To the Editor,
The global overload that health systems are undergoing since the start of the COVID‐19 pandemic has forced hospitals to explore sustainable alternatives to treat vulnerable patients that require closer monitoring and higher use of resources, such as Kidney Transplant Recipients (KTRs). 1 , 2 The use of telemedicine and hospital‐like infrastructures represent a valid option for most patients with mild‐moderate COVID‐19, as well as for patients in the recovery phase who cannot be discharged from hospital. 3 , 4 Herein, we present our experience with KTRs infected by SARS‐CoV‐2 in the Hotel Salut (Health Hotel, HH), which was set‐up within 2.5 km from the Hospital on March 25, 2020, coinciding with the main COVID‐19 outbreak in Spain. At full capacity, the HH could accommodate up to 300 patients across 6 floors of 50 single‐rooms each floor. The HH was equipped with both human and material resources from the Hospital Clínic of Barcelona, including 24‐hour medical and nurse attention, availability of high‐flux oxygen, a pharmacy and the same IT equipment.
By the end of May, 45 KTRs who were followed‐up at our center developed COVID‐19, of which 28 were hospitalized at the Hospital Clínic. Twelve patients were transferred to the HH according to the following criteria: (a) >6 days from symptoms onset, (b) temperature below 37.3°C, iii) Respiratory rate < 22 per minute and FiO2 < 0.35, iv) C‐Reactive Protein < 5 mg/dL or descending, LDH < 240 UI/L or descending, lymphocytes > 1000/mm3 or increasing, and v)without radiological progression. Baseline characteristics and treatment are highlighted in Table 1 and are described as median [interquartile range], frequencies, and percentages. Differences were explored the with Mann–Whitney test or Fisher's exact test with SPSS 25.0 (SPSS Inc). The study has been approved by the local Ethical Committee (code HCB/2020/0641). The treatment protocol used in the HH was the same as the one carried out in the Hospital, and already described by our group. 5 Mycophenolate and/or mTOR inhibitors were discontinued in all patients. Calcineurin inhibitors were also suspended in case lopinavir/ritonavir was prescribed. KTRs were transferred to HH after 8.0 [4.25‐13.50] days of hospitalization; at that stage none of them had fever and 20% were still needing oxygen. Hospital stay was significantly shorter for patients treated at HH than for those discharged directly from the hospital (12.50 [8.25‐19.50] days, P = .001). Median stay at the HH was 9.50 [6.50‐12.50] days, and only one patient was readmitted to the Hospital for respiratory deterioration 3 days after HH admission, being discharged from the hospital 9 days afterward. Evolution of clinical parameters reflected progressive recovery after infection (Figure 1). It should be noted that stay at HH also allowed the gradual reintroduction of immunosuppression despite the challenging interactions between calcineurin inhibitors (CNIs) and the antiviral agents. 6 , 7 Therefore, tacrolimus was restarted 9 [8‐ 11] days after withdrawal, with trough levels of 4.85 [3.92‐5.55]ng/mL at the time of HH discharge. The rest of immunosuppressant drugs were introduced gradually afterward, tapering the steroids simultaneously.
Table 1.
Baseline characteristics and treatment of KTRs total population. Comparison between KTRs who were transferred to the Hotel Salut (Health Hotel, HH) and those who were discharged directly from the Hospital
|
Total population (n = 28) |
Transferred to HH (n = 12) |
Discharged from the Hospital (n = 16) |
P‐value | |
|---|---|---|---|---|
| Age | 52.50 [46.25‐68] | 48.50 [43.75‐57.25] | 58 [47.25‐72.75] | .110 |
| Sex (% males) | 18/28 (64.3%) | 7/12 (58.3%) | 11/16 (68.8%) | .698 |
| Time from transplant | 56.46 [22.01‐125‐45] | 42.56 [12.21‐74.75] | 65.15 [26.11‐134.92] | .423 |
| Baseline immunosuppression | ||||
| TAC + MPA | 14/28 (50.0%) | 5/12 (41.7%) | 9/16 (56.3%) | .240 |
| TAC + mTORi | 9/28 (32.1%) | 6/12 (50.0%) | 3/16 (18.8%) | |
| Other | 5/28 (17.9%) | 1/12 (8.3%) | 4/16 (25.0%) | |
| Creatinine at baseline (mg/dL) | 1.55 [1.15‐2.18] | 1.93 [1.44‐2.54] | 1.29 [1.13‐2.10] | .093 |
| Positive PCR swab (%yes) | 23/28 (82.1%) | 9/12 (75.0%) | 14/16 (87.5%) | .624 |
| Symptoms (%yes) | ||||
| Fever | 26/28 (92.9%) | 10/12 (83.3%) | 16/16 (100.0%) | .175 |
| Cough | 18/28 (64.3%) | 9/12 (75.0%) | 9/16 (56.3%) | .434 |
| Dyspnea | 9/28 (32.1%) | 2/12 (16.7%) | 7/16(43.8%) | .223 |
| Gastrointestinal | 7/28 (25.0%) | 2/12 (16.7%) | 5/16 (31.3%) | .662 |
| Dysgeusia | 3/28 (10.7%) | 1/12 (8.3%) | 2/16 (12.5%) | 1 |
| Pneumonia | 25/28(95.3%) | 9/12 (75.0%) | 16/16 (100.0%) | .067 |
| AKI | 19/28 (67.9%) | 9/12 (75.0%) | 10/16 (62.5%) | .687 |
| Need of dialysis | 3/28 (10.7%) | 0/12 (0.0%) | 3/16 (18.8%) | .238 |
| Treatment | ||||
| Lopinavir/Ritonavir | 24/28 (85.7%) | 9/12 (75.0%) | 15/16 (93.8%) | .285 |
| Hydroxicloroquine | 27/28 (96.4%) | 12/12 (100.0%) | 15/16 (93.8%) | 1 |
| Azithromycin | 27/28 (96.4%) | 11/12 (91.7%) | 16/16 (100.0%) | .429 |
| Tocilizumab | 18/28 (64.3%) | 6/12 (50.0%) | 12/16 (75.0%) | .243 |
| Steroids (bolus) | 8/28 (28.6%) | 3/12 (25.0%) | 5/16 (31.3%) | 1 |
| ICU Admission | 8/28 (28.6%) | 3/12 (25.0%) | 5/16 (31.3%) | 1 |
| Death | 5/28 (17.9%) | 0/12 (0.0%) | 5/16 (31.3%) | .053 |
| Length of stay | ||||
| At the Hospital | 12.50 [8.25‐19.50] | 8 [4.25‐13.50] | 15.50 [12‐25.50] | .001 |
| At the Hotel | / | 9.50 [6.50‐12.50] | / | |
| Total | 18 [13‐24] | 19.00 [16.25‐24] | 15.50 [12‐25.50] | .631 |
Figure 1.
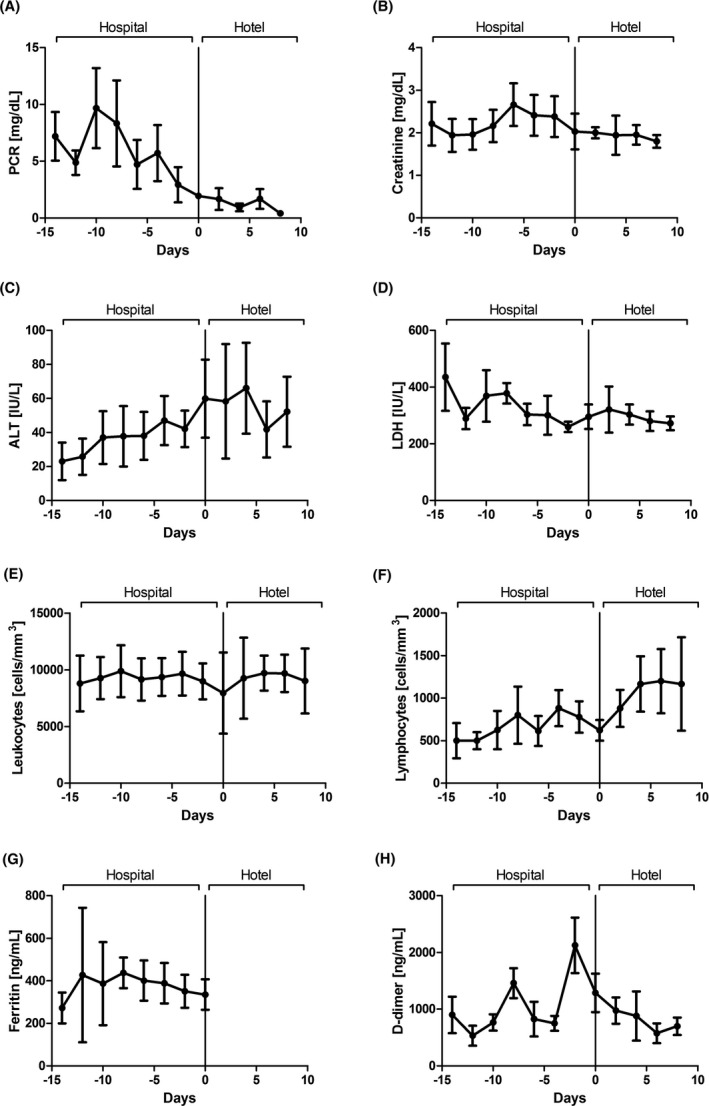
Evolution of COVID‐19‐related laboratory parameters before and after HH admission
In conclusion, although our study was conducted among a small proportion of all the COVID‐19 infected KTRs, treating them at a medicalized hotel facility allowed us to monitor their progress closely, thus obtaining positive clinical outcomes as well as the ability to safely reintroduce immunosuppression.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Clinical Transplantation.
ACKNOWLEDGMENTS
We would like to thank Catalonia Hotels & Resorts and all the Members of the H4H team: Andrea Arenas, Aleix Agelet, Pol Maymó, Eugenia Butori, Carmen Aranda, Marta Sala, Ana Fernández, Cristina Escobar, Laura Moreno, Adolfo Suarez, Susana Cano, Maribel Avalos, Anna Carbonell, Regina Garcia, Nuria Subirana, Jose Vicente Picón, Magali Rodriguez, Maria Martinez, Alba Martinez, Elisabeth Rosero, Maria Asenjo (Hospital at Home Unit, Medical and Nurse Direction); Almudena Sánchez, Aida Alejaldre, Sara Llufriu, Daniela Lopera, Patricia Buendia, Guadalupe Fernandez, Maria Navarro (Neurology Service, Institut Clinic de Neurociències); Miguel Ángel Torrente, Andrea Rivero, Marta Cervera, Desiré Vigo Conde, Alberto Fernández (Hematology Service, Institut Clinic d'Hematologia i Oncologia); Francis Espósito (Oncology Service, Institut Clinic d'Hematologia i Oncologia); Daniela Barreto (Radiation Oncology Service, Institut Clinic d'Hematologia I Oncologia); Agustí Toll, Daniel Morgado Josep Riera, Constanza Riquelme, Andrea Combalía (Dermatology Service, Institut de Medicina i Dermatologia); Ramón Estruch, Joaquim Fernàndez‐Solà, Marta Farré (Internal Medicine Service, Institut Clínic de Medicina i Dermatologia); Elena Guillén, Ana Santamaria, Lidia Gomez, Mònica Sorroche (Nephrology Service, Institut Clinic de Nefrologia i Urologia); Monica Peradejordi, Alberto Tello, Juan M López, Antonio Alcaraz (Urology Service, Institut Clinic de Nefrologia i Urologia); Roberto Gumucio, Belén Massó (Reumathology Service, Institut Clinic d'Especialitats Médico‐Quirùrgiques), Carolina Montoya (Traumatology and Orthopedics Service, Institut Clinic d'Especialitats Mèdico‐Quirúrgiques), Josep Miranda, Elena Salas, Carlos Garcia, (AGC); Gemma Martinez, Antoni Castells (Nursing and Medical Direction); Laura Perelló, Raquel Crespo, Ariadna Patricia Mejía (CDI); Roser Cadena, Maria Galisteo (DIR.Qualitat); Natalia Charines, Mª Carmen Hernández, Julia Prieto, Laia Sarto, Marta Jimenez, Maria Jesús Sánchez (ICGON); Immaculada Sebastián, Silvia Vidorreta (CDB); Anna Campreciós, Olga Hernando, Carmen Tares (A.QUIR); Ana Mancebo (ICMDM); Gemma Mercade (ICOF); Darwin Barboza, Emilia Abad (ICR); Anna Planell (CDB); Ana Labarta, Jaume Gas, Andrea Ocaña, and Eva Martinez (CAPSBE); all from Hospital Clínic de Barcelona, Barcelona, Spain.
Cucchiari D, Guillen E, Cofan F, et al; the Hospital Clínic 4H Team (Hospital at Home‐Health Hotel) . Taking care of kidney transplant recipients during the COVID‐19 pandemic: Experience from a medicalized hotel. Clin Transplant.2021;35:e14132. 10.1111/ctr.14132
Juan M Pericàs and David Nicolás Contributed equally.
Members of the Hospital Clínic 4H Team are listed in the Acknowledgements.
Contributor Information
David Cucchiari, Email: cucchiari@clinic.cat.
Fritz Diekmann, Email: fdiekman@clinic.cat.
the Hospital Clínic 4H Team (Hospital at Home‐Health Hotel):
Andrea Arenas, Aleix Agelet, Pol Maymó, Eugenia Butori, Carmen Aranda, Marta Sala, Ana Fernández, Cristina Escobar, Laura Moreno, Adolfo Suarez, Susana Cano, Maribel Avalos, Anna Carbonell, Regina Garcia, Nuria Subirana, Jose Vicente Picón, Magali Rodriguez, Maria Martinez, Alba Martinez, Elisabeth Rosero, Maria Asenjo, Almudena Sánchez, Aida Alejaldre, Sara Llufriu, Daniela Lopera, Patricia Buendia, Guadalupe Fernandez, Maria Navarro, Miguel Ángel Torrente, Andrea Rivero, Marta Cervera, Desiré Vigo Conde, Alberto Fernández, Francis Espósito, Daniela Barreto, Agustí Toll, Daniel Morgado, Josep Riera, Constanza Riquelme, Andrea Combalía, Ramón Estruch, Joaquim Fernàndez‐Solà, Marta Farré, Elena Guillén, Ana Santamaria, Lidia Gomez, Mònica Sorroche, Monica Peradejordi, Alberto Tello, Juan M López, Antonio Alcaraz, Roberto Gumucio, Belén Massó, Carolina Montoya, Josep Miranda, Elena Salas, Carlos Garcia, Gemma Martinez, Antoni Castells, Laura Perelló, Raquel Crespo, Ariadna Patricia Mejía, Roser Cadena, Maria Galisteo, Natalia Charines, Mª Carmen Hernández, Julia Prieto, Laia Sarto, Marta Jimenez, Maria Jesús Sánchez, Immaculada Sebastián, Silvia Vidorreta, Anna Campreciós, Olga Hernando, Carmen Tares, Ana Mancebo, Gemma Mercade, Darwin Barboza, Emilia Abad, Anna Planell, Ana Labarta, Jaume Gas, Andrea Ocaña, and Eva Martinez
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Cravedi P, Suraj SM, Azzi Y, et al. COVID‐19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020.20(11):3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hollander JE, Carr BG. Virtually Perfect? Telemedicine for Covid‐19. N Engl J Med. 2020;382(18):1679‐1681. [DOI] [PubMed] [Google Scholar]
- 4. Abuzeineh M, Muzaale AD, Crews DC, et al. Telemedicine in the Care of Kidney Transplant Recipients With Coronavirus Disease 2019: Case Reports. Transplant Proc. 2020;52(9):2620–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montagud‐Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS‐CoV‐2 infection in a Spanish single center cohort of kidney recipients. Am J Transplant. 2020;20(10):2958‐2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartiromo M, Borchi B, Botta A, et al. Threatening drug‐drug interaction in a kidney transplant patient with coronavirus disease 2019 (COVID‐19). Transpl Infect Dis. 2020;22(4):e13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López V, Vázquez T, Alonso‐Titos J, et al. Recommendations on management of the SARS‐CoV‐2 coronavirus pandemic (Covid‐19) in kidney transplant patients. Nefrologia. 2020;40(3):265‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


