Abstract
Background
COVID‐19 patients are considered at high risk of venous thromboembolism (VTE). The real nature of pulmonary artery occlusions (PAO) in COVID‐19 has been questioned, suggesting that it is caused also by in situ thrombi, rather than only by emboli (PE) from peripheral thrombi.
Methods
We searched MEDLINE for studies published until 6 June 2020 that included COVID‐19 patients or non‐COVID‐19 medical patients at VTE risk, treated with heparins, in whom VTE (PE and deep vein thrombosis, DVT) had been reported. Systematic review and results reporting were conducted in accordance with PRISMA guidelines. Data were independently extracted by two observers, and estimates were pooled using random‐effects meta‐analysis.
Results
We identified 17 studies including 3224 COVID‐19 patients and 7 including 11 985 non‐COVID‐19 patients. Two analyses were performed: in all COVID‐19 patients and only in those (n = 515) who, like non‐COVID‐19 patients, were screened systematically for DVT. The latter analysis revealed that the prevalence of DVT was 15.43% (95%CI, 4.08‐31.77) in COVID‐19 and 4.21% (2.27‐6.68) in non‐COVID‐19 patients (P = .0482). The prevalence of PE was 4.85% (40.33‐13.01) in COVID‐19 patients and 0.22% (0.03‐0.55) in non‐COVID‐19 patients (P = .0128). The percentage of PE among VTE events was 22.15% (5.31‐44.60) in COVID‐19 and 6.39% (3.17‐10.41) in non‐COVID‐19 patients (P = .0482). Differences were even more marked when all COVID‐19 patients were analysed.
Conclusions
The results of our meta‐analysis highlight a disproportion in the prevalence of PE among all VTE events in COVID 19 patients, likely reflecting PAO by pulmonary thrombi, rather than emboli from peripheral vein thrombi.
Keywords: COVID‐19, embolism and thrombosis, low‐molecular‐weight heparin, SARS coronavirus
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) is an acute disorder that is associated with SARS‐CoV‐2 infection, which, in its most severe presentation, is characterized by the development of interstitial pneumonia, acute respiratory distress syndrome (ARDS) and multiorgan failure. 1 , 2 , 3 As in other acute medical conditions, 4 , 5 COVID‐19 is associated with risk for venous thromboembolism (VTE), the severity of which is difficult to determine, due to the wide variation in the reported rates in different studies likely due to differences in clinical practice, diagnostic strategies, patient populations and, perhaps most importantly, the small number of enrolled patients. 6 , 7 , 8 , 9 Despite this uncertainty, the general perception is that the VTE risk is particularly high in COVID‐19 and, as a consequence, hospitalized COVID‐19 patients are often treated with higher doses of low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH) 6 , 10 than recommended for thromboprophylaxis. 4 , 5
The analysis of some reports on VTE in COVID‐19 revealed that the prevalence of pulmonary embolism (PE) exceeded that of deep vein thrombosis (DVT), leading us to hypothesize that in many instances filling defects of the pulmonary arteries detected by CT‐angiography scans could be attributable to in situ thrombosis, rather than to thrombo‐emboli originating from the peripheral venous circulation. 6 Our hypothesis was later corroborated by a study of a small series of patients with severe COVID‐19 in a tertiary care unit, which was based on accurate analysis of angio‐CT scans. 11 In addition, we postulated that, given the potential pathogenic difference between PE and pulmonary thrombi, likely caused by thrombo‐inflammation triggered by viral infection and the subsequent cytokine storm, the use of higher than recommended LMWH doses for thromboprophylaxis in COVID‐19 patients may not be justified. 6
In consideration of the uncertainties on the incidence of DVT and PE (or, perhaps better said based on our hypothesis, pulmonary artery occlusion, PAO), we elected to meta‐analyse the reported data in retrievable studies, published until 6 June 2020. Selected studies had to have enrolled patients on thromboprophylaxis with LMWH or UFH. The incidences of PE and DVT in COVID‐19 patients were compared with those reported in randomized clinical trials (RCT) of critically ill medical patients treated with low, prophylactic doses of LMWH. Sub‐analyses were performed of patients hospitalized in Intensive Care Units (ICU) or in non‐ICU wards. Our study allowed us to estimate the incidence of DVT and PE in a relatively high number of COVID‐19 patients, and to estimate the different ratios between the frequencies of PE and DVT in COVID‐19 patients in the different hospital settings, relative to those of similar, acutely ill medical patients without SARS‐Cov‐2 infection.
2. MATERIALS AND METHODS
2.1. Search strategy
We conducted a systematic review of the literature to summarize findings from studies of VTE risk in COVID‐19 and non‐COVID‐19 medical inpatients, who were on treatment with UFH or LMWH for VTE prevention. For COVID‐19 studies, we searched on MEDLINE for studies published until 6 June 2020, using the following keywords: “SARS‐COV‐2” OR “COVID 19” AND (“venous thromboembolism” OR “thrombosis” OR “pulmonary embolism” OR “deep vein thrombosis”). For non‐COVID‐19 studies, we searched on MEDLINE for studies published until June 6th 2020, using the following keywords: “medical illness” OR “medical inpatient” AND (“venous thromboembolism” OR “thrombosis” OR “pulmonary embolism” OR “deep vein thrombosis”). The systematic review and the reporting of results were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines.
2.2. Eligibility criteria
Studies of COVID‐19 were eligible if they included patients with SARS‐CoV‐2 infection, ascertained by polymerase chain reaction of respiratory specimens. Studies of medical non‐COVID‐19 inpatients were eligible if they included patients hospitalized because of other types of acute medical illnesses. Patients had to be hospitalized in ICU or non‐ICU wards and be treated with UFH or LMWH for thromboprophylaxis; PE and DVT had to be reported. Studies of thromboprophylaxis in surgical patients, post‐mortem, commentaries, non‐research letters, reviews, case reports, duplicates and studies not reporting relevant data were excluded from the analysis. In case of redundant publications on the same population, the most recent and complete report was included.
2.3. Study selection
Two independent reviewers (SB, MM) screened the identified records, at first by title and abstract and then by reading the full text to ascertain suitability before data extraction. Discordances between reviewers were straightened by discussion; in case an agreement could not be reached, a third reviewer (GMP) was responsible for the final decision.
2.4. Quality assessment
Two reviewers (SB, MM) assessed the quality of data in included studies according to the Cochrane Collaboration Risk of Bias Tool. 12
2.5. Outcomes
We reported the number of PE and DVT in both COVID‐19 and non‐COVID‐19 hospitalized patients receiving UFH or LMWH for VTE prevention. The primary outcome was the ratio between PE and all VTE events (PE + DVT) in COVID‐19 versus non‐COVID‐19 patients. Considering that all non‐COVID‐19 patients had undergone systematic screening for DVT by compression ultrasonography (CUS), we compared their data not only to those obtained in the total number of COVID‐19 patients, but also to those obtained in COVID‐19 patients who did undergo systematic DVT screening by CUS. Secondary outcomes were the prevalence of DVT and PE. We also separately analysed data from ICU and non‐ICU wards.
2.6. Statistical analysis
We calculated prevalences, expressed as proportions and 95% confidence intervals (CI), for all included studies. The meta‐analyses were conducted using the DerSimonian and Laird random‐effects model. 13 Meta‐analyses were performed after having transformed individual studies proportions, according to the Freeman‐Tukey double arcsine transformation. 14 The estimates obtained from meta‐analyses were back‐transformed and the pooled results expressed as proportions. The I 2 index was used to measure inconsistency among studies, and the presence of statistical heterogeneity was assessed by the chi‐squared test, with statistical significance set at P < .10. The chi‐square test was used to assess differences between ICU and non‐ICU subgroups. All statistical analyses were performed using Stata software (version 16, StataCorp LLC).
3. RESULTS
3.1. Study selection
Our search strategy identified 319 studies of COVID‐19 patients and 288 studies of non‐COVID‐19 patients. A total of 259 and 274 records were excluded after screening through titles and abstracts. Sixty COVID‐19 and 14 non‐COVID‐19 full‐text articles were assessed for eligibility. Of the 60 COVID‐19, 19 were excluded because of missing data on therapy or outcome, 17 because they were reviews or commentaries, 5 were case reports, 2 reported post‐mortem data, leaving 17 studies for qualitative and quantitative synthesis. 11 , 29 Two of them 16 , 18 reported data on ICU and non‐ICU patients separately and were therefore considered separately for quantitative analysis. Of the 14 non‐COVID‐19 studies, 3 were excluded because included patients who were not on UFH or LMWH, 2 did not report data for pre‐specified outcomes, 1 was a case report, 1 reported post‐mortem data, leaving 7 studies for qualitative and quantitative synthesis 30 , 31 , 32 , 33 , 34 , 35 , 36 (Figure 1).
FIGURE 1.
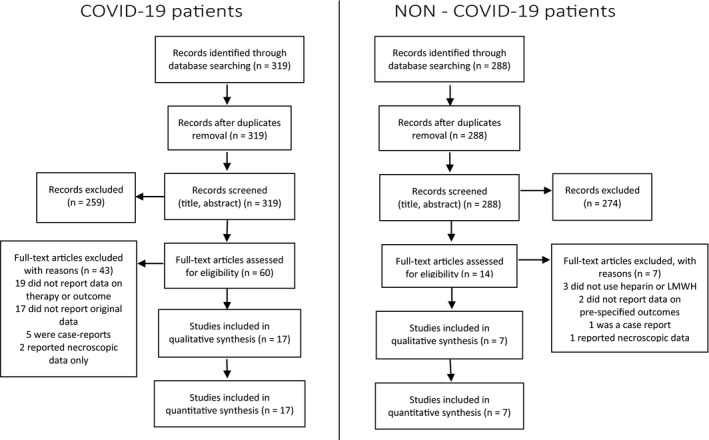
PRISMA flow diagrams for search of studies on COVID‐19 and Non‐COVID‐19 patients
COVID‐19 studies included a total of 3224 patients, 515 of whom underwent systematic DVT screening by CUS. The total population included 1644 non‐ICU patients and 1180 ICU patients; a total of 258 non‐ICU and 257 ICU patients underwent systematic DVT screening. One study included patients hospitalized either in non‐ICU or ICU, without a clear separation of the two populations in data reporting 15 ; therefore, it was not suitable for ICU or non‐ICU sub‐analysis. Non‐COVID‐19 studies included 11 985 patients, 1873 of whom hospitalized in ICU (Table 1).
TABLE 1.
Relevant details of the studies included in the meta‐analysis
| Study | Setting |
Patient No. |
Drug | Low dose, % | Full dose, % |
Age, y |
Systematic CUS for DVT |
Infection, % |
|---|---|---|---|---|---|---|---|---|
| COVID‐19 patients | ||||||||
| Al‐Samkari 15 | M, ICU | 400 | NA | 89 | 9 | 60,65 | No | 100 |
| Artifoni 22 | M | 71 | Enoxaparin | 100 | 0 | 64 | Yes | 100 |
| Cattaneo 6 | M | 64 | Enoxaparin | 100 | 0 | 70 | Yes | 100 |
| Demelo‐Rodriguez 23 | ICU | 156 | NA | 98 | 0 | 68 | Yes | 100 |
| Desborough 11 | ICU | 66 | Dalteparin | 100 | 0 | 59 | No | 100 |
| Fraissé 24 | ICU | 92 | NA | 47 | 53 | 61 | No | 100 |
| Galeano‐Valle 25 | M | 785 | Enoxaparin | 40 | 23 | 64 | No | 100 |
| Helms 26 | ICU | 150 | Enoxaparin,UFH | 70 | 30 | 63 | No | 100 |
| Hippensteel 27 | ICU | 91 | NA | 46 | 54 | 56 | No | 100 |
| Klok 28 | ICU | 184 | NA | 100 | 0 | 64 | No | 100 |
| Llitjos 29 | ICU | 26 | NA | 31 | 69 | 68 | Yes | 100 |
| Lodigiani 12 | M | 327 | NA | 40 | 23 | 68 | No | 100 |
| Lodigiani 12 | ICU | 61 | NA | 69 | 3 | 61 | No | 100 |
| Maatman 17 | ICU | 109 | NA | 94 | 6 | 61 | No | 100 |
| Middeldorp 18 | M | 123 | NA | 90 | 10 | 60 | Yes | 100 |
| Middeldorp 18 | ICU | 75 | NA | 91 | 9 | 62 | Yes | 100 |
| Poissy 19 | ICU | 107 | NA | ‐ | ‐ | 57 | No | 100 |
| Stoneham 20 | M | 274 | NA | ‐ | ‐ | 66 | No | 100 |
| Thomas 21 | ICU | 63 | Dalteparin | 100 | 0 | ‐ | No | 100 |
| Non‐COVID‐19 patients | ||||||||
| Cohen 30 | M | 4051 | Enoxaparin | 100 | 0 | 71 | Yes | 45.1 |
| Goldhaber 31 | M | 2284 | Enoxaparin | 100 | 0 | 66 | Yes | 22.8 |
| Leizorovicz 32 | M | 1759 | Dalteparin | 100 | 0 | 68 | Yes | 36.4 |
| Cook 33 | ICU | 1873 | Dalteparin | 100 | 0 | 61 | Yes | 14.9 |
| Riess 34 | M | 1624 | Certoparin | 100 | 0 | 79 | Yes | 26.8 |
| Samama 35 | M | 291 | Enoxaparin | 100 | 0 | 73 | Yes | 53.7 |
| Schellong 36 | M | 103 | Certoparin | 100 | 0 | 70 | Yes | NA |
All patients were treated with Unfractionated Heparin (UFH) or a low‐molecular‐weight Heparin, also in the studies in which the type of drug was not indicated (NA, not available).
Abbreviations: CUS, compressive ultrasonography; DVT, deep vein thrombosis; ICU, intensive care unit ward; M, medical (non‐ICU) ward.
3.2. Types and doses of heparin used
A total of 67% of COVID‐19 patients were treated with UFH or LMWH at low prophylactic doses, while 16% were on full, therapeutic doses. In the subgroup of COVID‐19 studies that implemented systematic DVT screening by CUS, 92% of patients were treated with UFH or LMWH at low prophylactic doses, while 7% were on full, therapeutic doses and 1% did not assume any anticoagulant. Non‐COVID‐19 patients were all on low prophylactic doses (Table 1).
3.3. Prevalence of PE and DVT in studies in which DVT was systematically screened by CUS
3.3.1. Patients in all hospital settings
The pooled prevalence of DVT in COVID‐19 was 15.43% (95%CI = 4.08‐31.77), which was significantly higher than that in non‐COVID‐19, 4.21% (2.27‐6.68; P = 0,046). The pooled prevalence of PE in COVID‐19 was 4.85% (0.33‐13.01), much higher than that in non‐COVID‐19 patients, 0.22% (0.03‐0.55, P = .013; Figure 2).
FIGURE 2.
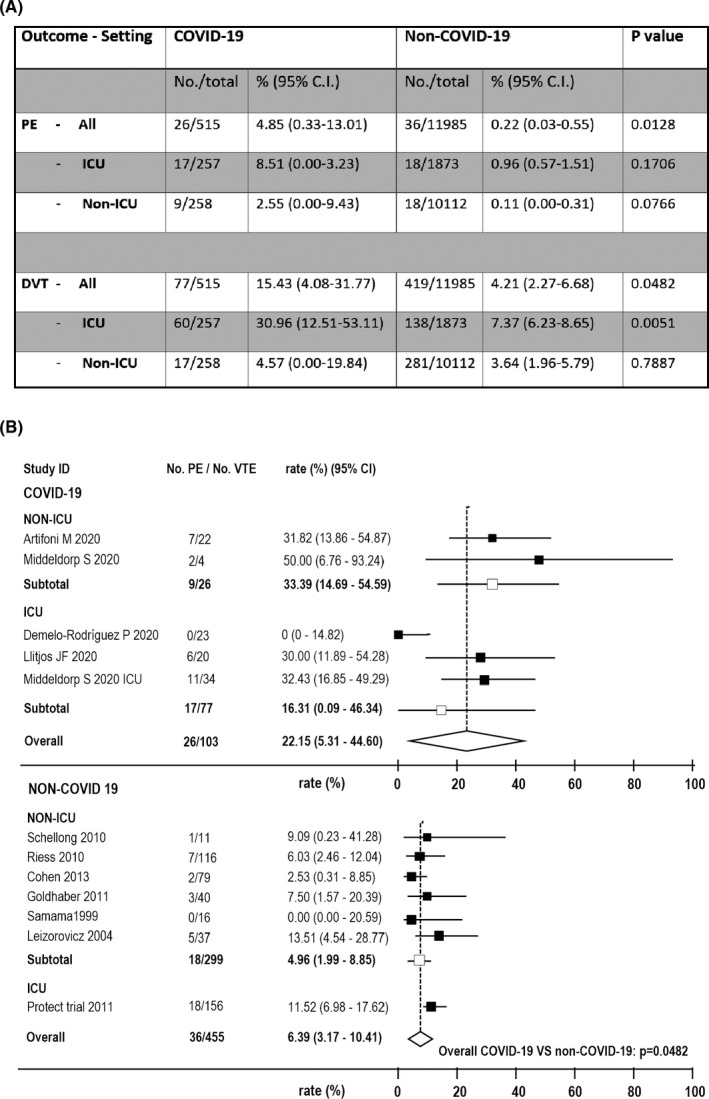
Outcomes of meta‐analysis of studies that systematically screened patients for deep vein thrombosis. A, prevalence of pulmonary embolism (PE) and deep vein thrombosis (DVT) in COVID‐19 and Non‐COVID‐19 patients in total and in different clinical settings (ICU, intensive care units; Non‐ICU, non‐intensive care units); B, forest plots representing the ratio of PE over all venous thromboembolism (VTE) events in COVID‐19 and Non‐COVID‐19 patients in total and as a function of the clinical settings. Five COVID‐19 studies (two Non‐ICU studies 18 , 22 and three ICU studies 16 , 21 , 27 ) and seven Non‐COVID‐19 studies 28 , 29 , 30 , 31 , 32 , 33 , 34 reported data for these outcomes
The pooled percentage of PE among all VTE was 22.15% (5.31‐44.60) in COVID‐19, compared with 6.39% (3.17‐10.41) in non‐COVID‐19 (P = .048; Figure 2).
3.3.2. Patients in non‐ICU
The pooled prevalence of DVT in COVID‐19 in non‐ICU was 4.57% (0.00‐19.84), which was similar to that in non‐COVID‐19, 3.64% (1.96‐5.79; P = 0,789). The pooled prevalence of PE in COVID‐19 was 2.55% (0.00‐9.43), which was numerically much higher than that in non‐COVID‐19, 0.11% (0.00‐0.31), although the difference did not reach statistical significance (P = .077; Figure 2).
The pooled percentage of patients with PE among all VTE was 33.4% (14.69‐54.59) in COVID‐19, compared with 4.96% (3.17‐10.41) in non‐COVID‐19 (P = .0002; Figure 2).
3.3.3. Patients in ICU
The pooled prevalence of DVT in COVID‐19 in ICU was 30.96% (12.51‐53.11), which was significantly higher than that in non‐COVID‐19, 7.37% (6.23‐8.65; P = .005). The pooled prevalence of PE was 8.51% (0.00‐3.23) in COVID‐19 and 0.96% (0.57‐1.51) in non‐COVID‐19, (P = .17; Figure 2).
The pooled percentage of patients with PE among all VTE was 16.31% (0.09‐46.34) in COVID‐19, compared with 11,52% (6.98‐17.62) in non‐COVID‐19 (P = .622).
3.4. Prevalence of PE and DVT in all selected studies
3.4.1. Patients in all hospital settings
The overall pooled prevalence of DVT in COVID‐19 was 6.10% (95%CI = 3.11‐9.91), similar to that in non‐COVID‐19, 4.21% (2.27‐6.68; P = 0,262). In contrast, the prevalence of PE in COVID‐19 was 7.65% (4.05‐12.19), much higher than that in non‐COVID‐19, 0.22% (0.03‐0.55, P < .0001; Figure 3).
FIGURE 3.
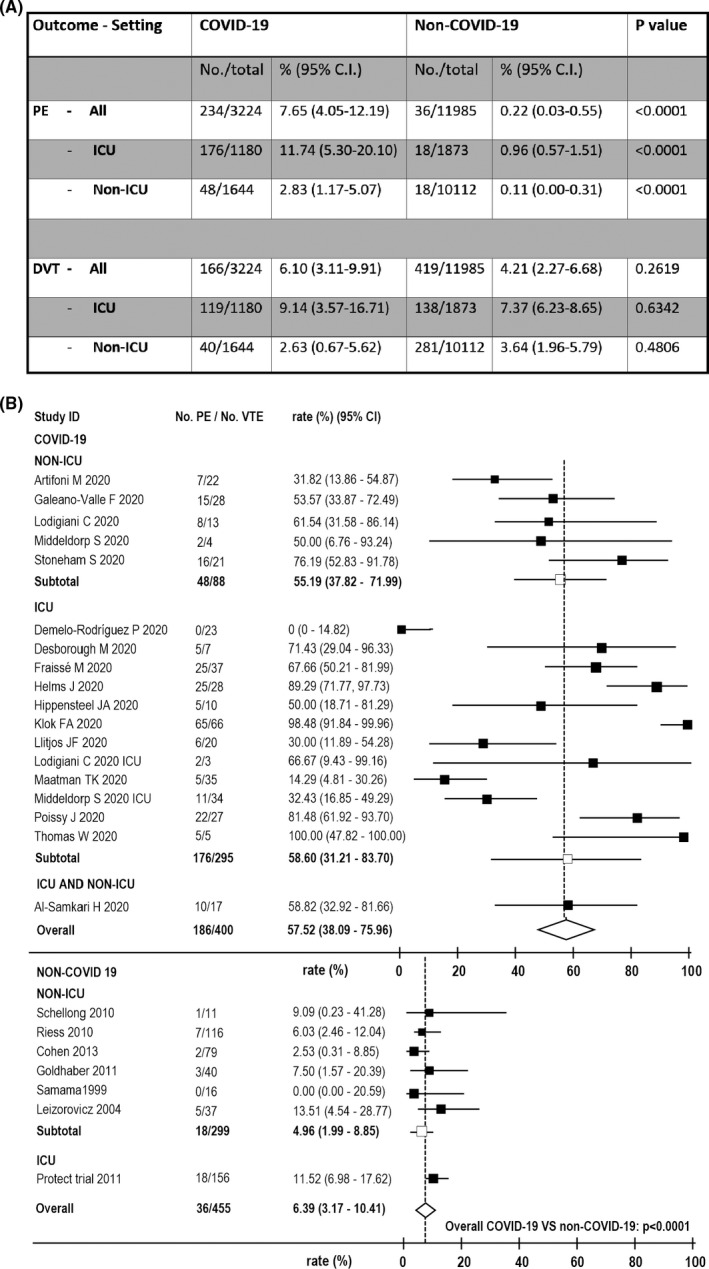
Outcomes of meta‐analysis of all included studies. A, Pooled prevalence of pulmonary embolism (pe) and deep vein thrombosis (DVT) in COVID‐19 and Non‐COVID‐19 patients in total and in different clinical settings (ICU, intensive care units; Non‐ICU, non‐intensive care units); B, forest plots representing the ratio of PE over all venous thromboembolism (VTE) events in COVID‐19 and Non‐COVID‐19 patients in total and as a function of the clinical settings. Seventeen COVID‐19 studies (five Non‐ICU studies 12 , 16 , 18 , 20 , 23 and twelve ICU studies 9 , 27 ) and seven Non‐COVID‐19 studies 28 , 29 , 30 , 31 , 32 , 33 , 34 reported data for these outcomes
The pooled percentage of PE among all VTE was 58.60% (31.21‐83.70) in COVID‐19, compared with 6.39% (3.17‐10.41) in non‐COVID‐19 (P < .0001; Figure 3).
3.4.2. Patients in non‐ICU
The pooled prevalence of DVT in COVID‐19 patients hospitalized in non‐ICU was 2.63% (0.67‐5.62), which was similar that in non‐COVID‐19, 3.64% (1.96‐5.79; P = 0,481). In contrast, the prevalence of PE in COVID‐19 was 2.83% (1.17‐5.07), much higher than that in non‐COVID‐19, 0.11% (0.00‐0.31, P < .0001; Figure 3).
The pooled percentage of PE among all VTE was 55,19% (37.82‐71.99) in COVID‐19, compared with 4.96% (1.99‐8.95) in non‐COVID‐19 (P < .0001; Figure 3).
3.4.3. Patients in ICU
The pooled prevalence of DVT in COVID‐19 patients hospitalized in ICU was 9.14% (3.57‐16.71), which was similar to that observed in non‐COVID‐19, 7.37% (6.23‐8.65; P = 0,634). In contrast, the prevalence of PE in COVID‐19 was 11.74% (5.30‐20.10), much higher than that in non‐COVID‐19, 0.96% (0.57‐1.51, P < .0001; Figure 3).
The pooled percentage of patients with PE among all VTE was 57.52% (38.09‐75.96) in COVID‐19, compared with 11.5% (6.98‐17.62; P = .0002) in the only trial 33 in non‐COVID‐19 that reported relevant data (Figure 3).
4. DISCUSSION
There is general perception that VTE risk in COVID‐19 is higher than that in other critical medical illnesses, not associated with SARS‐Cov‐2 infection, despite the fact that highly variable results have been reported. 15 , 19 , 37 , 38 , 39 , 40 Moreover, some studies reported an incidence of PE that was disproportionately high relatively to that of DVT. This observation lead to hypothesize that in some instances the observed pulmonary artery occlusion that was detected by imaging studies was caused by thrombi formed in loco, rather than emboli from thrombi in peripheral veins. 6 In the present analysis of data reported by published studies until 6 June 2020, we evaluated the percentage of PE among the reported episodes of VTE, the incidence of PE and of DVT among COVID‐19 patients under thromboprophylaxis with UFH or LMWH and compared them to those reported in RCT of thromboprophylaxis with heparins in critically ill medical patients not infected by SARS‐Cov‐2. In order for the data of COVID‐19 patients to be more accurately comparable to those of non‐COVID‐19 patients, who were all systematically screened for DVT by CUS, we analysed separately those studies in which COVID‐19 patients were also systematically screened for DVT and all studies of COVID‐19 patients.
The percentage of PE among VTE episodes was >3‐fold higher among systematically screened COVID‐19 patients and about 10‐fold higher in all COVID‐19 patients than in non‐COVID‐19 patients: all differences were statistically significant. When patients were separately considered according to their hospital settings (ICU versus non‐ICU), the percentages of PE were still significantly higher in COVID‐19, with the exception of those who had been systematically screened COVID‐19 hospitalized in ICU. It should be emphasized; however, that data relative to ICU are hampered by the fact that only one non‐COVID‐19 study reported relevant data. 33
Our analysis also allowed the comparison of the prevalence of DVT and PE in COVID‐19 and non‐COVID‐19 patients. The analysis of all retrieved studies revealed no statistically significant difference in the prevalence of DVT between COVID‐19 and non‐COVID‐19 patients both in non‐ICU (about 3%) and ICU (about 7%‐9%). When we analysed only COVID‐19 studies that systematically screened patients for DVT, we found similar results for COVID‐19 and non‐COVID‐19 patients who had been hospitalized in non‐ICU (about 3%‐4%), while the prevalence in ICU was significantly higher in COVID‐19 (about 31% vs about 7%). This result could indeed suggest that the risk of DVT is high in COVID‐19 patients in ICU, likely due to several factors that relate to the severity of their conditions and/or to the fact that many DVT could be line‐related. 11 The similar, relatively low prevalence of DVT in COVID‐19 and non‐COVID‐19 patients in non‐ICU would imply that the perception that COVID‐19 is associated with a particularly high risk is fallacious. In addition, considering that the vast majority of COVID‐19 patients were treated with thromboprophylaxis similarly to patients with other medical conditions, our data do not support the suggestions that high heparin doses are necessary to prevent DVT in COVID‐19, at least in non‐ICU. It is important to take in due account the higher bleeding risk that is associated with high heparin doses, especially considering that the incidence of major bleeding complications can be rather high in COVID‐19 patients. 15 A retrospective study of COVID‐19 patients hospitalized in non‐ICU wards in Italy showed that, compared with standard prophylactic doses, higher doses of UFH, LMWH or fondaparinux were not more effective in preventing death, but were associated with significantly higher incidence of major or clinically relevant bleeding. 41 Similar results were reported by a retrospective analysis of 4,389 COVID‐19 patients in USA, which compared therapeutic doses with prophylactic doses of anticoagulant drugs 42 Ongoing randomized clinical trials are comparing different heparin doses for thromboprophylaxis of COVID‐19. 8 , 43 , 44
As for PE, its prevalence was higher in COVID‐19 both when all retrieved studies and when only studies that screened patients systemically for DVT were considered. However, in the last series of studies, statistical significance was achieved only when all patients together, in non‐ICU and ICU, were considered, despite the much higher prevalence in both non‐ICU (about 20‐fold) and ICU (about 8‐fold) wards, likely due to the low number of COVID‐19 patients.
What are the reasons for such a dramatic difference in PE occurrence between COVID‐19 and non‐COVID‐19? In many studies of COVID‐19 patients, isolated PE (in the absence of concomitant DVT) was the predominant VTE manifestation. Isolated PE can be observed also in non‐COVID‐19 patients, but it accounts for only about 20% of the total PE episodes. 45 The reason could be either failure to detect DVT or higher ‘friability’ of the venous thrombi, resulting in their complete embolization to the lungs and disappearance from the deep venous system. However, both these possibilities are plausibly equally frequent in COVID‐19 and non‐COVID‐19 patients. Another possibility is that the frequent occlusions of pulmonary arteries that are observed in COVID‐19 patients are not only caused by pulmonary emboli originating from thrombi in peripheral veins, but also by pulmonary thrombi originating in loco, as results of a thrombo‐inflammatory process. This possibility was hypothesized by us 6 and later supported by clinical and post‐mortem studies. 11 , 46 Ackermann et al provided additional histological support to this hypothesis, reporting that lungs from COVID‐19 patients display diffuse alveolar damage with perivascular T‐cell infiltration, severe endothelial injury and widespread thrombosis with microangiopathy. 47 The high frequency of pulmonary artery occlusions does not seem to be associated with ARDS per se, because a much higher prevalence of PE was observed in COVID‐19 ARDS (11.7%) than non‐COVID‐19 ARDS (2.1%). 26 The distinction between PE and pulmonary thrombosis is not trivial, as the pathogenesis of the two conditions and, hence, their sensitivity to treatment, may be quite different. 6
In conclusion, the results of our meta‐analysis suggest that the perception of higher VTE risk in COVID‐19 patients does not seem to be supported by our data on the prevalence of DVT, at least in non‐ICU patients, and could be due to erroneous interpretation of the frequent pulmonary artery occlusions as PE, rather than in situ pulmonary thrombosis, whose medical treatment and prevention could be quite different.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Birocchi S, Manzoni M, Podda GM, Casazza G, Cattaneo M. High rates of pulmonary artery occlusions in COVID‐19. A meta‐analysis. Eur J Clin Invest. 2021;51:e13433. 10.1111/eci.13433
Simone Birocchi and Marco Manzoni contributed equally to this study.
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. J Am Med Assoc. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019‐nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):1‐4. 10.1111/eci.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141(2):e195S‐e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackman N. Coagulation abnormalities and thrombosis in patients infected With SARS‐CoV‐2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40(9):2033‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spyropoulos AC, Weitz JI. Hospitalized COVID‐19 patients and venous thromboembolism: a perfect storm. Circulation. 2020;142(2):129‐132. [DOI] [PubMed] [Google Scholar]
- 9. Ioannidis JPA. Coronavirus disease 2019: The harms of exaggerated information and non‐evidence‐based measures. Eur J Clin Invest. 2020;50(4):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: a comment. J Thromb Haemost. 2020;18(8):2060‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desborough MJR, Doyle AJ, Griffiths A, Retter A, Breen KA, Hunt BJ. Image‐proven thromboembolism in patients with severe COVID‐19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 14. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607‐611. [Google Scholar]
- 15. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS‐CoV2 infection. Blood. 2020;136(4):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maatman TK, Jalali F, Feizpour C, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783‐e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Middeldorp S, Coppens M, Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184‐186. [DOI] [PubMed] [Google Scholar]
- 20. Stoneham SM, Milne KM, Nuttall E, et al. Thrombotic risk in COVID‐19: a case series and case–control study. Clin Med. 2020;20(4):e76‐e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas W, Varley J, Johnston A, et al. Thrombotic complications of patients admitted to intensive care with COVID‐19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of D‐dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demelo‐Rodríguez P, Cervilla‐Muñoz E, Ordieres‐Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID‐19 pneumonia and elevated D‐dimer levels. Thromb Res. 2020;192:23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID‐19 patients: a French monocenter retrospective study. Crit Care. 2020;24:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galeano‐Valle F, Oblitas CM, Ferreiro‐Mazón MM, et al. Antiphospholipid antibodies are not elevated in patients with severe COVID‐19 pneumonia and venous thromboembolism. Thromb Res. 2020;192:113‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID‐19 [published online ahead of print, 2020 Jun 2]. Br J Haematol. 2020;190(3). 10.1111/bjh.16908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klok FA, Kruip M, van der Meer N, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Llitjos J‐F, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18(7):1743‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513‐523. [DOI] [PubMed] [Google Scholar]
- 31. Goldhaber SZ. Apixaban versus enoxaparin in medically ill patients. N Engl J Med. 2011;365(23):2167‐2177. [DOI] [PubMed] [Google Scholar]
- 32. Leizorovicz A, Cohen AT, Turpie AGG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874‐879. [DOI] [PubMed] [Google Scholar]
- 33. Cook D, Meade M, Guyatt G, et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305‐1314. [DOI] [PubMed] [Google Scholar]
- 34. Riess H, Haas S, Tebbe U, et al. A randomized, double‐blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non‐surgical patients: CERTIFY Study. J Thromb Haemost. 2010;8(6):1209‐1215. [DOI] [PubMed] [Google Scholar]
- 35. Samama MM, Cohen AT, Darmon J‐Y, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med. 1999;341(11):793‐800. [DOI] [PubMed] [Google Scholar]
- 36. Schellong SM, Haas S, Greinacher A, et al. An open‐label comparison of the efficacy and safety of certoparin versus unfractionated heparin for the prevention of thromboembolic complications in acutely ill medical patients: CERTAIN. Expert Opin Pharmacother. 2010;11(18):2953‐2961. [DOI] [PubMed] [Google Scholar]
- 37. Mei F, Fan J, Yuan J, et al. Comparison of venous thromboembolism risks between COVID‐19 pneumonia and community‐ acquired pneumonia patients. Arter Thromb Vasc Biol. 2020;40(9):2332‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ren B, Yan F, Deng Z, et al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID‐19 in Wuhan. Circulation. 2020;142(2):181‐183. [DOI] [PubMed] [Google Scholar]
- 39. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114‐128. [DOI] [PubMed] [Google Scholar]
- 40. Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID‐19 in a New York City Health System. J Am Med Assoc. 2020;324(8):20‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pesavento R, Ceccato D, Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non‐critically ill patients with Covid‐19: The Padua province experience. J Thromb Haemost. 2020;18(10):2629‐2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nadkarni GN, Lala A, Bagiella E, et al. Mortality, bleeding and pathology among patients hospitalized with COVID‐19: a single health system study [published online ahead of print, 2020 Aug 24]. J Am Coll Cardiol. 2020;76(16):1815–1826. 10.1016/j.jacc.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cattaneo M, Morici N. Is thromboprophylaxis with high‐dose enoxaparin really necessary for COVID‐19 patients? A new “prudent” randomised clinical trial. Blood Transfus. 2020;18(3):237‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tritschler T, Mathieu M‐E, Skeith L, et al. Anticoagulant interventions in hospitalized patients with COVID‐19: A scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020. 10.1111/jth.15094. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palareti G, Antonucci E, Dentali F, et al. Patients with isolated pulmonary embolism in comparison to those with deep venous thrombosis. Differences in characteristics and clinical evolution. Eur J Intern Med. 2019;69:64‐70. [DOI] [PubMed] [Google Scholar]
- 46. von der Thüsen J, van der Eerden M. Histopathology and genetic susceptibility in COVID‐19 pneumonia. Eur J Clin Invest. 2020;50(7):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]


