Abstract
Background
The liberal administration of hydroxychloroquine‐sulphate (HCQ) to COVID‐19 patients has raised concern regarding the risk of QTc prolongation and cardiac arrhythmias, particularly when prescribed with azithromycin. We evaluated the incidence of QTc prolongation among moderately and severely ill COVID‐19 patients treated with HCQ and of the existence of concomitant alternative causes.
Methods
All COVID‐19 patients treated with HCQ (between Mar 1 and Apr 14, 2020) in a tertiary medical centre were included. Clinical characteristics and relevant risk factors were collected from the electronic medical records. Individual patient QTc intervals were determined before and after treatment with HCQ. The primary outcome measure sought was a composite end point comprised of either an increase ≥60 milliseconds (ms) in the QTc interval compared with pre‐treatment QTc, and/or a maximal QTc interval >500 ms
Results
Ninety patients were included. Median age was 65 years (IQR 55‐75) and 57 (63%) were male. Thirty‐nine patients (43%) were severely or critically ill. Hypertension and obesity were common (n = 23 each, 26%). QTc prolongation evolved in 14 patients (16%). Age >65 years, congestive heart failure, severity of disease, C‐reactive protein level, hypokalaemia and furosemide treatment, were all associated with QTc prolongation. Adjusted analysis showed that QTc prolongation was five times more likely with hypokalaemia [OR 5, (95% CI, 1.3‐20)], and three times more likely with furosemide treatment [OR 3 (95% CI, 1.01‐13.7)].
Conclusion
In patients treated with HCQ, QTc prolongation was associated with the presence of traditional risk factors such as hypokalaemia and furosemide treatment.
What is known?
The liberal use of hydroxychloroquine‐sulphate in COVID‐19 patients has raised concern regarding the potential of QTc prolongation and cardiac arrhythmia.
Previous reports have shown a pronounced risk of QTc prolongation among hydroxychloroquine‐treated COVID‐19 patients, particularly in combination with azithromycin.
What is new?
In patients treated with hydroxychloroquine, QTc prolongation can be associated with traditional risk factors such as hypokalaemia and Furosemide treatment.
Addressing modifiable risk factors for QTc prolongation is of utmost importance prior to treatment with the relevant agents in order to effectively reduce the risk of QTc prolongation and the potentially fatal cardiac arrhythmias.
1. INTRODUCTION
The emerging outbreak of Corona virus disease 2019 (COVID‐19) because of the novel beta corona virus SARS‐CoV‐2 has spread globally at an alarming rate. In search of effective treatments, one strategy was drug repurposing of an approved drug for a different disease than that for which it was originally developed.
Among other options, the anti‐malarial drugs chloroquine (CQ) and hydroxychloroquine (HCQ) have resurfaced as promising drugs for the treatment of patients with COVID‐19 disease. 1 , 2 The accumulated long‐term experience with these drugs with regards to dosage, safety, adverse effects and drug interactions provided the leeway required for off‐label use in the setting of the new pandemic. Pressured to provide antiviral treatment options in the midst of the pandemic, the FDA had approved the use of CQ and HCQ for treating COVID‐19 disease. 3 An open‐label non‐randomised (nowadays controversial) trial also showed that HCQ treatment was associated with a significant reduction in viral shedding, an effect reportedly accentuated by concomitant treatment with azithromycin in six patients. 4 Despite the small sample studied and the major methodological limitations of the study, it had garnered substantial attention in the media and scientific community. This led to liberal use of HCQ in hospitals overwhelmed with the SARS‐CoV‐2 pandemic despite the paucity of evidence.
In the wake of the widespread use of HCQ, several authors have raised concerns regarding the potential for inducing cardiac arrhythmias. Significant QTc prolongation was attributed to treatment with HCQ in several papers, albeit mainly in patients receiving combined HCQ‐azithromycin treatment. 5 , 6 , 7 Resultantly it has been proposed that frequent electrocardiographic (ECG) monitoring be mandatory for patients with COVID‐19 who receive treatment with HCQ. As similar monitoring has not been previously recommended in other chronic illnesses such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) where HCQ is prescribed for months, this raises a major question regarding the actual risk of arrhythmias in this patient cohort.
This study aimed to quantify the evolution of ECG changes potentially attributable to HCQ among moderate and severely ill COVID‐19 patients and to seek concomitant causes. We hypothesised that QTc prolongation may be induced by the combination of severe acute illness and severe inflammation (reported in COVID‐19) in addition to medications.
2. METHODS
Following receipt of Institutional Review Board approval (0154‐20‐SZMC) with waiver of informed consent, data collection was performed during patient admission for the purpose of this observational study.
2.1. Clinical setting
The Shaare Zedek Medical Centre (SZMC) is a tertiary 1000‐bed teaching hospital. As the SARS‐CoV‐2 pandemic reached Jerusalem in March 2020, six departments were established to treat patients with COVID‐19 in the SZMC. These departments were isolated and set up with telemedicine and monitoring technologies to enable constant observation and monitoring of patients. Patients were admitted to these departments only after testing positive in a polymerase‐chain reaction (PCR) nasopharyngeal swab specimen for SARS‐CoV‐2. Patients were classified as having mild to moderate illness defined as mild respiratory symptoms (no oxygen support) up to mild pneumonia. Severe disease was defined as hypoxia, diffuse bilateral infiltrates on imaging, requirement of high‐flow nasal cannula or non‐invasive ventilation. Critically ill patients were those who required mechanical ventilation with or without multi‐organ failure.
Treating physicians were allowed to administer HCQ to any patient with confirmed COVID‐19 of moderate severity or worse unless contraindicated (QTc > 500 ms, known allergy to HCQ, significant liver disease). The treatment dose of HCQ was 400 mg twice daily on day 1, and 200 mg twice daily thereafter for 5 to 10 days. The dose was reduced by 50% in patients with creatinine clearance less than 30 mL/min. In cases where QTc interval increased by more than 60 ms or prolonged >500 ms, HCQ was withdrawn.
2.2. Inclusion/exclusion criteria
The EMRs of all adult patients admitted for treatment of confirmed COVID‐19 and treated with HCQ were screened (Mar‐1‐2020 to 14‐Apr‐2020). Inclusion was confirmed following manual verification of a positive PCR nasopharyngeal swab specimen tested for SARS‐CoV‐2 and receipt of >2 days of HCQ and at least one follow‐up ECG post‐treatment (Figure 1).
FIGURE 1.
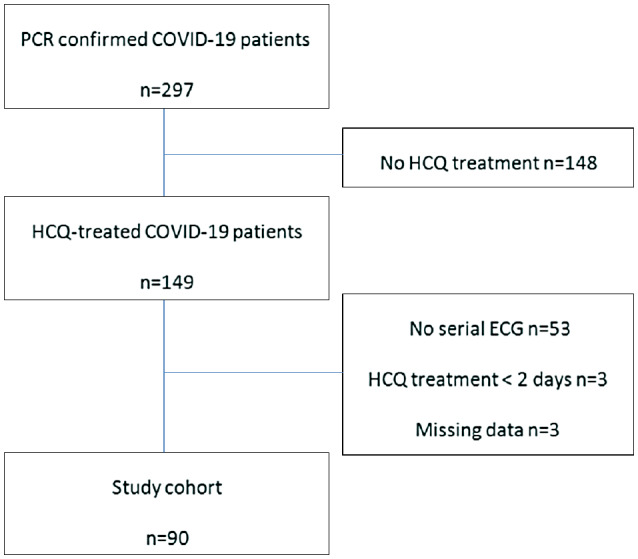
Study cohort
Patients with known arrhythmias such as atrial fibrillation or conduction blocks were not excluded from the analysis.
2.3. Data collection and variables
All relevant demographic, clinical and laboratory data (including additional medications which could potentially prolong QTc interval, prescribed throughout hospitalisation and overlapped with HCQ treatment) were collected from the electronic medical record (EMR). HCQ was defined as a QT prolonging agent. In‐line with the 2011 American Heart Association/American College of Cardiology scientific statement on prevention of TdP in hospital settings, a QTc of >470 ms for men and >480 ms for women was considered prolonged. A QTc >500 ms was considered highly abnormal for both men and women. 8 Drug‐induced QT prolongation was defined as a QTc of 500 ms or greater or an increase of 60 ms or greater in the QT interval compared with the premedication baseline interval. 8
2.4. QTc calculation
The calculation of QTc in all patients was performed in accordance with a special QTc calculation protocol written by a team of senior electrophysiologists based on the guidelines recommended in the literature. The QT was measured manually using the “tangent” method, looking mainly at leads II and V5. 9 A tangent was drawn to the steepest last limb of the presumed T wave to define the end of the T wave as the intersection of this tangent with the baseline. The QTc interval was calculated from the QT and R‐R intervals using Bazett's formula. The QRS interval was measured from the onset of the Q wave, or the R wave if no Q wave was visible, to the J point. The JTc interval was calculated by subtracting the QRS duration from the QTc interval (QTc interval‐QRS duration).
The protocol also accounts for the two special cases of atrial fibrillation and patients with wide complexes such as CLBBB––in cases of atrial fibrillation the average of QT interval and RR intervals for 5 to 10 beats was calculated, and QTc was then calculated according to Bazett's formula. in case of CLBBB or pacemaker rhythm, QTc was calculated by subtracting 50 ms from the corrected QT when QRS width was more than 100 ms, or subtracting 50 ms from the original value in a pacemaker provided the pacing rate is around 60 beats per second.
ECG was repeated at the convenience of the treating physician in order to reduce unnecessary contact with patients. The maximal QTc measurement observed in all follow‐up electrocardiograms was documented for each patient.
2.5. Outcome measures
The primary outcome measure was a composite end point consisting of either an increase ≥60 ms of the QTc interval post‐HCQ treatment (as compared with baseline QTc prior to treatment) and/or a maximal QTc interval longer than 500 ms The secondary outcome was the adjusted association of the occurrence of QT prolongation (yes/no) with HCQ treatment combinations.
2.6. Statistical analysis
All data were inserted to a Microsoft Excel (version 16.0) spreadsheet and were subsequently transferred for analysis to SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). Quantitative variables were compared using either the t‐test or the Mann‐Whitney test dependent on variable distribution. For categorical variables, the χ 2 test or Fisher's Exact Test was similarly chosen. Variables found to be associated with the dependent variable (the composite end point) in univariate analysis (ie, statistical significance determined as a two‐sided P < .05), were further evaluated for co‐linearity in order to avoid inclusion of correlated variables as independent variables in the regression model. Finally, the association of the selected variables with the dependent variable (QTc prolongation) was studied by multivariate regression analysis.
3. RESULTS
Two hundred and ninety‐seven patients with confirmed COVID‐19 disease were screened during the 45‐day study period. Among these patients, overall 149 were treated with HCQ during admission. One patient was initially treated with CQ but this treatment was replaced with HCQ 3 days later because of hallucinations. Overall 90 patients met inclusion criteria. The rest were excluded because of lack of serial ECG recordings, missing data or completion of less than 2 days of treatment (Figure 1). HCQ was prescribed for a median of 7 days (IQR 6‐8).
The demographic and clinical characteristics of the study cohort are presented in Table 1. The median age of the patients was 65 (IQR 55‐75) years and 57 (63%) were male. Fifty‐one patients (57%) had mild to moderate disease and 39 (43%) had severe or critical illness. The mean CRP level was 15 (±11) mg/dL. Twenty‐six patients (29%) were treated with HCQ alone and 31 patients (34%) were treated with HCQ and azithromycin. Thirty‐three patients were treated with HCQ and other QTc prolonging medications including seven patients who were treated with levofloxacin (Table 1). The mortality rate in the study cohort was 12.2% (11 patients), all were critically ill. Nine patients had atrial fibrillation. Two had a pacemaker and one had CLBBB.
TABLE 1.
Characteristics of COVID‐19 patients treated with hydroxychloroquine for COVID‐19 infection
| Characteristic | n (%) |
|---|---|
| Age, median (IQR) y | 65 (55‐75) |
| Age >65 | 45 (50) |
| Female gender | 33 (36.7) |
| BMI, mean (±SD) | 29.8 (±6.7) |
| Severity of illness | |
| Mild to moderate | 51 (56.7) |
| Severe/Critical | 39 (43.4) |
| QT prolonging medications prescribed during hospitalisation | |
| Loop diuretics (primarily furosemide) | 32 (35.6) |
| HCQ only | 26 (28.9) |
| HCQ and Azithromycin | 31 (34.4) |
| HCQ and other agents a | 33 (36.6) |
| ≥2 QT‐prolonging medications (including HCQ) | 65 (72.2) |
| Beta blockers treatment b | 19 (21) |
| Co‐morbidities | |
| Ischemic heart disease | 11 (12.2) |
| Diabetes mellitus | 9 (10) |
| Hypertension | 23 (25.6) |
| Congestive heart failure | 15 (16.7) |
| Chronic Kidney disease | 5 (5.6) |
| Obesity | 23 (25.6) |
| Electrocardiographic features, median (IQR) | |
| Baseline QTc, ms | 422 (405‐444) |
| Post‐treatment maximal QTc, ms | 445 (427‐470) |
| ΔQTc, ms | 23 (7‐33) |
| Day of maximal QTc | 4 (3‐5) |
| Selected laboratory parameters during hospitalisations, mean (±SD) | |
| eGFR c on admission in ml/min/1.73 m2 | 83.7 (32.6) |
| feGFR c on maximal QTc day in mL/min/1.73 m2 | 90.2 (38.2) |
| Highest CRP during HCQ treatment, mg/dL | 15.2 (11.1) |
BMI, body mass index, HCQ, Hydroxychloroquine‐sulphate; IQR, interquartile range; QTc, corrected QT interval (ms, milliseconds); ΔQTc, change in corrected QT interval (ms, milliseconds).
Other agents included quinolones (n = 7), antipsychotic medications (n = 16), anti‐depressive agents (n = 5), amiodarone (n = 5).
Beta blockers treatment refers to chronic (premorbid) state
Cockcroft‐Gault formula estimated GFR (eGFR).
3.1. Primary outcome measure—QTc interval prolongation
The median number of post‐treatment ECGs per patient was three (IQR 2‐4). Thirty‐two patients had only one follow‐up ECG. Repeat ECGs were performed on days 1 to 5 post administration of HCQ. The median QTc interval at baseline was 422 ms (IQR 405‐444). The median QTc interval calculated from the maximal values post‐HCQ treatment was 445 ms (IQR 427‐470). The median day of maximal QT prolongation was 4 (IQR 3‐5) (Table 1).
A significant QTc prolongation of more than 60 ms was noted in 11 (12%) patients. QTc prolongation greater than 500 ms was identified in seven (7.8%) patients. The overall composite end point was identified in overall 14 patients (16%).
The increase in the QTc interval seemed somewhat higher among patients treated with combination therapy as compared with HCQ treatment only but the difference was not found to be statistically significant (Figure S1).
Overall, 11 patients died. The median age of non‐survivors was 86 (IQR 79‐90). Median BMI and CRP was 30 (IQR 21‐33) and 24 (9‐35), respectively. Five had CHF, six had atrial fibrillation. Nine were treated with multiple QTc prolonging agents including azithromycin, ciprofloxacin and anti‐psychotics. Six had met the composite end point. Two had an increase of more than 60 ms in the QTc interval, three had a maximal QTc longer than 500 ms and in one patient both end points were reached. In all patients HCQ was withdrawn.
Three of the patients had hypokalaemia. Five were treated with multiple QT prolonging drugs.
Sudden clinical deterioration and asystole was documented in a 71‐year‐old morbidly obese male patient with diabetes. A 90‐year‐old female patient with congestive heart failure, atrial fibrillation and hyperkalaemia had ventricular tachycardia.
Ventricular fibrillation occurred in one 79‐year‐old patient with multiple co‐morbidities (IHD, diabetes and end‐stage kidney disease) not meeting the composite end point.
3.2. Secondary outcome measure: Adjusted association of the occurrence of QT prolongation in HCQ‐treated patients
Univariate analysis revealed that in COVID‐19 patients treated with HCQ, age above 65 years, severe or critical illness, congestive heart failure, hypokalaemia, furosemide treatment and increased CRP level were all significantly associated with the composite end point (Table 2). Hypomagnesemia was not detected in any of the patients.
TABLE 2.
Univariate analysis and adjusted multivariate regression analysis of variables associated with QT prolongation > 60 ms and/or QT prolongation > 500 ms post‐treatment with HCQ (the composite end point)
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age >65 (n = 45) | 4.5 (1.2‐17.5) | .03 | 2.6 (0.6‐12) | .2 |
| Female sex (n = 33) | 2 (0.6‐6.4) | .2 | — | |
| Severe or critical illness a (n = 39) | 4 (1.2‐14) | .03 | — | |
| BMI ≥30 (n = 23) | 2.9 (0.6‐13.5) | .2 | — | |
| IHD (n = 11) | 2.3 (0.5‐9.9) | .4 | — | |
| CHF (n = 15) | 3.7 (1.01‐13.2) | .04 | 3 (0.6‐14.6) | .17 |
| Hypertension | 1.8 (0.5‐6) | .35 | ||
| Baseline QTc ≥450 ms | 2.5 (0.7‐8.5) | .16 | — | |
| Hypokalaemia (≤3.5) (n = 27) | 4 (1.2‐13) | .02 | 5 (1.3‐20) | .02 |
| CRP mg/dL (n = 82) a | 1.06 (1.01‐1.1) | .01 | — | |
| eGFR b on maximal QTc day | 1.02 (0.99‐1.04) | .11 | ||
| Furosemide (n = 32) | 4.1 (1.3‐13.7) | .03 | 3.7 (1.01‐13.7) | .04 |
| Beta blockers treatment (n = 19) | 1.6 (0.4‐5.9) | .5 | — | |
| HCQ monotherapy (n = 26) | 0.6 (0.2‐2.5) | .8 | — | |
| HCQ and Azithromycin (n = 31) | 0.5 (0.1‐1.9) | .4 | — | |
| HCQ and other QT prolonging agents c (n = 28) | 2.6 (0.8‐8.3) | .1 | — | |
BMI, body mass index; CHF, congestive heart failure; GFR, Glomerular filtration rate; HCQ, hydroxychloroquine‐sulphate; IHD, ischemic heart disease; OR, odds ratio.
Due to collinearity between the terms Furosemide treatment, severity of illness and CRP level, the latter two variables were not included in the regression model (see results).
Cockcroft‐Gault formula estimated GFR (eGFR).
HCQ was defined as a QT prolonging agent. Other agents included quinolones (n = 7), antipsychotic medications (n = 16), anti‐depressive agents (n = 5), amiodarone (n = 5).
Co‐linearity testing showed a strong interaction between furosemide treatment, CRP and severe or critical disease. Although CRP had a strong correlation with meeting the composite end point (P = .01), the OR was not as significant as that of furosemide––OR 1.06 (95% CI 1.01‐1.1), versus OR 4.1 (1.3‐13.7), respectively. Moreover, among the three, CRP is a marker of severity of illness and not a cause of QT prolongation and was, therefore, not included in the final model. Severity of illness was determined by both patient and treatment‐related variables and was, therefore, not considered a reliable measure of stress. Conversely, furosemide may induce an electrolyte imbalance thereby triggering ECG alterations. The latter was, therefore, selected for inclusion in the model. No co‐linearity was found between furosemide treatment and hypokalaemia (data not shown) and additional interactions were not detected. The final model included furosemide and hypokalaemia.
Multivariate analysis revealed that hypokalaemia and furosemide therapy were strongly associated with QTc prolongation (Table 2).
4. DISCUSSION
The current study reports significant QTc interval prolongation in 16% of the patients treated with HCQ with/without other agents. However, multivariate analysis in this small dataset also suggested that in COVID‐19 patients treated with HCQ, concomitant hypokalaemia and furosemide treatment were strongly associated with QTc prolongation.
Our study findings support those of previous studies that showed a mild increase in the QTc interval among patients treated with HCQ and azithromycin and to a higher degree with quinolones. 7 In our cohort QTc prolongation was not significantly higher in patients receiving HCQ in combination with other drugs than with HCQ treatment alone but our study was not powered to seek this outcome. Similarly, a recent multicentre randomised controlled trial conducted in Brazil reported more frequent events of QTc interval prolongation among patients who received HCQ, either with azithromycin or alone, than patients who did not receive either agent. 10
The presence of hypokalaemia did not correlate with furosemide treatment. Hypokalaemia may be a common finding in COVID‐19 patients, a finding that has been mainly attributed to urinary potassium loss secondary to angiotensin converting enzyme 2 (ACE2) degradation in recent reports. 11 Gastrointestinal loss of potassium may also play and important role in inducing hypokalaemia as diarrhoea may occur in 2% to 50% of COVID‐19 patients. 12
The use of loop diuretics is an independent risk factor for QTc prolongation. 13 It is also one of the variables comprising the Tisdale Risk Score which predicts the risk of QT prolongation >500 ms in hospitalised patients. 14 A similar finding was demonstrated in a recent report. 7
Furosemide was often administered to patients in our cohort, most often to those who were severely ill. Diuretics may be appropriate in ARDS and are the mainstay of treatment of heart failure. The pulmonary infiltrates that are almost pathognomonic of COVID‐19 disease tempt clinicians treating these complex patients, particularly those who have been trained to treat these diseases, to administer diuretics. However, our findings suggest that treatment with loop diuretics is not harmless in this scenario and that administration of such treatment, particularly when combined with HCQ, should not be empirical. Testing for beta natriuretic factor levels and performing an echocardiograph may be of value. Treatment should be accompanied by appropriate monitoring of blood potassium and magnesium levels as well as periodic ECGs.
Severely ill patients are at increased risk of QTc prolongation because of multiple risk factors [12 15 ]. These patients are often treated with QTc prolonging drugs other than loop diuretics (eg, amiodarone, anti‐emetics and anti‐psychotics). Acute delirium is common among patients with COVID‐19 disease. 16 In our cohort 16 patients (18%) received anti‐psychotic medications (Table 1, footnote). Although we did not identify an association between such treatment and QTc prolongation, our cohort may have been too small to detect it. Mercuro et al also showed an association between QTc prolongation >500 ms and severe COVID‐19 infection with more than two inflammatory systemic response syndrome criteria 7 Other studies have also suggested that critically ill patients with increased levels of IL‐1, IL‐6, TNF‐ɑ and CRP are prone to arrhythmia. 17 , 18 , 19 We also found that severely ill patients had a higher mean CRP than did mild to moderately ill patients (20 ± 13.8 vs 11 ± 6.4, respectively, P < .01) and higher values were significantly associated with the composite end point (Table 2). Finally, there remains the possibility that COVID‐19 myocardial involvement in the form of subclinical or clinical myocarditis may contribute to the occurrence of QTc prolongation and fatal arrhythmia. 20
Among the study patients eleven died. All were elderly, severely ill patients with markedly elevated CRP and a high BMI. It is difficult to delineate the cause of death as in most cases it is multi‐factorial (multiple co‐morbidities, respiratory insufficiency, renal failure and secondary infections) and this was not the aim of our study. However, QTc prolongation is a risk factor for cardiac death and indeed, six patients who did not survive had met the composite end point of the study. Although fatal arrhythmia was documented in only two, most of the cohort patients were not continuously monitored, thus, the likelihood of under‐diagnosis of severe arrhythmias was high.
Our study has several limitations, including the inherent limitations of a retrospective design, a single centre study and its small sample size. However, similar observations have been reported elsewhere. 5 , 7 Some patients had only one follow‐up ECG reflecting the initial technical difficulties of many medical teams that were learning to manage mass isolation at the outset of the pandemic. In addition, patients excluded from the study because of missing serial ECGs may have had mild cardiac disease and this may have contributed to selection bias. Finally, the lack of a control group of patients without HCQ treatment makes it difficult to ascertain whether the QTc prolongation is related to treatment with HCQ or is a consequence of the disease itself. Nevertheless, serial electrocardiograms with successive QT measurements are not routinely performed for COVID‐19 patients which makes creating a control group a rather difficult task.
In conclusion, our study shows that QTc prolongation among HCQ‐treated patients was associated with traditional, modifiable risk factors such as hypokalaemia and furosemide treatment which are both commonly observed in COVID‐19 patients. Importantly, the present study was based on data from patients admitted during the first weeks of the pandemia in Israel, when treatment guidelines were based on preliminary reports from China and Italy suggesting HCQ to be beneficial. Since then, accumulating data based on better‐designed studies, do not support the use of this drug. 21 , 22 In our institute, HCQ is no longer recommended in COVID‐19.
DISCLOSURES
The authors declare they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Bashar Fteiha—Study conception and design, Collection of data, analysis and interpretation of results, drafting the manuscript. Hani Karameh—Study conception and design, Collection of data, analysis and interpretation of results, drafting the manuscript. Ramzi Kurd—Collection of data. Batsheva Ziff‐Werman—Collection of data. Itamar Feldman—Collection of data. Itamar Feldman—Collection of data. Alon Bnaya—Collection of data. Sharon Einav—Critical revision of manuscript. Amir Orlev—Critical revision of Manuscript. Eli Ben‐Chetrit—Study conception and design, drafting of manuscript, analysis and interpretation of data, critical revision.
Supporting information
Fig S1
Fteiha B, Karameh H, Kurd R, et al. QTc prolongation among hydroxychloroquine sulphate‐treated COVID‐19 patients: An observational study. Int J Clin Pract. 2021;75:e13767. 10.1111/ijcp.13767
Bashar Fteiha and Hani Karameh contributed equally to this work.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 3. Re H.Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 Coronavirus disease [response letter to Bright R]. Washington, DC: United States Food and Drug Administration; 2020. [Google Scholar]
- 4. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;65: 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020. [DOI] [PubMed] [Google Scholar]
- 6. Simpson TF, Kovacs RJ, Stecker EC. Ventricular arrhythmia risk due to hydroxychloroquine‐azithromycin treatment for COVID‐19. Am Coll Cardiol. 2020. [Google Scholar]
- 7. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vink AS, Neumann B, Lieve KVV, et al. Determination and interpretation of the QT interval. Circulation. 2018;138:2345‐2358. [DOI] [PubMed] [Google Scholar]
- 10. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild‐to‐Moderate Covid‐19. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID‐19). 2020. [DOI] [PMC free article] [PubMed]
- 12. D'Amico F, Baumgart DC, Danese S, Peyrin‐Biroulet L. Diarrhea during COVID‐19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020;18(8):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heemskerk CPM, Pereboom M, van Stralen K, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol. 2018;74:183‐191. [DOI] [PubMed] [Google Scholar]
- 14. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandes FM, Silva EP, Martins RR, Oliveira AG. QTc interval prolongation in critically ill patients: Prevalence, risk factors and associated medications. PLoS One. 2018;13:e0199028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID‐19: ICU delirium management during SARS‐CoV‐2 pandemic. Crit Care. 2020;24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adlan AM, Panoulas VF, Smith JP, Fisher JP, Kitas GD. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J Rheumatol. 2015;42:421‐428. [DOI] [PubMed] [Google Scholar]
- 18. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front Cardiovasc Med. 2015;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID‐19 (Coronavirus Disease 2019) Treatment. J Am Coll Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med. 2020;382:2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In‐Hospital Mortality in Patients With COVID‐19 in New York State. JAMA. 2020;323:2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


