two of the cysteine variants introduced within the A2-domain (Fig. 2C). In particular, A1A2A3-L1591C and A1A2A3-V1636C both resulted in significant reductions in mean resident times (MRT) compared to WT-A1A2A3 (11.2 ± 0.7 mins and 14.7 ± 1 mins versus. 22.8 ± 1.1 mins, respectively, p <0.05; Fig. 2E).
Figure 2. The effect of cysteine mutagenesis on the clearance of A1A2A3-VWF.
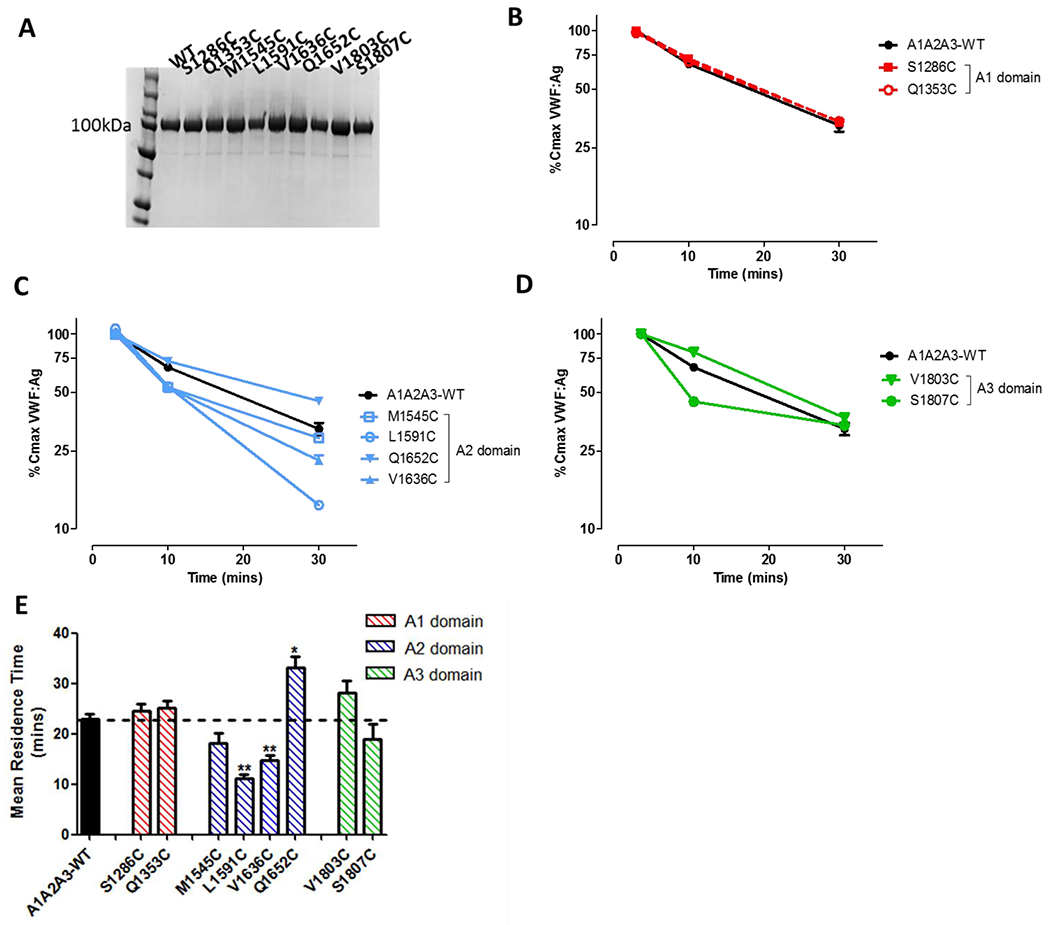
(A) All novel A1A2A3-VWF cysteine variants were secreted and purified from HEK293F cells. (B, C and D) Clearance of non-PEGylated A1A2A3 cysteine variants were examined in VWF−/− mice, with 3-4 mice per time point. (E) The mean resident time (MRT) of all non-PEGylated variants was calculated using one phase decay fit and graphed as ± SEM. (* p <0.05, ** p <0.01, respectively).
PEGylation of A1A2A3-VWF modulates VWF clearance in a site-specific manner.
The novel engineered cysteine residues on the A1A2A3 variants were next used to direct conjugation of a 40-kDa PEG polymer in a site-specific manner. In keeping with the concept that the A-domains do not contain any free cysteine residues, no PEG conjugation was observed for wild-type A1A2A3. In contrast, for each of the A1A2A3 cysteine variants, PEGylation was confirmed with conjugation efficacy of greater than 89% (Table 1). PEGylation of the A1A2A3-V1636C variant was significantly less efficient (79.3%) and consequently no further studies were performed using this variant.
Table 1. Percentage PEGylation efficacy of A1A2A3 cysteine variants and wild-type A1A2A3.
The PEGylation efficacy for each of the A1A2A3 variants as measured by analytical size exchange chromatography following PEG maleimide conjugation and purification. No conjugation of PEG to WT-A1A2A3 was observed.
| A1A2A3 Variant | % PEGylated species |
|---|---|
| WT A1A2A3 | 0 |
| S1286C | 99.3 |
| Q1353C | 90.5 |
| M1545C | 94.7 |
| L1591C | 96.1 |
| V1636C | 79.3 |
| Q1652C | 91.1 |
| V1803C | 89.8 |
| S1807C | 94.7 |
Following site-specific PEGylation variants were purified by size exclusion chromatography and in vivo clearance studies were repeated in VWF-deficient mice. Despite all A1A2A3 variants having a single additional novel cysteine residue, and having been conjugated with a 40-kDa PEG, we observed that PEGylation modulated the clearance of A1A2A3-VWF in a site-dependent manner. For example, within the A1-domain, PEGylation at S1286C markedly inhibited VWF clearance compared to WT-A1A2A3 (MRT 133.4 ± 9.2 mins versus 26.4 ± 1.3 mins, p <0.01; Figs 3A and 3D). In contrast, PEGylation at Q1353C within the A1-domain had only a small inhibitory effect on in vivo clearance of A1A2A3-VWF (Figs 3A and 3D).
Figure 3. PEGylation of A1A2A3-VWF modulates VWF clearance in a site-specific manner.
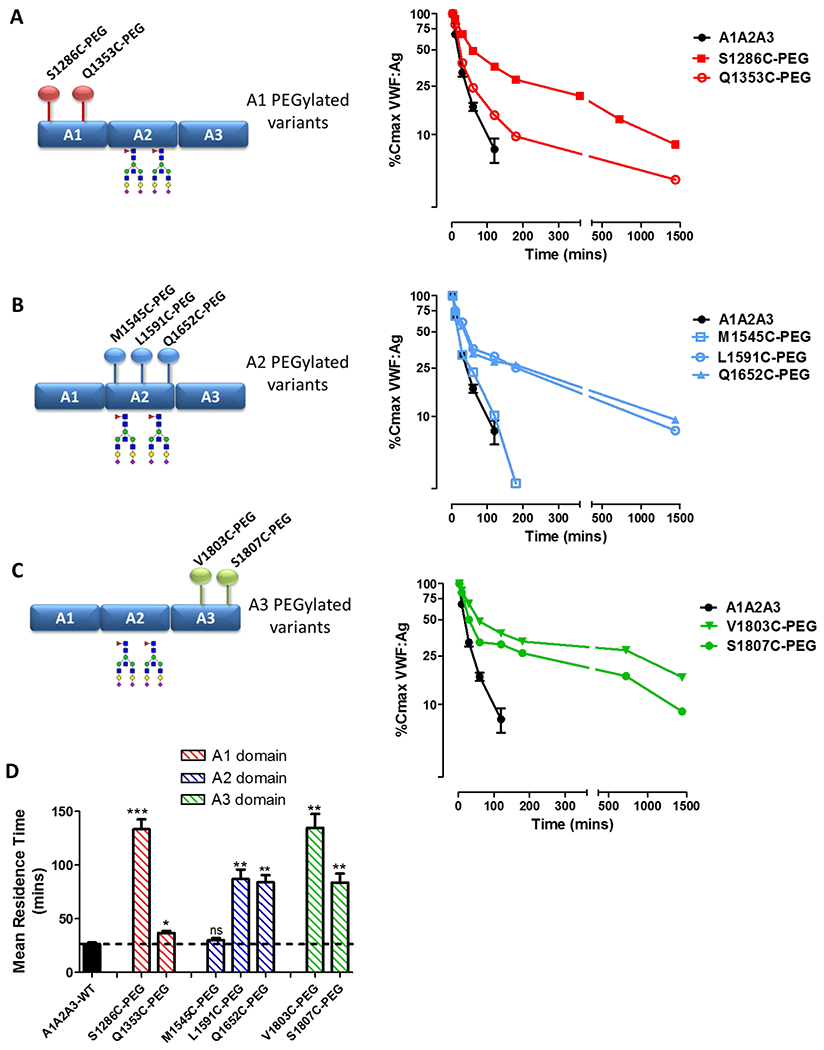
Following site-specific PEGylation, the clearance of A1A2A3-VWF variants were assessed in VWF−/− mice. (A) The clearance of A1-domain variants, (B) A2-domain variants and (C) A3-domain variants were all compared to non-PEGylated A1A2A3-WT. (D) PEGylation served to markedly increase the MRT of A1A2A3-VWF in a site-dependent manner. All data is graphed as mean values ± SEM, 3-5 mice per time point (* p <0.05, ** p <0.01, *** p <0.0001 respectively).
Similarly, for the A2 PEGylated A1A2A3 variants, significant variation in clearance kinetics was seen depending upon the location of the surface PEG moiety. PEGylation at both L1591C and Q1652C within A2 markedly attenuated A1A2A3-VWF clearance compared to wild type A1A2A3 (Figs 3B and 3D). Conversely, conjugation of PEG at M1545C, had no significant effect upon A1A2A3-VWF clearance in vivo (Fig. 3B). Finally, despite the fact that the A3-domain has not previously been shown to directly modulate VWF clearance, PEGylation at both V1803C and S1807C within A3 significantly attenuated A1A2A3-VWF clearance compared to wild type (Figs 3C and 3D). The magnitude of the effect on clearance was more marked with the V1803C variant (134.7 ± 13.0 vs. 26.4 ± 1.3 mins, respectively, p <0.01; Fig. 3D). Collectively, these results demonstrate that PEGylation of A1A2A3-VWF can be used to prolong the in vivo survival of A1A2A3-VWF. However, our findings further highlight that this effect varies depending upon the site of PEGylation, suggesting important roles for specific sites within A1A2A3 in modulating VWF clearance.
In keeping with this, we observed that addition of surface PEGylation can have site specific effects on A1A2A3 biology. For example, PEGylation at the S1286 in the A1 domain and V1803 in the A3 domain served to ablate binding to GPIbα in vitro. Interestingly, PEGylation at the neighbouring site S1807 in the A3 domain only attenuated binding approximately 50% (Supplementary Fig. 2). Binding of the extended half-life variants to collagen types III and IV was also investigated. PEGylation within the A1 domain (VWF S1286C-PEG) did not affect binding to collagen III (new Supplementary Fig. 3A). However, PEGylation within the A3 domain (V1803C-PEG and S1807C-PEG) significantly attenuated collagen III binding compared to WT-A1A2A3-VWF (Supplementary Figure 3B and 3C) Finally, all PEGylated variants with extended half-lives retaining similar binding affinity for collagen IV compared to WT-A1A2A3-VWF (new Supplementary Figure 3A–C).
PEGylation of A1A2A3-VWF attenuates binding to macrophages
Previous studies have demonstrated that macrophages are important in clearing A1A2A3-VWF. Consequently, to investigate the biological mechanisms underpinning the half-life variability observed between specific PEGylated A1A2A3-VWF variants, binding to THP-1 macrophages was assessed using flow cytometry. Expression of VWF clearance receptor LRP1 on differentiated THP-1 macrophages was confirmed by flow cytometry (supplementary Figure 4A). Inhibition of LRP1 significantly attenuated binding of WT-A1A2A3-VWF suggesting VWF adhesion occurs in LRP1-dependant manner (supplementary Figure 4B). In keeping with the in vivo clearance data, all non-PEGylated cysteine variants in the A1-, A2- and A3-domains displayed macrophage binding similar to that of WT-A1A2A3-VWF (Figs. 4A, 4B and 4C, respectively). PEGylation within the A1-domain at S1286C ablated binding to THP-1 macrophages (Fig. 4A). In contrast, PEGylation at Q1353C in A1 resulted in a modest reduction in macrophage binding. These in vitro data are again consistent with the in vivo clearance data which demonstrated that PEGylation at S1286C had a marked effect in attenuating VWF clearance. Furthermore, significantly reduced macrophage binding was also seen for the A2 and A3 PEGylated variants associated with reduced in vivo clearance (L1591C-, Q1652C-, V1803C-, and S1807C-A1A2A3 respectively) (Figs 4B and 4C).
Figure 4. PEGylation of A1A2A3-VWF attenuates binding to macrophages.
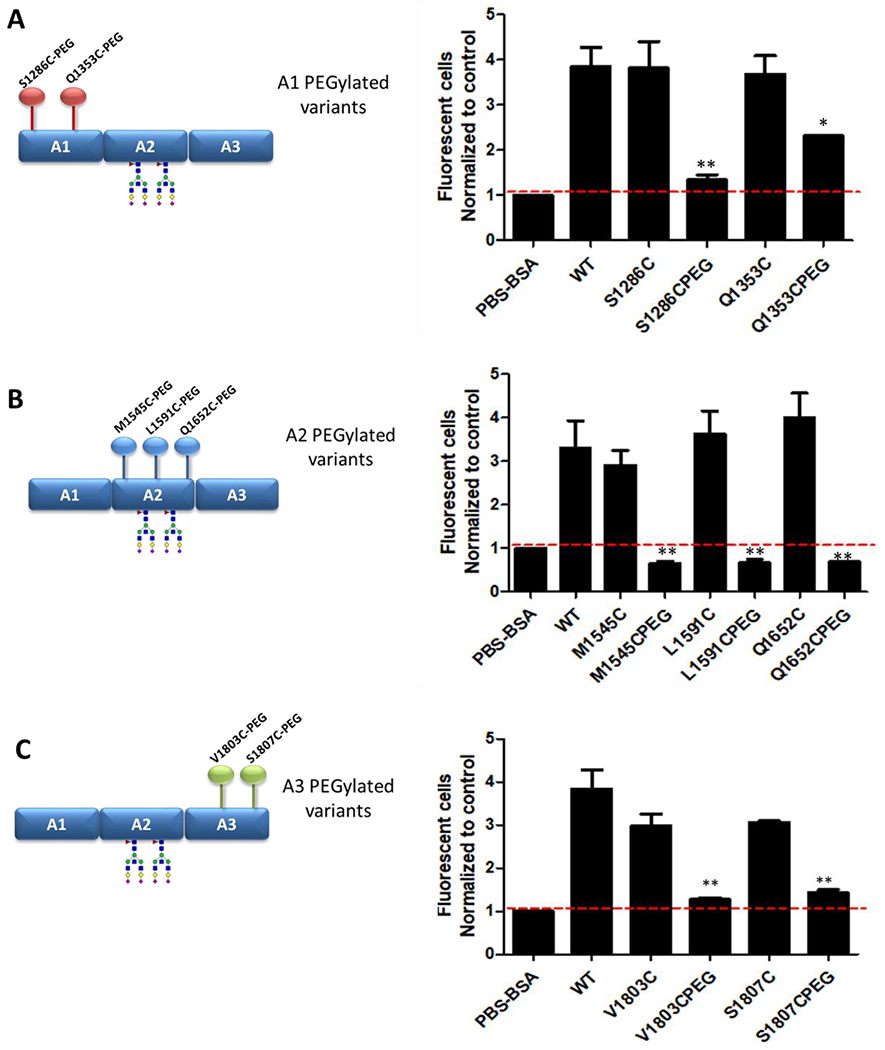
Flow cytometry was used to assess binding to THP-1 macrophages for each of the non-PEGylated and PEGylated (A) A1 variants, (B) the A2 variants and (C) the A3 variants. Data is graphed as mean fluorescent number of cells normalised to the control (PSA-BSA) ± SEM (* p <0.05, ** p <0.01, respectively).
Site-specific PEGylation of A1A2A3-VWF modulates LRP1 binding
In recent studies, we and others have reported that the macrophage LRP1 scavenger receptor plays a critical role in regulating the clearance of both full-length and A1A2A3-VWF.15,18 The extracellular domain of LRP1 is composed of a number of repeated clusters (I, II, III, and IV) that regulate ligand binding. The majority of LRP1 ligands described to date bind via the cluster II and cluster IV repeats.9 To further elucidate the variable reduced rates of clearance for specific A1A2A3-VWF PEGylated variants, binding of these variants to LRP1 cluster II and IV was investigated. Interestingly, PEGylation of A1A2A3-VWF served to inhibit VWF-LRP1 interaction but in a site-dependent manner. For example, in keeping with the markedly reduced clearance rate observed for S1286-PEG, this A1 variant demonstrated no binding to either LRP1 cluster II and IV, compared to non-PEGylated S1286C and WT-A1A2A3 controls respectively (Figs 5A and 5B). Conversely however, PEGylation at Q1353C within the A1-domain inhibited binding to LRP1 cluster II (Fig. 5A) but had no significant effect on cluster IV binding (Fig. 5B). Given that this PEGylated variant resulted in only a minor increase in VWF half-life compared to S1286C-PEG, these findings suggest that the VWF binding to LRP1 cluster IV rather than cluster II may be more important in regulating the rate of clearance. Consistent with this hypothesis, PEGylation at M1545C within the A2-domain partially inhibited binding to LRP1 cluster II (Fig. 5C) but had no significant effect on cluster IV binding (Fig. 5D). Interestingly, this PEGylated A2 variant was also not associated with any significant increase in A1A2A3-VWF survival. The two remaining A2 variants, L1591C-PEG and Q1652C-PEG displayed binding to LRP1 cluster IV that was comparable to their non-PEGylated controls (Fig. 5D). In contrast however, both of these PEGylated variants demonstrated a marked reduction in binding to LRP1 cluster II (Fig. 5C) which may in part contribute to the moderate increase in plasma half-life observed for these variants compared to M1545C-PEG and WT-A1A2A3. Finally, PEGylation at both of the A3-domain sites (V1803C and S1807C) resulted in ablation of LRP1 binding for both cluster II (Fig. 5E) and cluster IV (Fig. 5F), respectively.
Figure 5. Site-specific PEGylation of A1A2A3-VWF modulates binding to LRP1 cluster II and IV.
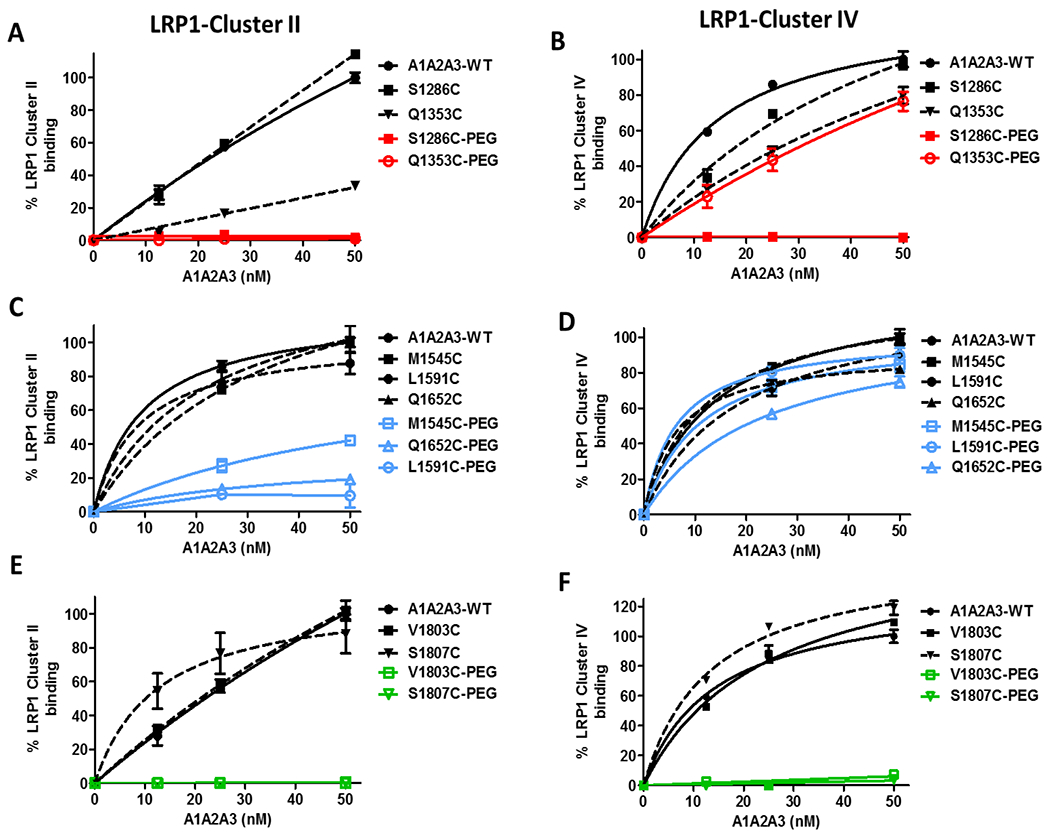
Immunosorbant assays were used to examine binding of A1A2A3-VWF variants with clearance receptor LRP1 ligand binding clusters II (A, C, E) and IV (B, D, F). A1A2A3-VWF binding was graphed as a percentage of A1A2A3-WT set to 100%. All assays were performed in triplicate, and results presented as the mean values ± SEM.
Introduction of novel site-directed N-glycosylation does not affect A1A2A3-VWF clearance
On the basis of having identified specific sites within A1A2A3 that may influence LRP1-mediated macrophage clearance, we sought to examine whether introduction of novel N-linked glycans at these positions might also extend VWF half-life in vivo. Within native A1A2A3-VWF there are two N-linked glycans within the A2-domain, at N1515 and N1574 (Fig. 6A). Importantly, these structures have previously been shown to protect VWF from LRP1-mediated macrophage clearance.19,25 Based on the data from the site-directed PEG studies, novel NLG consensus sequences were engineered at specific sites within A1A2A3 (S1286 in the A1-domain; V1803 and S1807 in the A3-domain) (Fig. 6A). The A1A2A3-N1286 variant failed to express. For the A1A2A3-N1803 and -N1807 variants, N-glycan occupancy was confirmed by capillary gel electrophoresis (Fig. 6B) The N1807-NLG variant was confirmed with 54% occupancy. This partial occupancy is consistent with the doublet band appearance by western blot in Fig. 6B. However, the N1803-NLG had a high occupancy frequency with 75% of the protein species confirming the presence of the novel NLG structure. In vivo clearance rates for each of these two variants was then assessed in VWF−/− mice. Despite the fact that conjugation of PEG at V1803 and S1807 markedly attenuated A1A2A3-VWF clearance in vivo, insertion of novel N-linked glycans at these same sites failed to extend the plasma half-life of A1A2A3-VWF (Figs 6C and 6D). Moreover, binding of the A1A2A3-N1803 and –N1807 variants to LRP1 cluster IV were not significantly different from to A1A2A3-WT (Figs 6E and 6F).
Figure 6. Site-specific N-linked glycosylation does not alter A1A2A3-VWF clearance or affinity for LRP1.
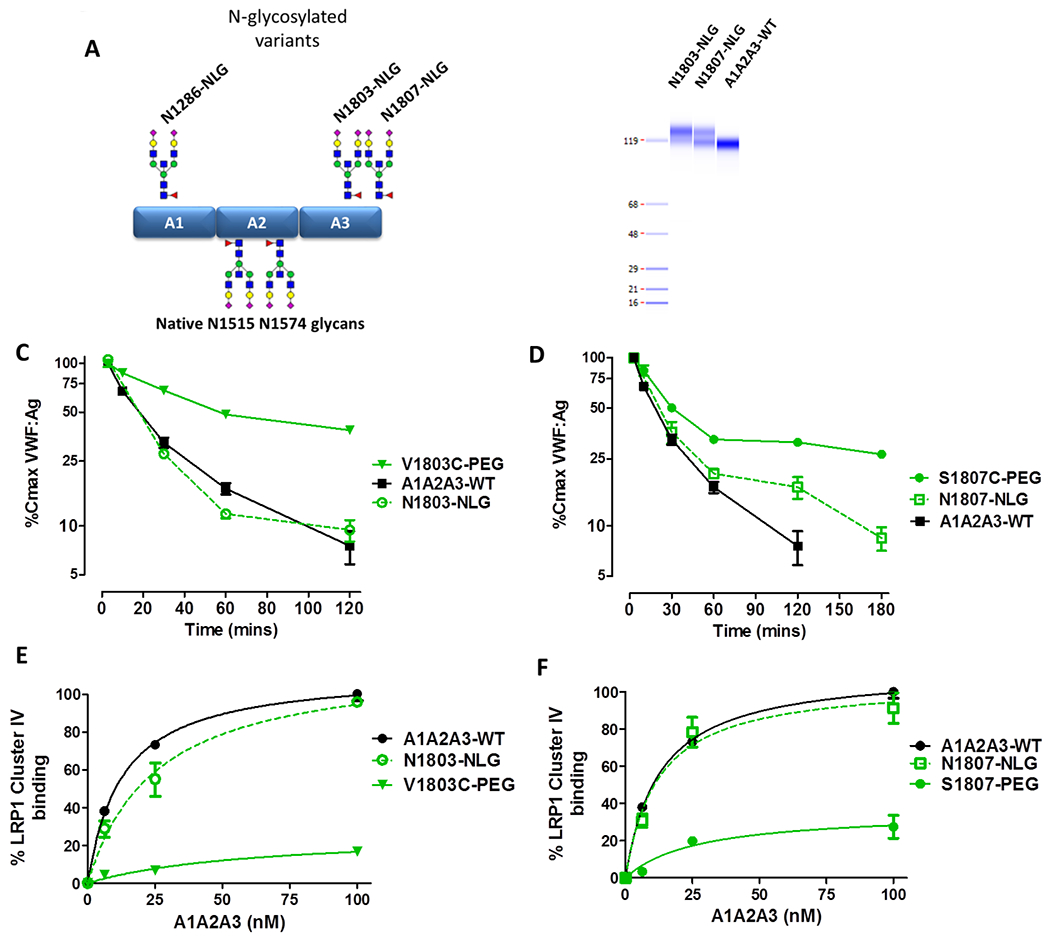
(A) Schematic illustration depicting the location of the three potential novel NLG sites with A1A2A3-VWF, N1286, N1803 and N1807. (B) N-linked occupancy was confirmed for N1803 and N1807 by capillary gel electrophoresis, as indicated by the presence of a higher molecular weight band. Clearance of N1803-NLG (C) and N1807-NLG (D) were assessed in VWF−/− mice. Immunosorbant assays were used to access binding to LRP1 cluster IV ligand binding domain for 1803-NLG (E) and 1807-NLG (F). All data is graphed as mean values ± SEM. For clearance studies, 3-5 mice per time point were used (* p <0.05, ** p <0.01, *** p <0.0001 respectively).
Discussion
Accumulating recent evidence has demonstrated that the A-domains of VWF play an important role in regulating VWF clearance. In particular, the A-domains appear to be of specific importance in modulating macrophage-mediated clearance of VWF. Of note, the isolated A1-domain of VWF has been reported to bind directly to both LRP1 and SR-A1.16,19 Importantly however, the other A-domains may also have a role to play in regulating VWF clearance in vivo. For example, we recently reported that N-linked glycans within the A2-domain protect VWF against premature clearance via the macrophage LRP1 receptor.13,18 Given that many VWF mutations associated with increased clearance are clustered within the A1A2A3 region, understanding the biological mechanisms through which the A-domains influence VWF clearance are of direct translational relevance.14,20
The data derived from the site-directed PEGylation studies presented in this manuscript further support the hypothesis that the A-domains of VWF contain critical receptor-recognition sites that are important in regulating VWF clearance in vivo. Although the structure of the A1A2A3-domain complex has not been defined, our data suggest that more than one clearance recognition site is likely to be located within the A-domains. Further studies will be required to elucidate the precise locations of these sites, and how they specifically function to modulate macrophage-mediated clearance.
Previous studies have reported that a number of different cysteine point mutations within VWF are associated with significantly elevated VWFpp/VWF;Ag ratios and enhanced clearance in type 1 VWD patients. These include, R1308C, R1342C, R1374C, Y1584C and R2464C.26–29 In addition, we recently demonstrated that a cysteine mutation at R1205 in the D3-domain (also described in a VWD patient) results in markedly increased clearance, even more rapid than that of the archetypal R1205H Vicenza variant.12 Importantly, we further demonstrated that the accelerated clearance of VWF-R1205C was macrophage-mediated. Given these cumulative data, it is interesting that in the present study the insertion of novel cysteine residues at selected sites in the A1- (S1286C, Q1353C) and A3- (V1803C, S1807C) domains had no significant effect of the rate of A1A2A3 clearance. Conversely, we observed that introduction of novel cysteine residues at several sites within the A2-domain (M1545, L1591 and V1636) resulted in accelerated A1A2A3 clearance. The molecular mechanisms through which these cysteine mutations impact upon A1A2A3 clearance in vivo remain unclear. However, the long-range disulphide bonds in A1 and A3 may contribute to the ability of these domains to tolerate insertion of cysteine residues. In contrast, the A2-domain possesses a vicinal disulphide bond which has been reported to facilitate conformational changes and thus enable exposure of the buried ADAMTS13 cleavage site.30 We have previously shown that conformational changes in the A2-domain induced by removal of N-linked glycans, at N1515 and N1574, resulted in rapid macrophage-mediated VWF clearance. However, this effect was abolished in a structurally-constrained A2 variant, suggesting the presence of a cryptic macrophage recognition site within the A2-domain.19 Although A1A2A3-M1545C, -L1591C and -V1636C variants were all associated with enhanced clearance in vivo, we observed no increased macrophage binding for any of these three variants. Collectively, these findings raise the possibility that VWF cysteine variants may at least in part direct enhanced clearance through macrophage-independent pathways.
The novel engineered cysteine residues were utilised to target PEGylation to specific sites within A1A2A3. Given the large number of free cysteines in full length VWF, this targeted cysteine-mediated PEGylation approach is unlikely to be successful in developing an extended half-life (EHL) VWF therapy. Moreover, previous work by Turecek et al, demonstrated that a lysine-mediated PEGylation of full length VWF significantly prolonged survival compared to non-modified VWF.31 However this non-targeted and uncontrolled PEGylation approach also resulted in a significant reduction in VWF activity.
Surprisingly, we observed that not all PEGylated sites served to inhibit A1A2A3 clearance. For example, PEGylation at two different sites within the A1-domain (S1286C and Q1353C), had significantly different effects. Whereas PEGylation at S1286C resulted in a 5-fold increase in A1A2A3 MRT in vivo, conversely Q1353C-PEG was associated with only a modest 1.3-fold increase in MRT. Interestingly, the S1286C site is adjacent to a known VWD type 1C mutation S1285P, which has been reported to be associated with a significantly elevated VWFpp/Ag ratio, second only to the archetypal VWD 1C mutation, Vicenza.32 In addition, we observed that PEGylation at S1286C served to ablate macrophage binding in vitro, and specifically inhibited LRP1 interaction. Taken together, these findings suggest that this specific region within the A1-domain may be important in regulating LRP1-mediated macrophage clearance of VWF.
We recently reported in vitro, dose-dependent binding of the isolated A1-domain of VWF to THP-1 macrophages.19 In contrast, no significant binding was seen for the isolated A2- or A3-domains. In addition, Wohner et al. subsequently showed that the isolated A1-domain (but not the A2- or A3-domains) of VWF is sufficient to enable binding to LRP1 in vitro. Given these findings, it is interesting that we observed that PEGylation at specific sites within the A2- (L1591, Q1652) and A3- (V1803, S1807) domains was able to significantly attenuate A1A2A3 clearance in vivo, as well as inhibit binding to THP-1 macrophages and purified LRP1 in vitro. Importantly, the magnitude of these effects varied depending on the specific PEGylation site within A2 or A3 respectively. For example, A1A2A3-S1807-PEG in the A3-domain ablated binding to LRP1 and markedly attenuated VWF clearance, to a similar extent as that observed with the A1 variant, A1A2A3-S1286-PEG. Cumulatively, these novel data suggest that although the isolated A2- and A3-domains may not have direct roles in modulating LRP1 or macrophage binding, they may still be important in modulating VWF clearance. We hypothesise that A2 and A3 may function to shield binding sites for LRP1 and/or other macrophage clearance receptors within the A1-domain. This hypothesis is consistent with our previous data, defining a protective role for the glycans at N1515 and N1574 in the domain.19,33
The findings from our PEGylation studies suggest that both cluster II and cluster IV of LRP1 are involved in mediating VWF binding. Importantly, we also observed that PEGylation at some sites had differential effects in attenuating either cluster II or cluster IV binding. Additional studies will be necessary to define the molecular mechanisms underpinning these findings, and to elucidate the relative importance of individual LRP1 clusters in modulating the physiological and/or pathological VWF clearance in vivo. Nonetheless, our data suggest that PEGylated variants that ablated binding to both LRP1 cluster II and cluster IV (A1A2A3-S1286-PEG, A1A2A3-V1803-PEG and A1A2A3-S1807-PEG) were associated with the most significant increase in VWF half-life in vivo. Conversely, variants which only demonstrated inhibition in LRP1 cluster II but not cluster IV binding (A1A2A3-Q1353-PEG, A1A2A3-L1591-PEG and A1A2A3-Q1652), had more moderate increases in VWF half-life.
Finally, it is interesting that in contrast to PEGylation at the same sites, novel N-linked glycosylation at N1803 and N1807 had no effect on either LRP1 binding or indeed A1A2A3 clearance in vivo. In contrast to the 40-kDa branched PEG moiety, these additional N-linked glycan structures are smaller in size (approximately 5 kDa). Consequently, we hypothesise that the potential shielding effect of PEG is likely to be significantly more effective than that of the smaller N-linked glycans.
In conclusion, our findings support the hypothesis that the A-domains of VWF contain critical receptor-recognition sites that are important in regulating macrophage-mediated clearance in vivo. Furthermore, site specific PEGylation of A1A2A3-VWF suggests that specific regions within the A1- and A3-domains may be key in facilitating interaction with LRP1 and thus regulating VWF clearance via macrophage-LRP1 in vivo. These results also provide insight into how macrophage-LRP1 mediated VWF clearance may be attenuated using site-directed PEGylation.
Supplementary Material
Footnotes
Conflict-of-interest disclosure:
J.S.O’D has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma and Octapharma. He has also served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim and Pfizer. J.S.O.D has also received research grant funding awards from Baxter, Bayer, Pfizer and Novo Nordisk.
N. C., V. T., J. C., C. P., O. C, M. L. and D. P. are Pfizer employees.
References
- 1.Lenting PJ, Christophe OD, Denis C V. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125(13):2019–28. [DOI] [PubMed] [Google Scholar]
- 2.Terraube V, O’Donnell JS, Jenkins P V. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16(1):3–13. [DOI] [PubMed] [Google Scholar]
- 3.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavin M, Aguila S, Dalton N, et al. Significant gynecological bleeding in women with low von Willebrand factor levels. Blood Adv. 2018;2(14):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins PV, Rawley O, Smith OP, O’Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br. J. Haematol 2012;157(6):653–63. [DOI] [PubMed] [Google Scholar]
- 6.Rietveld IM, Lijfering WM, le Cessie S, et al. High levels of coagulation factors and venous thrombosis risk: strongest association for factor VIII and von Willebrand factor. J. Thromb. Haemost 2019;17(1):99–109. [DOI] [PubMed] [Google Scholar]
- 7.Lenting PJ, Casari C, Christophe OD, Denis C V. von Willebrand factor: the old, the new and the unknown. J. Thromb. Haemost 2012;10(12):2428–37. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan J, Preston R, Robson T, O’Donnell J. Emerging Roles for von Willebrand Factor in Cancer Cell Biology. Semin. Thromb. Hemost 2018;44(02):159–166. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan JM, Ward S, Lavin M, O’Donnell JS. von Willebrand factor clearance - biological mechanisms and clinical significance. Br. J. Haematol 2018;183(2):185–195. [DOI] [PubMed] [Google Scholar]
- 10.van Schooten CJ, Shahbazi S, Groot E, et al. Macrophages contribute to the cellular uptake of von Willebrand factor and factor VIII in vivo. Blood. 2008;112(5):1704–12. [DOI] [PubMed] [Google Scholar]
- 11.Lenting PJ, Westein E, Terraube V, et al. An experimental model to study the in vivo survival of von Willebrand factor. Basic aspects and application to the R1205H mutation. J. Biol. Chem 2004;279(13):12102–9. [DOI] [PubMed] [Google Scholar]
- 12.Rawley O, O’Sullivan JM, Chion A, et al. von Willebrand factor arginine 1205 substitution results in accelerated macrophage-dependent clearance in vivo. J. Thromb. Haemost 2015;13(5):821–6. [DOI] [PubMed] [Google Scholar]
- 13.Chion A, OSullivan JM, Drakeford C, et al. N-linked glycans within the A2 domain of von Willebrand factor modulate macrophage-mediated clearance. Blood. 2016;128(15):1959–1968. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan JM, Ward S, Lavin M, O’Donnell JS. von Willebrand factor clearance - biological mechanisms and clinical significance. Br. J. Haematol 2018;183(2):185–195. [DOI] [PubMed] [Google Scholar]
- 15.Rastegarlari G, Pegon JN, Casari C, et al. Macrophage LRP1 contributes to the clearance of von Willebrand factor. Blood. 2012;119(9):2126–34. [DOI] [PubMed] [Google Scholar]
- 16.Wohner N, Muczynski V, Mohamadi A, et al. Macrophage scavenger receptor SR-AI contributes to the clearance of von Willebrand factor. Haematologica. 2018;103(4):728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward SE, O’Sullivan JM, Drakeford C, et al. A novel role for the macrophage galactose-type lectin (MGL) receptor in mediating von Willebrand factor clearance. Blood. 2017;blood-2017-06-787853. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan JM, Aguila S, McRae E, et al. N-linked glycan truncation causes enhanced clearance of plasma-derived von Willebrand factor. J. Thromb. Haemost 2016;14(12):2446–2457. [DOI] [PubMed] [Google Scholar]
- 19.Chion A, O’Sullivan JM, Drakeford C, et al. N-linked glycan sites within the A2 domain of von Willebrand factor modulate macrophage-mediated clearance. Blood. 2016; [DOI] [PubMed] [Google Scholar]
- 20.Casari C, Lenting PJ, Wohner N, Christophe OD, Denis C V. Clearance of von Willebrand factor. J. Thromb. Haemost 2013;11 Suppl 1:202–11. [DOI] [PubMed] [Google Scholar]
- 21.Emsley J, Cruz M, Handin R, Liddington R. Crystal Structure of the von Willebrand Factor A1 Domain and Implications for the Binding of Platelet Glycoprotein Ib. J. Biol. Chem 1998;273(17):10396–10401. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Zhou Y-F, Zhang C-Z, et al. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. U. S. A 2009;106(23):9226–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bienkowska J, Cruz M, Atiemo A, Handin R, Liddington R. The von willebrand factor A3 domain does not contain a metal ion-dependent adhesion site motif. J.Biol.Chem 1997;272:25162–25167. [DOI] [PubMed] [Google Scholar]
- 24.Mei B, Pan C, Jiang H, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood. 2010;116(2):270–9. [DOI] [PubMed] [Google Scholar]
- 25.Preston RJS, Rawley O, Gleeson EM, O’Donnell JS. Elucidating the role of carbohydrate determinants in regulating hemostasis: insights and opportunities. Blood. 2013;121(19):3801–10. [DOI] [PubMed] [Google Scholar]
- 26.Casonato A, Gallinaro L, Cattini MG, et al. Reduced survival of type 2B von Willebrand factor, irrespective of large multimer representation or thrombocytopenia. Haematologica. 2010;95:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Schooten CJM, Denis CV, Lisman T, et al. Variations in glycosylation of von Willebrand factor with O-linked sialylated T antigen are associated with its plasma levels. Blood. 2007;109(6):2430–7. [DOI] [PubMed] [Google Scholar]
- 28.Castaman G, Lethagen S, Federici AB, et al. Response to desmopressin is influenced by the genotype and phenotype in type 1 von Willebrand disease (VWD): results from the European Study MCMDM-1VWD. Blood. 2008;111(7):3531–9. [DOI] [PubMed] [Google Scholar]
- 29.Davies JA, Collins PW, Hathaway LS, Bowen DJ. von Willebrand factor: evidence for variable clearance in vivo according to Y/C1584 phenotype and ABO blood group. J. Thromb. Haemost. JTH 2007;6(1):97–103. [DOI] [PubMed] [Google Scholar]
- 30.Luken BM, Winn LYN, Emsley J, Lane DA, Crawley JTB. The importance of vicinal cysteines, C1669 and C1670, for von Willebrand factor A2 domain function. Blood. 2010;115(23):4910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turecek PL, Scheiflinger F, Siekmann J, et al. Biochemical and Functional Characterization of PEGylated rVWF. Blood. 2006;108(11):1021–1021.16569765 [Google Scholar]
- 32.Eikenboom J, Federici AB, Dirven RJ, et al. VWF propeptide and ratios between VWF, VWF propeptide, and FVIII in the characterization of type 1 von Willebrand disease. Blood. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath RT, van den Biggelaar M, Byrne B, et al. Altered glycosylation of platelet-derived von Willebrand factor confers resistance to ADAMTS13 proteolysis. Blood. 2013;122(25):4107–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


