Abstract
The essential scope of the coronavirus infectious disease 2019 (COVID-19) pandemic is focused on developing effective treatments and vaccines for acute SARS-CoV-2 infection. There is also a critical need to develop interventions to prevent the complications of COVID-19, which occur with an alarming frequency in older adults. Since severe pathologic effects of infection occur with increasing age, COVID-19 falls under the geroscience concept that all diseases in older adults have a common and major underlying cause of declining function and resilience. Geroscience posits that manipulation of aging will simultaneously delay the appearance or severity of major diseases because they share the same risk factor: aging and the multiple processes involved in aging. Drug combinations that target multiple aging processes and the cytokine networks associated with them would not necessarily limit SARS-CoV-2 infection rates but would prevent severe pathologic consequences of the disease in older adults by maintaining a more youthful-like resilience to infection-related complications. A drug cocktail aimed at controlling cytokine actions would complement current clinical treatments and vaccine effectiveness for COVID-19 and serve as a prototype for future age-related infectious disease pandemics wherein the elderly population is especially vulnerable.
Keywords: COVID-19 complications, cytokines, geroscience, interactive cytokines, antiaging drug cocktail, aging resilience
COVID-19 Is a Geroscience Disease
The immediate problem of the coronavirus infectious disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 is that hundreds of thousands of people develop a range of symptoms. SARS-CoV-2 infection initiates through person-to-person respiratory spread and lung infection but then can spread to infect other tissues and organs, leading to systemic pathology engaging the immune system locally in the respiratory tract and at other sites throughout the body (Wadman and others 2020). People >60 years of age exhibit poor outcome of SARS-CoV-2 infection with dramatically enhanced COVID-19 symptoms compared with younger age groups (Nikolich-Zugich and others 2020). Studies to examine the biological aging processes that increase the risk of developing severe COVID-19 and mortality are essential for identifying interventions against several outcomes in aged people. Details on the pathology of SARS-CoV-2 infection in older people who have died are becoming more apparent, showing systemic involvement of infection. Importantly, the basic biology of aging underlies the functional performance of all organs in the body, wherein the field of geroscience aims to understand, at the cellular and molecular level, the interconnections between aging and disease, with a focus on understanding the mechanisms by which aging contributes to disease as a major risk factor (Sierra and Kohanski 2017). The geroscience concept posits that manipulation of aging will simultaneously delay the appearance or severity of major diseases because they share the same underlying major risk factor: aging and the multiple processes involved in aging (Kennedy and others 2014; Wahl and others 2019).
The geroscience approach assumes that all diseases that affect primarily older adults have a common and major underlying cause of declining function and resilience that is part of the multifactorial process associated with aging, including production of proinflammatory cytokines, persistent and systemic inflammation, and immune senescence (Sadighi Akha 2018; Furman and others 2019). This relationship has been established for chronic diseases, and is now a reality for infectious diseases, since decreased resilience to pathologic effects of infection occurs with increasing age (Sierra 2020). Currently in the United States, ∼80% of COVID-19 deaths have occurred in patients 65 years of age and older, and fatal outcomes are even more severe in patients >85 years (Garg and others 2020).
COVID-19 Disease Processes Are Aging Processes
Recent progress in the field of aging biology has allowed researchers to develop robust behavioral, genetic, and pharmacologic approaches to expand the lifespan of multiple species. Importantly, interventions that extend lifespan often result in improvements in multiple aspects of healthy aging, resulting in significant delays in the appearance of pathology and frailty (Ladiges and Liggitt 2017). The geroscience approach posits that to prevent age-related pathology associated with COVID-19 in the elderly, molecular and cellular processes of aging must be targeted. But how do we target aging processes, and what processes should we focus on since aging is complex and multifactorial in nature (Ladiges 2014)? We must first understand how processes of aging increase the susceptibility of older individuals to severe complications of COVID-19. Over the years, numerous aging processes have been identified and substantiated. We have selected 5 to discuss in this overview based on their prominent role in advancing age. They include inflammation and senescence/cytokine imbalance, autophagy impairment, insulin resistance, epigenetic dysfunction, and vascular impairment.
Inflammation is a hallmark of aging and generally refers to a low-grade chronic systemic condition in the absence of overt infection that is considered to be a significant risk factor for morbidity and mortality in the elderly (Franceschi and Campisi 2014). Senescent cells with its senescence-associated secretory phenotype are thought to perpetuate aging and age-related diseases as they release proinflammatory cytokines, chemokines (Schafer and others 2017), and tissue damaging proteases that negatively affect the surrounding tissue microenvironment (Kirkland and Tchkonia 2017). Prolonged inflammation and accumulation of senescence cells have been shown to contribute to age-related diseases such as cardiovascular disease and pulmonary fibrosis that provide a pathologic background for the development of severe COVID-19 complications (McHugh and Gil 2018; Garg and others 2020). The inflammatory state observed in the elderly might also contribute to a “cytokine storm” seen in older patients with COVID-19, which incites severe lung pathology (Jose and Manuel 2020). Therefore, reducing senescent cells and chronic inflammation in the elderly might help reduce the risk of COVID-19 pneumonia and other systemic complications.
Autophagy involves the degradation and recycling of damaged or aggregated proteins, lipids, and other cellular components such as organelles (Taylor and Dillin 2011). Damaged proteins accumulate with increasing age in association with endoplasmic reticulum (ER) stress and the promotion of the unfolded protein response, leading to impaired autophagy and the development of various underlying pathologies (Levine and Kroemer 2008; Garg and others 2020). Chemical chaperones that target the ER can enhance healthy aging in model organisms (Rubinsztein David and others 2011; Abdellatif and others 2018) by increasing efficiency of protein folding and decreasing ER stress, resulting in a more youthful autophagic response (Kolb and others 2015). Another key regulator of autophagy is the mammalian target of rapamycin (mTOR) pathway. The inhibition of mTOR leads to enhanced autophagy by promoting proteasome-mediated turnover and proteostasis (Johnson and others 2013). Therefore, targeting autophagy through the ER stress and mTOR pathways could promote cellular homeostasis and decrease underlying pathology and subsequent risk for COVID-19 complications.
Glucose homeostasis is essential in maintaining health with increasing age. Chronic overstimulation of insulin with increasing intake of glucose is associated with insulin resistance, defined as a subnormal response to a given dose of insulin. The detrimental effects of insulin resistance include obesity and metabolic dysfunction, resulting in increased oxidative stress and leading to hypertension, chronic inflammation, and type 2 diabetes, all of which increase the risk for COVID-19 complications (Verdile and others 2015; Jiang and others 2016; Garg and others 2020). Interventions that maintain the homeostasis of insulin signaling and glucose metabolism have been shown to prevent or even reverse the defects associated with systemic aging (Anderson and Weindruch 2012; Harrison and others 2014). As a result, treatments that restrict glucose intake could maintain glucose and insulin homeostasis, thereby reducing risk of developing COVID-19 comorbidities (Novelle and others 2016; Gibbs and others 2017).
Aging has long thought to be an irreversible road toward physiologic decline. However, it is remarkably plastic allowing the possibility of targeting nongenetic regulation. Specifically, aging is associated with profound epigenetic changes, resulting in alterations of gene expression and disturbances in broad genome architecture and the epigenomic landscape (Brunet and Berger 2014). In contrast to DNA mutations, epigenetic alterations represent reversible changes, offering the potential for a “rejuvenating” therapeutic effect. Of the various epigenetic alterations occurring with age, the influence of histone acetylation, a process balanced by the activity of histone acetyltransferases and histone deacetylases (HDACs) on lifespan regulation, has been the most characterized (McIntyre and others 2019). Since HDACs are associated with numerous hallmarks of aging and age-related disease, HDAC inhibitors offer a novel therapeutic strategy (Hull and others 2016; McIntyre and others 2019). HDAC inhibitors promote the expression of genes associated with an overall slowing of cell growth and proliferation associated with healthy aging (Pasyukova and Vaiserman 2017), so they could increase resilience to COVID-19 disease-related complications.
Vascular dysfunction and the progression of vascular disease contribute to age-related conditions such as heart disease (Donato and others 2018), which increases the risk of severe cardiac pathology with SARS-CoV-2 infection (Garg and others 2020). Studies have shown that an increased burden of vascular pathology correlates with other systemic age-related conditions such as cognitive impairment and dementia (Gorelick and others 2011). With vascular breakdown, there is leakage of fibrinogen into the parenchyma, resulting in formation of clots (Ryu and others 2015). Replenishing or rejuvenating the aged/dysfunctional vascular cells is critical to effective repair. A major weapon of endothelial cells to fight vascular pathology is endothelial nitric oxide synthase (eNOS), an enzyme that generates the vascular protective molecule nitric oxide (NO). Inhibition of mTOR has been shown to be effective in correcting vascular pathology associated with aging by activating eNOS and increasing secretion of NO, which allows dilation of blood vessels to increase blood circulation (Galvan and Hart 2016). Therefore, promoting healthy vascular function by targeting mTOR could promote healthy aging and reduce the risk of developing various age-related comorbidities and COVID-19 pathologies.
These processes of aging are all interrelated, and interaction is most likely mediated by dysregulated cytokine networks. For example, autophagy has important effects in the innate immune response in aging and age-related diseases by influencing cytokine secretion, antigen presentation, and lymphocyte function (Cuervo and Macian, 2014). Under youthful circumstances, autophagy activity is upregulated so that the removal of immune mediators is expedited (Shi and others 2012). However, the autophagy response becomes blunted with increasing age, so that immune mediators remain active and prolong an inflammatory response (Zhong and others 2016). Emerging data have revealed that nuclear factor kappa-b signaling, activated by events such as oxidative stress, serves as the major innate immune signaling pathway that can initiate senescence and stimulate production of senescence-associated secretory proteins (Salminen and others 2012). An imbalance between generation of reactive oxygen species and antioxidant defense systems represents the primary cause of endothelial dysfunction leading to vascular damage. A final example of dysfunctional cytokine interaction with increasing age is the epigenetic regulation of cytokine secretion. For example, it has been reported that HDAC activity, which increases with increasing age, suppresses macrophage plasticity, favoring a proinflammatory phenotype (Cabanel and others 2015). Therefore, it is apparent that a loosely defined network of cytokines linking with “aging pathways” can play a major role in mediating and perpetuating aging, and in the process can provide an ideal environment for SARS-CoV-2 to inflict severe and lethal pathology.
Antiaging Cocktails Can Be Designed to Prevent COVID-19 Complications
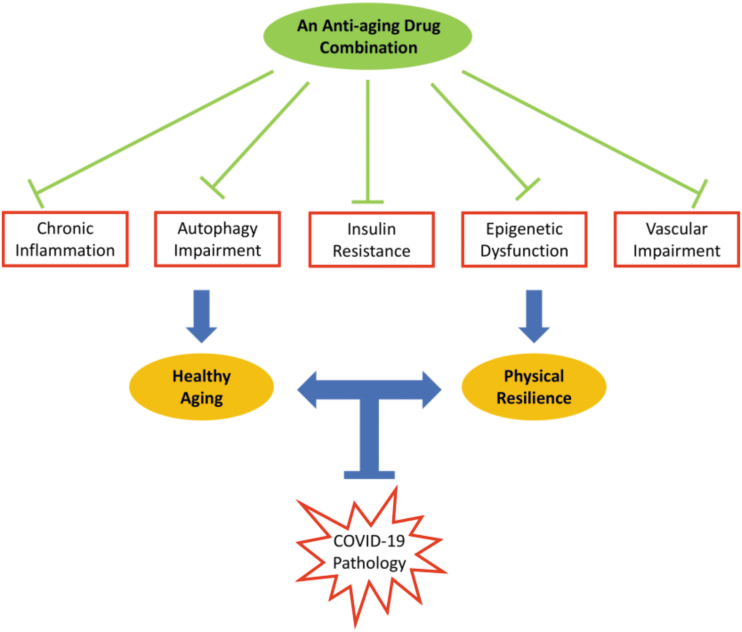
Aging processes already discussed are an example of what fit within the geroscience concept that stipulates there is a concordance of aging and COVID-19 pathologic complications in the elderly. The geroscience approach is to target these processes simultaneously with multiple drugs that each individually target 1 or more of the aging processes (Fig. 1). The drug “cocktail” would have to be designed so that each drug would complement one another and robustly enhance a delay of aging and age-related disease not achievable with monotherapeutic approaches (Sharma and others 2019). The cocktail would need to prevent decline in age-related organ functionality to increase resilience to COVID-19 complications. Although this approach by itself would not necessarily limit SARS-CoV-2 infection rates, it would protect older adults against the more severe pathologic consequences of the disease by maintaining a more youthful-like homeostasis against declining health with increasing age. A drug cocktail would provide a sustainable geroscience approach that would complement current clinical treatments and vaccine effectiveness for COVID-19 disease and serve as a prototype for future age-related infectious disease pandemics wherein the elderly population is especially vulnerable.
FIG. 1.
A drug combination with antiaging properties that targets multiple aging processes will enhance resilience to aging to prevent pathologic consequences of COVID-19 in the elderly. COVID-19, coronavirus infectious disease 2019.
Conclusions
Severe disease complications of COVID-19 are age related. Since geroscience is based on the concept that manipulation of aging will simultaneously delay the severity of age-related disease, then aging processes must be involved in the pathogenesis. Aging is multifactorial, so combinations of drugs that target multiple aging processes would be more effective than individual drugs. Such a drug cocktail would not necessarily limit SARS-CoV-2 infection rates but would prevent severe pathologic consequences of the disease in older adults by maintaining a more youthful-like resilience to infection-related complications. Antiaging drug cocktails could be designed to be preventive or therapeutic to complement vaccine effectiveness or clinical treatments for COVID-19. In addition, the geroscience concept could serve as a prototype for the next age-related infectious disease pandemic wherein the older adult population is especially vulnerable.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the National Institute of Allergy and Infectious Diseases: UM1 AI148684, Uo1 AI151698, R01 AI127463 and the National Institute on Aging: R01 057381, R01 067193, U19 AG017221.
References
- Abdellatif M, Sedej S, Madeo F, Kroemer G. 2018. Cardioprotective effects of autophagy induction in sepsis. Ann Transl Med Suppl 1:61–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. 2012. The caloric restriction paradigm: Implications for healthy human aging. Am J Hum Biol 24(2):101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Berger SL. 2014. Epigenetics of aging and aging-related disease. J Gerontol Ser A 69(Suppl. 1):S17–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanel M, Brand C, Oliveira-Nunes MC, Cabral-Piccin MP, Lopes MF, Brito JM, de Oliveira FL, El-Cheikh MC, Carneiro K. 2015. Epigenetic control of macrophage shape transition toward an atypical elongated phenotype by histone deacetylase activity. PLoS One 10(7):e0132984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Macian F. 2014. Autophagy and the immune function in aging. Curr Opin Immunol 29:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Machin DR, Lesniewski LA. 2018. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 123(7):825–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A 69(Suppl. 1):S4–S9 [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. 2019. Chronic inflammation in the etiology of disease across the life span. Nat Med 25(12):1822–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan V, Hart MJ. 2016. Vascular mTOR-dependent mechanisms linking the control of aging to Alzheimer's disease. Biochim Biophys Acta 1862(5):992–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. 2020. Hospitalization rates and characteristics of patients hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. 69(15):458–464 [DOI] [PMC free article] [PubMed]
- Gibbs VK, Brewer RA, Miyasaki ND, Patki A, Smith DL Jr. 2017. Sex-dependent differences in liver and gut metabolomic profiles with acarbose and calorie restriction in C57BL/6 mice. J Gerontol Ser A 73(2):157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. 2011. Vascular contributions to cognitive impairment and dementia. Stroke 42(9):2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. 2014. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13(2):273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EE, Montgomery MR, Leyva KJ. 2016. HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. BioMed Res Int 2016:8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S-Z, Lu W, Zong X-F, Ruan H-Y, Liu Y. 2016. Obesity and hypertension. Exp Ther Med 12(4):2395–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose RJ, Manuel A. 2020. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 8:E46–E47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. 2014. Geroscience: linking aging to chronic disease. Cell 159(4):709–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. 2017. Cellular senescence: a translational perspective. EBioMedicine 21:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. 2015. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol 61:45–52 [DOI] [PubMed] [Google Scholar]
- Ladiges W. 2014. The quality control theory of aging. Pathobiol Aging Age Related Dis 4:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladiges W, Liggitt D. 2017. Testing drug combinations to slow aging. Pathobiol Aging Age Related Dis 8(1):1407203–1407203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132(1):27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Gil J. 2018. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 217(1):65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RL, Daniels EG, Molenaars M, Houtkooper RH, Janssens GE. 2019. From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs. EMBO Mol Med 11(9):e9854–e9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. 2020. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience 42(2):505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelle MG, Ali A, Diéguez C, Bernier M, de Cabo R. 2016. Metformin: a hopeful promise in aging research. Cold Spring Harb Perspect Med 6(3):a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasyukova EG, Vaiserman AM. 2017. HDAC inhibitors: A new promising drug class in anti-aging research. Mech Ageing Dev 166:6–15 [DOI] [PubMed] [Google Scholar]
- Rubinsztein David C, Mariño G, Kroemer G. 2011. Autophagy and aging. Cell 146(5):682–695 [DOI] [PubMed] [Google Scholar]
- Ryu JK, Petersen MA, Murray SG, Baeten KM, Meyer-Franke A, Chan JP, Vagena E, Bedard C, Machado MR, Coronado PER, Prod'homme T, Charo IF, Lassmann H, Degen JL, Zamvil SS, Akassoglou K. 2015. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun 6(1):8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha AA. 2018. Aging and the immune system: an overview. J Immunol Methods 463:21–26 [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Kaarniranta K. 2012. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24(4):835–845 [DOI] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. 2017. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8:14532–14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Wang J, Jiang Z, Klug J, Darvas M, Imai D, Snider T, Niedernhofer L, Ladiges W. 2019. The rationale for testing drug combinations in aging intervention studies. Aging Pathobiol Therap 1:1–4 [Google Scholar]
- Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. 2012. Activation of autophagy by inflammatory signals limits IL-1a production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13(3):255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F. 2020. Geroscience and the coronavirus pandemic: the whack-a-mole approach is not enough. J Am Geriatr Soc 68(5):951–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Kohanski R. 2017. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. GeroScience 39(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Dillin A. 2011. Aging as an event of proteostasis collapse. Cold Spring Harbor Perspect Biol 3(5):a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P. 2015. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer's disease. Mediators Inflamm 2015:105828–105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. 2020. A rampage through the body. Science 368(6489):356–360 [DOI] [PubMed] [Google Scholar]
- Wahl D, Solon-Biet SM, Cogger VC, Fontana L, Simpson SJ, Le Couteur DG, Ribeiro RV. 2019. Aging, lifestyle and dementia. Neurobiol Dis 130:104481. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR,McGeough MG, Ellisman MH, Seki E, Gustafsson AB, Hoffman HM, Diaz-Meco MT, Moscat J, Karin M. 2016. NF-κB restricts inflammasome activation via elimination of dam-aged mitochondria. Cell 164(5):896–910 [DOI] [PMC free article] [PubMed] [Google Scholar]