Abstract
Transplantation in potential candidates who have recently recovered from COVID‐19 is a challenge with uncertainties regarding the diagnosis, multi‐organ systemic involvement, prolonged viral shedding in immunocompromised patients, and optimal immunosuppression. A 42 year male with alcoholic hepatitis underwent a successful deceased donor liver transplantation 71 days after the initial diagnosis of COVID‐19. At the time of transplant, he was SARS‐CoV‐2 PCR negative for 24 days and had a MELD score of 33. His post‐operative course was complicated by acute rejection which responded to intense immune‐suppression using T‐cell depletion and steroids. He was discharged with normal end‐organ function and no evidence of any active infection including COVID‐19. Prospective organ transplant recipients who have recovered from COVID‐19 can be considered for transplantation after careful pre‐transplant evaluation, donor selection, and individualized risk‐benefit analysis.
Keywords: coronavirus, COVID‐19, liver transplantation
1. INTRODUCTION
Community spread and the subsequent rapid upsurge of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) associated Coronavirus Disease 2019 (COVID‐19) in New York started in second week of March 2020. 1 Because of the diagnostic and treatment uncertainties involving COVID‐19 in recipients and donors, overall transplant volumes fell drastically in New York and other states. 2 Here, we describe the considerations, challenges, and outcomes associated with a successful liver transplantation in a patient who had recovered from COVID‐19.
2. CASE REPORT
On April 13, 2020, a 42 year man was hospitalized at Westchester Medical Center, New York with progressive jaundice, ascites, and a diagnosis of acute alcoholic hepatitis. He also tested positive for SARS‐CoV‐2 by polymerase chain reaction (PCR). He had fevers and dyspnea without hypoxemia or infiltrates on chest X‐ray. He was empirically treated with 7 days of steroids for alcoholic hepatitis, with complete resolution of fever. During the hospitalization he had highest body mass index of 24, nadir lymphocyte count of 494/mL, highest d‐dimer of 14 mg/L, highest C‐ reactive protein of 7.8 mg/dL, and a highest lactate dehydrogenase of 406 U/L. He received COVID‐19 convalescent plasma 12 days into his hospitalization. Serial SARS‐CoV‐2 PCR tests of nasopharyngeal specimen, analyzed using Cepheid or Roche platforms, were positive on days 1, 11, 21, 25, 28 and became negative after day 47 of the initial diagnosis. His model for end‐stage liver disease (MELD) score was 34 on admission, peaked at 36 and then decreased to 29, coincident with un‐detectability of SARS‐CoV‐2. After weighing the risks of death without transplant and potential unknown risks associated with recent COVID‐19, he was listed as a liver transplant candidate on day 53. He subsequently underwent successful deceased donor liver transplant surgery 71 days after the initial diagnosis of COVID‐19. At the time of transplantation he had a MELD score of 33, a negative measured SARS‐CoV‐2 antibody, no clinical or CT‐chest findings suggestive of COVID‐19 and type 2 hepatorenal syndrome. The patient's organ donor was evaluated for COVID‐19 based on clinical presentation, epidemiological risk assessment for possible exposure to SARS‐CoV‐2, a review of rates of ongoing community transmission in the donor's area, chest CT findings, and SARS‐CoV‐2 PCR from nasopharyngeal and bronchoalveolar lavage (BAL) specimens. Prior to transplant, the patient provided informed consent to accept the unknown risks associated with COVID‐19 or its potential impact on graft function. Biopsy of explanted liver showed parenchymal extinction, 30% macrovascular steatosis in viable hepatocytes and no evidence of cirrhosis.
Post‐operatively, his immune‐suppression included tacrolimus and high dose steroids. In the setting of recent COVID‐19 and with contemporaneous expert guidance recommending minimization of immunosuppression, he received no induction therapy or mycophenolate. He received standard peri‐operative antibiotic prophylaxis and post‐transplant prophylaxis with sulfamethoxazole/trimethoprim and valganciclovir. Tacrolimus levels were maintained between 10 and 15 ng/mL. Immediate graft liver function was excellent, with near‐normalization of liver enzymes by day 6 post‐transplantation. However, on day 7 he developed worsening liver function, with aspartate aminotransferase/alanine aminotransferase (AST/ALT) rising to 403/1558 U/L and total bilirubin rising to 6.9 mg/dL. Initial work‐up showed no signs of any active infection, donor derived infection, or vascular or biliary complication. Repeat nasopharyngeal and stool PCR tests were negative for SARS‐CoV‐2. He received three doses of methylprednisolone 500 mg for clinical diagnosis of acute cellular rejection with some improvement in liver function tests. Biopsy of the liver allograft on post‐operative day 10 revealed centrilobular necrosis along with patchy positive CD4d immunostaining in the endothelial cells, suggestive of partially‐treated severe acute rejection. The patient was treated with thymoglobulin 1 mg/kg for 5 days, in addition to continuation of steroids and tacrolimus. This resulted in further normalization of liver chemistries. The patient was discharged with normal end‐organ function and no evidence of any active infection, including COVID‐19, on day 25 post‐transplantation. (Figure 1).
FIGURE 1.
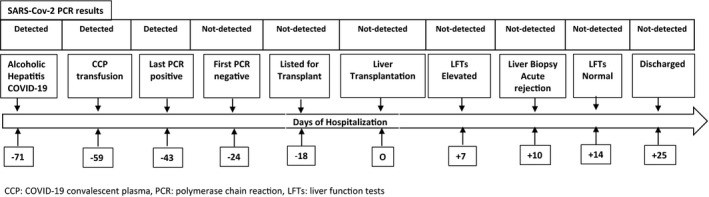
Clinical course of patient with recovered COVID‐19 and subsequent Liver transplantation
3. DISCUSSION
The management of COVID‐19 in transplant recipients remains a challenge with uncertainties regarding the diagnosis, multi‐organ systemic involvement, prolonged viral RNA detection in immunocompromised patients and optimal immunosuppression. 3 There is further uncertainty regarding the safety of performing a solid organ transplantation in a recipient who has recently recovered from COVID‐19. There is limited current knowledge about the latency of SARS‐CoV‐2 and potential sites of involvement that are not easily detected by commercially available PCR based tests. COVID‐19 in a new transplant recipient may develop at various steps post‐transplantation with the risk of SARS‐CoV‐2 transmission from the donor, from health care workers during hospitalization, from community transmission after discharge and an unknown risk of worsening/reactivation of a recent infection in the recipient. Once transplant recipients develop COVID‐19, they are at a higher risk of dying and/or developing end‐organ dysfunction. 4 , 5
In this report, we describe the clinical course of a young patient with acute alcoholic hepatitis who underwent a successful liver transplant after recovering from recent COVID‐19. This follows the unpublished reports of lung transplantation in few patients in China, Europe, and United States who had recovered from COVID‐19. Before transplant surgery, our recipient was screened for infection caused by SARS‐CoV‐2 using serial PCRs and clinical and radiological data. Donor was screened for COVID‐19 using epidemiological and clinical history, molecular testing (from nasopharyngeal and BAL specimens) and radiological testing. Based on all the available evidence for our patient, we suspect that a combination of a cautious approach to minimize immunosuppression in a young recipient may have led to severe acute rejection which ultimately responded to T‐cell depletion. Other known risk factors associated with early rejection after liver transplantation including cytomegalovirus (CMV) mismatch, sex mismatch, human leukocyte antigen (HLA) mismatch, prolonged cold ischemia time, and biliary complications were not present. 6 Subsequent intense immunosuppression with T‐cell depletion, high doses of steroids, and tacrolimus was not associated with any clinical or molecular evidence of reactivation of SARS‐CoV‐2.
Prospective organ transplant recipients who have recovered from COVID‐19, should be considered for transplantation after careful pre‐transplant evaluation, donor selection, and individualized risk‐benefit analysis.
AUTHORS CONTRIBUTIONS
AD, RB, CN, SN conceived the report. AD, AS collected the relevant data. AD, RB, SN, RN, TD, DW helped with interpretation of the data. AD, CN, DW wrote the manuscript. All authors provided critical feedback and helped with the final manuscript.
Dhand A, Bodin R, Wolf DC, et al. Successful liver transplantation in a patient recovered from COVID‐19. Transpl Infect Dis.2021;23:e13492. 10.1111/tid.13492
REFERENCES
- 1. https://www.cdc.gov/covid‐data‐tracker/#cases. Accessed 24 July 2020.
- 2. Boyarsky BJ, Po‐Yu Chiang T, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fishman JA. The immunocompromised transplant recipient and SARS‐CoV‐2 infection. JASN. 2020; 31(6):1147‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US Epicenter. Am J Transplant. 2020;20:1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhry ZS, Williams JD, Vahia A, Fadel R, et al. Clinical characteristics and outcomes of COVID‐19 in solid organ transplant recipients: a case‐Control Study. Am J Transplant. 2020. Accepted author manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dogan N, Hüsing‐Kabar A, Schmidt HH, Cicinnati VR, Beckebaum S, Kabar I. Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. J Int Med Res. 2018;46(9):3979–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]


