Summary
Background: Coagulopathy and thromboembolic events are common in Covid‐19 patients and are poor prognostic factors. Controversy exists regarding the potential of anticoagulation (AC) to reduce mortality and incidence of thromboembolic events in Covid‐19 patients. The current systematic review and meta‐analysis investigated the association between anticoagulants and mortality in adult hospitalized COVID‐19 patients using the available published non‐randomized studies. Methods: Google Scholar, PubMed, Scopus, the Cochrane Library and Clinical Trials.gov were searched for relevant studies. A meta‐analysis of adjusted and unadjusted estimates was performed. The relative risk was used as a measure of effect. The random‐effects model was used to pool estimates using the generic inverse variance method. Results: Sixteen studies were included in the quantitative data synthesis. Results showed a statistically significant association between AC and mortality (RR = 0.56, 95% CI 0.36; 0.92, p = 0.02). Both therapeutic (Relative risk [RR] = 0.4, 95% CI 0.27; 0.57) and prophylactic AC (RR = 0.54, 95% CI 0.41; 0.71) were associated with lower risk of mortality. Pre‐admission AC was not associated with mortality (RR = 0.84, 95% CI 0.49; 1.43, p > 0.05) while prophylactic AC was associated with higher risk of mortality compared to therapeutic AC (RR = 1.58, 95% CI 1.34; 1.87, p < 0.001). Conclusion: Findings support the association of AC with mortality in Covid‐19 patients. The results, synthesized from mostly low‐quality studies, show that prophylactic and therapeutic AC might reduce mortality in Covid‐19 patients. Findings suggest that therapeutic doses might be associated with better survival compared to prophylactic doses.
Keywords: anticoagulants, Covid‐19, meta‐analysis, mortality, thromboprophylaxis, systematic review
Abbreviations
- AC
anticoagulation
- DIC
disseminated intravascular coagulation
- DOAC
direct oral anti‐coagulant
- DTI
direct thrombin inhibitors
- DVT
deep vein thrombosis
- HR
hazard ratio
- LMWH
low‐molecular‐weight heparin
- NOAC
novel oral anticoagulants
- NOS
Newcastle–Ottawa scale
- NRS
non‐randomized study
- OR
odds ratio
- PE
pulmonary embolism
- RCT
randomized clinical trial
- RR
relative risk
- RoB
risk of bias
- RT‐PCR
real‐time polymerase chain reaction
- VTE
venous thromboembolism
- UFH
unfractionated heparin
1. INTRODUCTION
Coronavirus disease 2019 (Covid‐19 or SARS‐CoV‐2), first reported in Wuhan City, is now a global pandemic and is responsible for 4,45,535 deaths globally. 1 Coagulopathy and thromboembolic events are characteristic of Covid‐19 and are considered as poor prognostic factors. The respiratory system is the main target of SARS‐CoV‐2, although other body systems may also be involved. Thus, symptoms might vary from respiratory distress to multiple organ failure. 2 The extent of immune and inflammatory processes disruption is the main determinant of Covid‐19 pathogenesis and severity. 3 In severe Covid‐19, a storm of overproduced proinflammatory cytokines results in a consequent risk of hypercoagulation, vascular hyperpermeability, multi‐organ failure and even death. 4 , 5 These findings are supported by the high reported prevalence of venous thromboembolism (VTE), pulmonary embolism (PE) and pulmonary in situ thrombosis. 5 , 6 In addition to thromboembolic events, the interplay between coagulation and inflammation has a significant impact on disease progression and can negatively affect the disease management outcomes. 5 , 7 Recent evidence suggests that the lung damage caused by SARS‐CoV‐2 represents a cytokine‐storm reaction similar to anaphylaxis. In light of such evidence, the cytokine storm should be given the same priority afforded to traditional cases of anaphylaxis. Randomized clinical trials should also investigate the efficacy of monoclonal antibodies in Covid‐19 patients. 8 Different anticoagulants, whether administered orally or parenterally, suppress the synthesis or interfere with the function of clotting factors within the body. 9 Unfractionated heparin (UFH), low‐molecular‐weight heparin (LMWH), fondaparinux, warfarin, direct thrombin inhibitors and novel oral anticoagulants (NOAC) are anticoagulants with different characteristics targeting the coagulation cascade at different points. 9 , 10 Multiple studies investigated the benefit of anticoagulation in Covid‐19 patients. In a small retrospective cohort study on 44 Covid‐19 patients, using LMWH resulted in higher lymphocyte and lower interleukin‐6 levels compared to control patients, indicating an improvement in coagulation parameters and normalization of immunity. 11 In another study, initiation of heparin was associated with improved oxygenation in 27 patients with Covid‐19 infection. 12 Controversy exists regarding the dose, timing, risk‐benefit ratio and duration of AC in Covid‐19 patients. Moreover, the question remains to be answered whether all hospitalized Covid‐19 patients would benefit from anticoagulation. The current systematic review and meta‐analysis investigated the association between AC and outcomes in hospitalized Covid‐19 patients.
2. METHODS
2.1. Research question and eligibility criteria
The research question for this systematic review was: ‘Is the use of therapeutic or/and prophylactic AC associated with mortality and incidence of venous thromboembolism in hospitalized adult Covid‐19 patients?’ Mortality was defined as death during hospitalization, while venous thromboembolism was defined as deep vein thrombosis (DVT) or/and pulmonary embolism (PE).
The research question (Appendix S2) was broken down and formulated using the Population, Intervention, Control, Outcome and Study criteria framework. 13 Studies were included if they met the following inclusion criteria: (i) Case‐control or cohort studies, (ii) hospitalized adult patients with confirmed or suspected Covid‐19 and (iii) the use of therapeutic or prophylactic AC. Exclusion criteria were: (i) lack of a control group and (ii) failure to provide information regarding outcomes of anticoagulation in Covid‐19 hospitalized patients. No randomized clinical trials were identified when the search was conducted.
2.2. Data sources and search strategy
A quantitative systematic review in compliance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines was performed (Figure 1). 14 The following electronic databases were searched for relevant articles: Google Scholar, PubMed, Scopus, the Cochrane Library and Clinical Trials.gov. Concept maps were developed through discussion to produce the search terms (Appendix S2). In brief, three key concepts were identified: (1) anticoagulation, (2) Covid‐19 and (3) human study. For each of the three concepts, authors mapped the relevant keywords and relevant control terms such as Medical Subject Headings (MeSH). The wild card symbols (* and ?) were used to enhance search results. A systematic literature search was performed on the 22nd of June and repeated on the 5th of July (Appendix S3).
FIGURE 1.
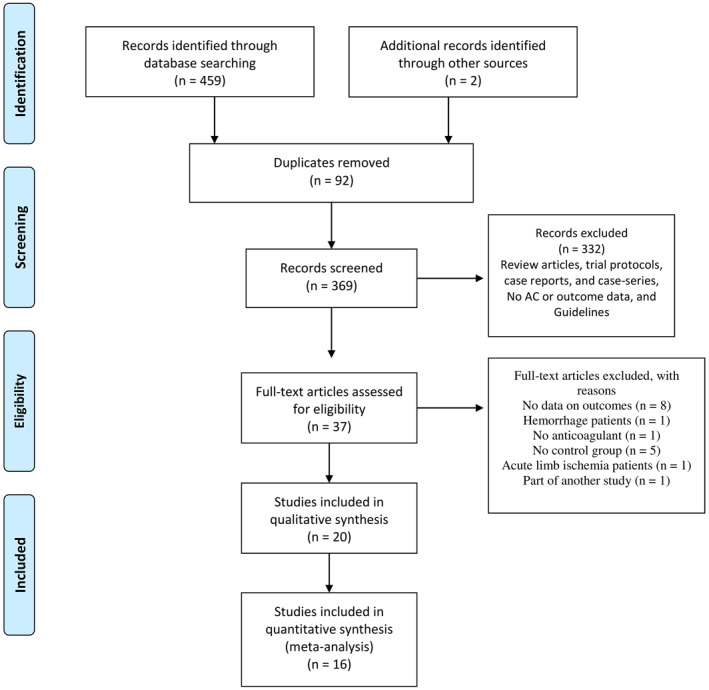
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram of the study selection process for the systematic review and meta‐analysis. AC: Anticoagulation
Literature search for published studies (prospective or retrospective) was performed. Review articles and articles in non‐English language were excluded from the analysis. In‐press articles, editorial letters and pre‐prints were included in the systematic review if they met the eligibility criteria. All included literature (peer‐reviewed, grey literature and non‐peer‐reviewed material) were subjected to the same rigorous methodological evaluation to ensure consistency. Bibliographies of selected articles and articles citing the selected articles were screened for inclusion. Studies were included even if their primary outcome was not investigating the efficacy of AC. The search was conducted using a combination of controlled vocabulary (MeSH) and title and/or abstract text words. To ensure accuracy, an initial literature review was conducted to identify sentinel articles that should be retrieved when the actual search is performed. Search results from each database were initially exported to Mendeley®, and duplicate citations were identified and discarded. The detailed search strategy (concept maps, keywords and sentinel studies) is described in Appendices S1, S3 and S13, respectively. Two authors (Mona Sobhy and Nada Magdy) independently conducted the searching and identified the eligible studies. Discrepancies were resolved by a third author (Ahmed M. Kamel).
2.3. Data extraction
Full‐text papers were retrieved for the eligible studies. Reasons for excluding studies at this stage are described in Appendix S4. Two authors (Nada Magdy and Mona Sobhy) screened and agreed on the included studies and assessed the risk of bias, with a third author as arbitrator (Ahmed M. Kamel). Once the relevant articles were identified, data were extracted by two authors (Nada Magdy and Mona Sobhy) and cross‐checked by a third author (Ahmed M. Kamel) for completeness and accuracy. The following information was extracted: the first author, publication year, study type, subject characteristics (such as age and gender and comorbidities), disease severity, study duration and sample size. Data related to anticoagulation included the name of the used anticoagulant, dosage, purpose (prophylactic vs. therapeutic), dosage form, duration and nature of use (pre‐admission vs. after admission). The adjusted and unadjusted estimates for the outcomes of interest were also extracted. Unadjusted estimates included the number of events and non‐events per group, unadjusted odds ratio (OR) or hazard ratio (HR). Adjusted estimates included the adjusted HR (aHR) or the adjusted OR (aOR). Estimates extracted from studies that had propensity‐matched groups were also treated as adjusted estimates. Authors of relevant articles were contacted for any unreported data essential for the analysis.
2.4. Risk of bias assessment
The risk of bias (RoB) and quality of individually selected studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS). 15 The RoB was evaluated based on the ability of the study to investigate the association between AC and mortality using adjusted data. The NOS was developed to assess the quality of non‐randomized studies such as cohort and case‐control studies (Appendix S2). A ‘star system’ was developed in which a study is judged on three broad perspectives: the selection of the study groups, the comparability of the groups and the ascertainment of either the exposure or outcome of interest for case‐control and cohort studies, respectively. The quality of each study was graded based on the three abovementioned domains with a maximum possible score of 9. The NOS rating for each study was then converted to the Agency for Healthcare Research and Quality standard. 16
Quality assessment was based on the use of adjusted estimates for the meta‐analysis and mortality as the main outcome of interest. For studies that did not provide estimates of mortality, the risk of bias assessment was based on the incidence of VTE (PE, DVT). Well‐designed studies that did not primarily adjust the outcome of interest for confounders (using propensity matching or multivariate analysis) were considered of poor quality as per the NOS criteria.
2.5. Outcomes
Mortality was the primary outcome in the current meta‐analysis. Secondary outcomes included incidences of PE, DVT and VTE (defined as combined DVT and PE). For studies that reported the effect size for several independent subgroups (e.g., oral and parenteral AC), rather than an overall estimate, the effect size was calculated by pooling either the raw data (e.g., counts) or adjusted estimates across subgroups assuming a correlation of 0. Studies that reported multiple comparisons (e.g., prophylactic AC and therapeutic AC) to the same reference group (no AC) were treated as independent groups when subgroup analysis was performed. That is, the subgroup was used as the unit of analysis. However, these comparisons were pooled (assuming a correlation of 0.5) when looking at the overall effect of AC. In other terms, the study was used as the unit of the analysis. A pooled effect size for VTE was calculated by pooling the results of the individual outcomes (PE and DVT) for studies that did not report an overall incidence of VTE (also assuming a correlation of 0.5, which produces the least biased estimates).
A separate meta‐analysis was performed to compare mortality and incidence of VTE between patients who received prophylactic (low or preventive dose) and therapeutic AC (curative, intermediate or full dose) after hospital admission on mortality and incidence of VTE. The analysis was also performed separately for adjusted and unadjusted data.
2.6. Subgroup analysis
The analysis was initially performed using the study as the unit of analysis. Effect sizes for various doses of the same anticoagulant within the same study (compared to no AC) were pooled to produce one estimate for each study before pooling the results across studies. Second, subgroup analysis was performed based on the strength of AC (therapeutic or prophylactic or unknown). The subgroup was used as the unit of analysis in such case. The analysis was performed separately for patients who started AC after admission and patients who were already on anticoagulants at the time of admission (for other indications such as cardiac problems).
2.7. Publication bias
Publication bias was evaluated by visual examination of funnel plots. Egger's regression test was not used to test the asymmetry of the funnel plot due to the small sample size of the included studies. 17 When there was visual evidence of funnel plot asymmetry, potentially missing studies were imputed using the ‘trim and fill’ method. 18
2.8. Sensitivity analysis
Sensitivity (influence) analysis was performed by removing individual studies (based on the risk of bias) and examining the effect size after exclusion to assess the robustness of the results. The pooled estimates were reported for mortality after excluding low and fair‐quality studies to minimize the risk of bias as recommended by the Cochrane collaboration. 19
2.9. Data analysis
The random‐effects model (using the Paule‐Mandel method as the tau estimator) was used to pool the effect sizes due to the heterogeneity of study populations included in the analysis. 20 , 21 The random effect model does not rely on the assumption that a true effect size is the same in all combined studies. The generic inverse variance method was used for weighting.
Relative risk (RR) was used as the unit of effect size for mortality and incidence of VTE. The RR was used because it is more interpretable compared to OR, especially in meta‐analysis settings. The counts and percentages were used to calculate the RR and standard error when available. OR from case‐control studies and HR from survival analysis were transformed to RR based on the approximation suggested by VanderWeele. 22 The rare disease assumption was not used when converting OR and HR to RR. Studies with no events in both groups were excluded from the analysis. The total effect (TE) and the corresponding standard error (SeTE) were calculated on natural logarithmic scale (ln) using the following formulas:
Effect sizes were pooled using the generic inverse variance method, and the pooled 95% confidence interval (95% CI) was calculated and used for hypothesis testing. 23 The adjusted estimates were used for the analysis as the RoB assessment was performed based on the adjusted outcomes. However, the analysis was repeated, when data was available, using the unadjusted data, and the results from both analyses were reported. The I2 was used to assess heterogeneity. The I2 statistic represents the percentage of variability caused by heterogeneity across studies rather than chance. 24 In cases of moderate to substantial heterogeneity, with I2 values greater than 50%, we explored and reported the potential causes. The Cochrane Q statistic was used to test the statistical significance of heterogeneity. 24 Forest plots were used to visualize the meta‐analysis results. The pooled effect size was back‐transformed for interpretation purposes. A p value < 0.05 was considered statistically significant. The analysis was performed using Comprehensive Meta‐Analysis Software v3 25 and R software v3.6.3. 26
3. RESULTS
3.1. Literature review and study selection
An initial review of databases returned 461 studies with 92 duplicates (Figure 1). Another 332 records were excluded after reviewing titles and abstracts. The details of the excluded studies are listed in Appendices S4 and S5. Full‐text articles were retrieved for 37 studies. After excluding another 17 studies (by reviewing the full‐text for eligibility), 20 and 16 studies were included in qualitative and quantitative data synthesis, respectively (Appendix S6). Two studies reported only the unadjusted estimates for DVT. 27 , 28 One study reported only the estimate for PE, 29 and one reported only the unadjusted estimates for VTE. 30 Quantitative analysis was performed only for estimates of mortality, as less than three studies provided estimates for the incidence of VTE. Thus, the former four studies were excluded from the quantitative analysis.
3.2. Risk of bias assessment
The systematic review included 19 studies (16 retrospective cohorts and 3 prospective cohorts) and one case‐control study. Twelve (60%) studies were of low quality mainly due to comparability issues. Only 3 (15%) and 5 (25%) studies were of fair and good quality, respectively (Table 1).
TABLE 1.
Quality assessment of the included cohort studies based on the modified NOS, ordered alphabetically by author
| Study (Author, Year) | Score per modified NOS domain | Total score | Study quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| Cohort studies | ||||||||||
| Ayrebe et al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 | Poor |
| Bousquet G. et al., 2020 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5 | Fair |
| Da‐xiong Zeng et al., 2020 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | Poor |
| Fauvel et. Al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | Good |
| Fraissé M. et al., 2020 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 4 | Poor |
| Giacomelli A. et al., 2020 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 | Poor |
| Gonzalez‐Porras J.R. et al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | Good |
| Klok F.A. et al., 2020 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | Poor |
| Li W. et al., 2020 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | Good |
| Llitjos J‐F et al., 2020 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | Poor |
| Middeldorp S. et al., 2020 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 4 | Poor |
| Paranjpe I. et al., 2020 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 4 | Poor |
| Rossi R. et al., 2020 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 4 | Poor |
| Sivaloganathan H. et al., 2020 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | Fair |
| Tang N. et al., 2020 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | Poor |
| Tremblay D. et al., 2020 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | Good |
| Trinh MA. et al., 2020 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | Good |
| Vincenzo R. et al., 2020 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 5 | Poor |
| Zhang L. et al., 2020 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Poor |
| Case‐control studies | ||||||||||
| Koleilat et. al. | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 4 | Poor |
| Modified NOS scale domains for cohort studies: | ||||||||||
| Selection 1. Representativeness of the exposed cohort 2. Selection of the non‐exposed cohort 3. Ascertainment of exposure 4. Demonstration that outcome of interest was not present at start of study | ||||||||||
| Comparability 5. Comparability of cohorts on the basis of the design or analysis | ||||||||||
| Outcome 6. Assessment of outcome 7. Was follow‐up long enough for outcomes to occur 8. Adequacy of follow‐up of cohorts | ||||||||||
| Modified NOS scale domains for case‐control studies: | ||||||||||
| Selection 1‐ case definition 2‐ Representativeness of cases 3‐selection of controls 4‐ Definition of controls | ||||||||||
| Comparability 5‐ comparability of cases and controls | ||||||||||
| Exposure 6‐ ascertainment of exposure 7‐ same method of ascertainment for cases and controls 8‐ non‐response rate | ||||||||||
Abbreviation: NOS, Newcastle–Ottawa Scoring System.
3.3. Characteristics of the included studies and patients
Characteristics of the included studies and patients are included in Appendices S7 and S8, respectively. The outcomes and comparisons extracted from each study are shown in Table 2. The average/median age was >50 years in 19 studies and was not specified in one study. 31 Covid‐19 status was confirmed using real‐time polymerase chain reaction in all but one study. 32
TABLE 2.
Outcomes reported by the included studies that compared AC to No AC, Ordered alphabetically by author
| Study (Author) | Adjusted estimates | Unadjusted estimates | Comparison (AC purpose) | Comparator | Setting | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | VTE | PE | DVT | Mortality | VTE | PE | DVT | ||||
| Ayrebe et al. 40 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Unknown | No AC | Hospital |
| Bousquet G. et al. 41 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Hospital |
| Bousquet G. et al. 41 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Prophylactic AC | No AC | Hospital |
| Bousquet G. et al. 41 , a | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic | Prophylactic | Hospital |
| Zeng et al. 56 | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Prophylactic LMWH or heparin | No AC | Hospital |
| Fauvel et. al. 29 , b | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | VKA, NOAC, heparin | No AC | Pre‐admission |
| Fauvel et al. 29 , b | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | Prophylactic heparin, LMWH | No AC | Hospital |
| Fauvel et al. 29 , b | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | Intermediate heparin, LMWH | No AC | Hospital |
| Fauvel et al. 29 , a , b | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | Intermediate heparin, LMWH | Prophylactic heparin, LMWH | Hospital |
| Fraissé M. et al. 32 , a | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | Therapeutic AC | Prophylactic AC | Hospital |
| Giacomelli A. et al. 37 | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | AC | No AC | Pre‐admission |
| Gonzalez‐Porras J.R. et al. 42 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Low dose LMWH | No heparin | Hospital |
| Gonzalez‐Porras J.R. et al. 42 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | High dose LMWH | No heparin | Hospital |
| Gonzalez‐Porras J.R. et al. 42 , a | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | High dose LMWH | Low dose LMWH | Hospital |
| Klok F.A. et al. 36 | ✓ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Pre‐admission |
| Koleilat et al. 28 , b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | Prophylactic LMWH, heparin, apixaban | No AC | Hospital |
| Koleilat et al. 28 , b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | Therapeutic heparin, DOAC, bivalirudin | No AC | Hospital |
| Koleilat et al. 28 , a , b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | Therapeutic heparin, DOAC, bivalirudin | Prophylactic LMWH, heparin, apixaban | Hospital |
| Li W. et al. 39 | ✓ | ✓ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Oral NOAC and warfarin (unknown) | No AC | Hospital |
| Li W. et al. 39 | ‐ | ✓ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Parenteral LMWH (unknown) | No AC | Hospital |
| Llitjos J‐F et al. 45 , a | ‐ | ‐ | ‐ | ‐ | ✓ | ✓ | ✓ | ‐ | Therapeutic AC | Prophylactic | Hospital |
| Middeldorp S. et al. 30 , b | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | Therapeutic AC | No AC | Pre‐admission |
| Paranjpe I. et al. 31 | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Hospital |
| Rossi R. et al. 34 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | DOAC | No AC | Pre‐admission |
| Sivaloganathan H. et al. 38 | ‐ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Pre‐admission |
| Tang N. et al. 43 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Hospital |
| Tremblay D. et al. 35 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | No AC | Pre‐admission |
| Trinh MA. et al.(1) 44 , a | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic AC | Prophylactic | Hospital |
| Vincenzo R. et al. 33 | ✓ | ‐ | ‐ | ‐ | ✓ | ‐ | ‐ | ‐ | Therapeutic NOAC, VKA | No AC | Pre‐admission |
| Zhang et al. 27 , b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ✓ | Prophylactic LMWH | No AC | Hospital |
Note: Studies that provided information for more than one comparison were reported more than once based on the number of comparisons.
Abbreviations: AC, anticoagulation; DOAC, direct oral anticoagulants; DVT, deep venous thrombosis; LMWH, low molecular weight heparin; NOAC, novel oral anticoagulants; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Studies that provided data to compare therapeutic and prophylactic AC.
Not included in the meta‐analysis.
Direct oral anticoagulants (DOACs) and warfarin were being used before admission in two studies. 33 , 34 Pre‐admission anticoagulants were not specified in two studies, 35 , 36 , 37 while patients were on a variety of anticoagulants prior to admission in one study. 38 UFH and LMWH were the main anticoagulants used in hospitals, although DOAC were also used in some of the included studies. 28 , 29 , 37 , 39 Heparin was used exclusively in one study although the dose was not specified. 40 Only three of the included studies were pre‐prints (Appendix S6). A summary of the pooled mortality estimates is shown in Figure 2.
FIGURE 2.
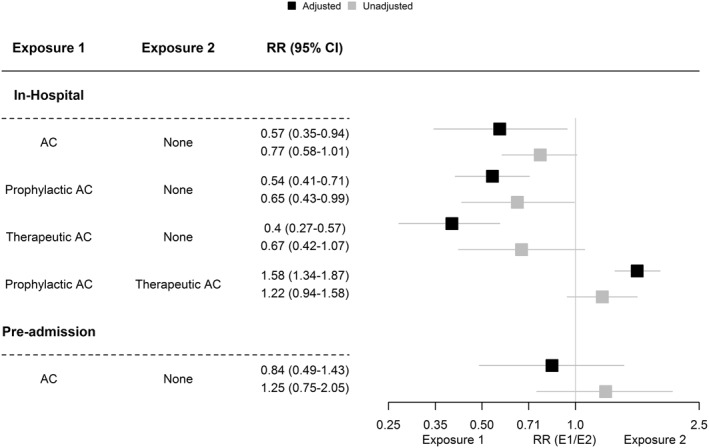
Summary of mortality pooled estimates in the current systematic review. AC, anticoagulation; CI, confidence interval; E1, exposure 1; E2, exposure 2; RR, risk ratio; SeTE, standard error; TE, total effect
3.4. Association of in‐hospital AC with mortality and incidence of VTE
Meta‐analysis of adjusted estimates (Figure 3) from five studies with 4229 patients 7 , 39 , 40 , 41 , 42 revealed a statistically significant association between AC and mortality (RR = 0.56, 95% CI 0.36; 0.92, p = 0.0218). Between‐study heterogeneity was 87%. The estimate reported by Tang and colleagues was identified as an outlier in mortality analysis and subsequently removed. 43
FIGURE 3.
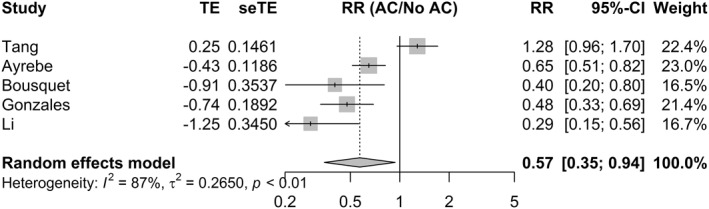
Random‐effects model for the association between in‐hospital AC and mortality. AC, Anticoagulation; CI, confidence interval; RR, risk ratio; SeTE, standard error; TE, total effect
After exclusion, the heterogeneity between studies (I2) decreased to 56%, and the effect size, still significantly increased (RR = 0.48, 95% CI 0.35–0.67, p < 0.001) indicating a beneficial effect for in‐hospital AC (Figure S1) on mortality. The pooled effect size (Figure S2) was robust to the leave‐one‐out sensitivity analysis. The association remained statistically significant after restricting the analysis to good and fair quality studies (RR = 0.42, 95% CI = 0.31; 0.56, p < 0.001) with no heterogeneity observed between studies (Figure S3 I 2 = 0, p = 0.43). Adding two studies using the trim and fill method did not affect the pooled effect size (Figure S4).
The pooled unadjusted estimate for mortality (Figure S5) from six studies (n = 3671) was statistically significant at the 0.1 level (RR = 0.77, 95% CI 0.58; 1.01, p = 0.06). No outliers were detected, and influence analysis showed that omitting either study conducted by Tang 43 or Paranjape 31 resulted in a statistically significant effect size at the 0.05 level (Figure S6).
Subgroup analysis (Figure 4) after excluding the study by Tang and colleagues 43 (n = 3780) revealed a statistically significant association between prophylactic AC and mortality (RR = 0.54, 95% CI 0.41; 0.71). The association between therapeutic AC and mortality (RR = 0.4, 95% CI 0.27; 0.57) was also significant. No heterogeneity was observed between studies for prophylactic and therapeutic AC. Using unadjusted estimates (Figure S7) resulted in similar effect size for prophylactic AC (RR = 0.65, 95% CI 0.43; 0.99, p = 0.043) but not for therapeutic AC (RR = 0.67, 95% CI 0.42; 1.07, p = 0.096).
FIGURE 4.
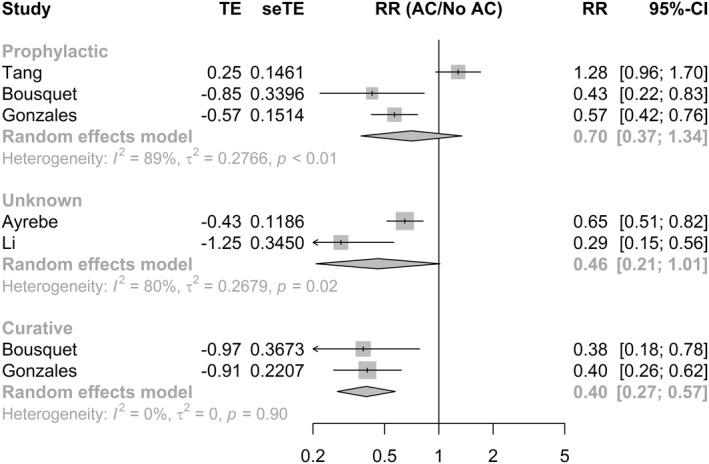
Subgroup analysis for adjusted estimates of mortality. AC, anticoagulation; CI, confidence interval; RR, risk ratio; SeTE: standard error; TE, total effect
Two studies reported the un‐adjusted 30 and adjusted 39 estimates for the incidence of VTE in Covid‐19 patients. The former reported a lower incidence of VTE (0% vs. 22%). Similar findings were reported by the latter for both prophylactic and therapeutic doses of anticoagulants. One study reported the adjusted and unadjusted estimates for PE 29 in s sample of 1284 patients. The former reported that AC (both pre‐admission and in‐hospital) was associated with a lower incidence of PE. The detailed findings for these studies are reported in Appendix S7. None of the studies reported the adjusted estimates for DVT, and only two studies reported unadjusted estimates. 27 , 28 A meta‐analysis of these estimates was not performed, given that only two studies reported unadjusted estimates.
3.5. Association between pre‐admission AC and both mortality and incidence of VTE
Three studies (n = 763) reported adjusted estimates for the association between pre‐admission AC and mortality (Figure 5). There was no statistically significant association between pre‐admission AC and mortality (RR = 0.84, 95% CI 0.49; 1.43, p > 0.05). The influence analysis did not alter the pooled estimate (Figure S8). One study was of good quality 35 while the remaining two were of poor quality. 33 , 34 (Figure 6)
FIGURE 5.
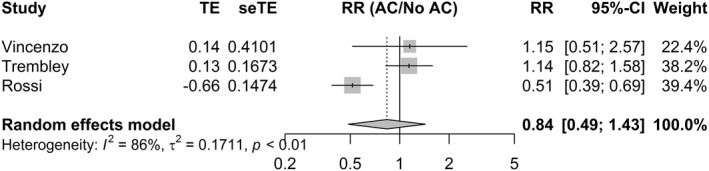
Random‐effects model for the association between pre‐admission AC and mortality. AC, anticoagulation; CI, confidence interval; RR, risk ratio; SeTE: standard error; TE, total effect
FIGURE 6.
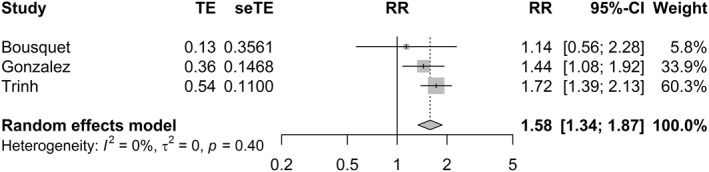
Random‐effects model for the association between the dose of the used anticoagulant and mortality (RR > 1 favours therapeutic doses and RR < 1 favours prophylactic doses, CI, confidence interval; RR, risk ratio; SeTE, standard error; TE, total effect)
Six studies (n = 3817) provided unadjusted estimates for the association between pre‐admission AC and mortality (Figure S9). The pooled estimate was not statistically significant (RR = 1.25, 95% CI 0.75; 2.05, p > 0.05), similar to what was observed with the adjusted estimate. The observed heterogeneity was high (I2 = 90%), and influence analysis (Figure S10) using the leave‐one‐out method did not alter the results, although the heterogeneity decreased to 33.2% and was not statistically significant after excluding the study by Tremblay, 35 which was identified as an outlier (RR = 0.97, 95% CI 0.67; 1.39). The trim and fill method (after exclusion) did not alter the pooled estimate (Figure S11). Four of the studies described the pre‐admission AC as therapeutic. 33 , 35 , 36 , 38 AC was used for cardio‐active treatment and chronic conditions in the remaining two studies. 34 , 37
3.6. Association between the dose of the used anticoagulant and both mortality and incidence of VTE
Three studies (n = 869) provided the adjusted estimates for mortality in patients who received low and high doses of anticoagulants 41 , 42 , 44 while four studies 41 , 42 , 44 , 45 provided unadjusted estimates (n = 895). The pooled estimate for mortality (Figure 6) favoured therapeutic AC when the adjusted estimates were used for the analysis (RR = 1.58, 95% CI 1.34; 1.87, p < 0.001), and no heterogeneity was observed between studies (I2 = 0%). The estimate was robust to the leave‐one‐out sensitivity analysis (Figure S12). Pooled analysis of unadjusted estimates (Figure S13), on the other hand, was not statistically significant (RR = 1.22, 95% CI 0.94; 1.58, p = 0.127) with low observed heterogeneity (I2 = 44%). Omitting the study conducted by Gonzalez 42 resulted in a statistically significant favourable effect for therapeutic AC (RR = 1.5, 95% CI 1.15; 1.94, p = 0.003) and no heterogeneity between studies (I2 = 0%).
The association between AC and the incidence of VTE was reported in two studies. 32 , 45 The association between AC and incidence of PE and DVT was reported in one study each. 28 , 29 Meta‐analysis was not performed for these estimates due to the small number of studies.
4. DISCUSSION
Based on evidence from a recently published meta‐analysis, the pooled estimate for mortality during an average follow‐up of 17.5 days was 6.6% (95% 2.8%; 15%). The pooled estimates for the incidence of VTE were 30.3% and 13.4% in ICU and ward settings, while the incidence of PE was estimated at 15.7% and 5.6%, respectively. 46
Preliminary data showed that prophylactic anti‐coagulation is associated with lower mortality in Covid‐19 patients, especially in patients with elevated levels of D‐dimer and who are on mechanical ventilation. 31 , 43 Therapeutic, prophylactic and pre‐admission anti‐coagulation were also associated with a lower risk of pulmonary embolism. 29 Zhang reported a beneficial effect for AC in patients with Padua risk score ≥4, although it was not statistically significant. 27 Tang et al. 43 concluded that using therapeutic doses of heparin for seven or more days was associated with better prognosis in patients with severe infection, meet sepsis‐induced coagulopathy score criteria, or have markedly elevated D‐dimer. A large cohort study evaluated the role of AC in mechanically ventilated Covid‐19 patients in the Mount Sinai Health System, USA. Results showed an in‐hospital mortality rate of 29.1% in patients who used anticoagulants compared to 62.7% in patients who did not receive anticoagulants. 31
Current guidelines recommend using thromboprophylaxis for all hospitalized patients with confirmed or highly suspected Covid‐19 irrespective of VTE risk assessment score in context to the recognition of the clotting dysregulation issue. 47 , 48 The use of prophylactic UFH or LMWH after discharge has also been proposed. Empiric use of therapeutic AC and thrombolytics was also suggested as a rescue approach for critically ill patients. 49 , 50 Less interest had been invested in using warfarin and DOACs in Covid‐19 patients; because of the potential drug–drug interaction with antiviral medications. 51 , 52
The majority of the current recommendations were based on expert opinions and uncontrolled studies. Evidence to support such a statement from non‐randomized studies is emerging. Li and colleagues reported that oral and parenteral anti‐coagulation reduced the risk for thromboembolism, although the exact dose of anticoagulant was not specified, 39 a finding supported by other studies included in the current systematic review. 27 , 29 Gonzalez, Bousquet and Ayrebe reported a beneficial effect for AC on mortality in Covid‐19 patients. 40 , 41 , 42 Several studies also reported a favourable effect for in‐hospital AC on mortality, 39 , 41 , 42 and others reported such beneficial effect in only selected subgroups of patients such as mechanically ventilated patients. 31
The current meta‐analysis showed a favourable effect for in‐hospital AC on mortality in Covid‐19 patients, and the association persisted when non‐adjusted estimates were used. The study by Tang was identified as an outlier as the author reported higher overall mortality (although non‐significant) with the use of prophylactic AC, a fact which was not observed in any other included study. The study by Tang classified patients based on the duration of anti‐coagulation, which might introduce misclassification bias. 43 Selection bias might have also confounded the results as only patients with severe Covid‐19 were included in the analysis. 43
Regarding pre‐admission AC, Tremblay 35 reported higher rates of mortality in patients who were on pre‐admission therapeutic AC. After adjusting the results for age, sex, race, Charlson Comorbidity Index and obesity, no statistically significant difference was observed between groups. On the other hand, Rossi 34 reported lower rates of mortality for patients who were on chronic treatment DOAC. Other studies did not show a statistically significant beneficial effect for pre‐admission AC. 38 , 44 Selection bias might have influenced the estimates reported in these studies for two main reasons. First, patients are usually initiated on therapeutic anticoagulants mainly for coagulation and cardiovascular problems, which can introduce confounding by indication and bias. Secondly, some studies do not take hospital‐related factors (e.g., other medications being administered during hospitalization) into consideration, and the observed effect in such cases might be confounded by factors that were not studied during the hospital stay. The findings of this meta‐analysis support the hypothesis that pre‐admission therapeutic AC is not associated with mortality. These results are similar to results initially reported by Klok et al., who found that therapeutic AC before admission was not associated with mortality using a competing risk model. 36 The estimate, in the current meta‐analysis, was robust when the unadjusted estimates were used. This might shed some light on the true estimate of the association between these factors and the confounding introduced by the research question itself.
To the best of our knowledge, this is the first meta‐analysis to investigate, separately, the effect of pre‐admission and in hospital AC on mortality and incidence of VTE using only case‐control and cohort studies. This might reduce the bias that may result from including all AC settings in one meta‐analysis as patients who were initiated on anticoagulants before admission are more likely to do so for various comorbidities that might influence the reported outcomes.
The current systematic review and meta‐analysis also investigated the effect of high versus low dose AC and showed that prophylactic AC might be associated with higher mortality than therapeutic AC. The results were derived from only three studies that provided the adjusted estimates. The benefit of therapeutic AC must be weighed against various adverse outcomes associated with anticoagulants' use, specifically bleeding, which is the major adverse effect of concern. 9 , 10 Bleeding cannot be neglected as an adverse effect of AC, especially in the presence of thrombocytopenia status in Covid‐19 infected patients associated with infection severity and mortality. 53 Additionally, reported cases of bleeding were investigated in patients with Covid‐19 infection. 54 , 55 Findings regarding the association between the dose of the used anticoagulant and mortality are also inconclusive as the unadjusted estimates yielded different results and, thus, should be interpreted with caution.
5. LIMITATIONS
More than half of the included studies were of low‐quality, which is reflected by the NOS tool. Although the adjusted estimates were used for the primary analysis, selection bias and confounding can influence the reported results due to the non‐randomized and mostly retrospective nature of the included studies. Moreover, some studies did not specify the dose 40 of the used anticoagulants, and some studies were restricted to specific subgroups such as patients on mechanical ventilation who might not be representative of hospitalized Covid‐19 patients. 35 , 44 Various anticoagulants and doses were used across studies, and some studies did not report the exact doses used. Moreover, the pooled estimates were not calculated for VTE due to the small sample size.
6. CONCLUSION
The current systematic review and meta‐analysis, which included only controlled non‐randomized studies, provide evidence to support the association of AC with mortality in Covid‐19 patients. The results, synthesized from mostly low‐quality studies, show that prophylactic AC may reduce mortality in Covid‐19 patients. They also show that therapeutic AC might offer an advantage over prophylactic AC. Randomized clinical trials are highly encouraged to produce high‐quality evidence regarding the safety and efficacy of AC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
Data extraction: Mona Sobhy, Nada Magdy, Analysis and interpretation of the data: Ahmed M. Kamel, Nada Magdy, Drafting of the article: Ahmed M. Kamel, Mona Sobhy, Nirmeen Sabry, Samar Farid, Critical revision for important intellectual content: Nirmeen Sabry, Samar Farid, Final approval of the article: Nirmeen Sabry, Samar Farid, Collection and assembly of data: Mona Sobhy, Ahmed M. Kamel.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Supplementary Material 1
Supplementary Material 2
ACKNOWLEDGEMENTS
We would like to thank Dr. Helena Sivaloganathan for providing essential data for the analysis. This research did not receive any grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report, 51. World Health Organization. 2020; https://apps.who.int/iris/bitstream/handle/10665/331475/nCoVsitrep11Mar2020-eng.pdf?sequence=1&isAllowed=y. Accessed July 20, 2020.
- 2. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol. 2020;215:108427. 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuppalli K, Rasmussen AL. A glimpse into the eye of the COVID‐19 cytokine storm. EBioMedicine. 2020;55:102789. 10.1016/j.ebiom.2020.102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Chest. 1995;108(5):1303‐1314. 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 5. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46‐e47. 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buonaguro FM, Ascierto PA, Morse GD, et al. Covid‐19: Time for a paradigm change. Rev Med Virol. 2020;30(5). 10.1002/rmv.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harter K, Levine M, Henderson S. Anticoagulation drug therapy: a review. West J Emerg Med. 2015;16(1):11‐17. 10.5811/westjem.2014.12.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: a review of the pharmacology, dosing, and complications. Curr Emerg Hosp Med Rep. 2013;1(2):83‐97. 10.1007/s40138-013-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe covid‐19 patients: a retrospective clinical study. medRxiv;28:20046144. 10.1101/2020.03.28.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Negri EM, Piloto B, Morinaga LK, et al. Heparin therapy improving hypoxia in COVID‐19 patients ‐ a case series. medRxiv. 10.1101/2020.04.15.20067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16. 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O'Connell D, Peterson J. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Ottawa, Canada: Ottawa Hosp Res Inst; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16. Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. http://www.ncbi.nlm.nih.gov/pubmed/22479713. [PubMed] [Google Scholar]
- 17. Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Stat Med. 2001;20(4):641‐654. 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 18. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta‐analysis. J Am Stat Assoc. 2000;95(449):89. 10.2307/2669529. [DOI] [Google Scholar]
- 19. Lundh A, Gøtzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8. 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21. Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Res Synth Methods. 2016;7(1):55‐79. 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VanderWeele TJ. Optimal approximate conversions of odds ratios and hazard ratios to risk ratios. Biometrics. 2019;76(3):746–752. 10.1111/biom.13197. [DOI] [PubMed] [Google Scholar]
- 23. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. John Wiley & Sons; 2009. 10.1002/9780470743386s. [DOI] [Google Scholar]
- 24. Thompson SG, Higgins JPT. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. http://doi.wiley.com/10.1002/sim.1186%0Afile:///Files/8F/8F67F2E0-726D-48C8-AF56-3CC1CC367697.pdf%0Apapers3://publication/doi/10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25. Borenstein M, Hedges L, Higgins JPT, Rothstein HR. Comprehensive Meta‐Analysis Version 3. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 26. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R A Lang Environ Stat Comput R Found Stat Comput; 2020. [Google Scholar]
- 27. Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation;120:046702. 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 28. Koleilat I, Galen B, Choinski K, et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease (COVID‐19). J Vasc Surg Venous Lymphat Disord. 10.1016/j.jvsv.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J. 2020;13:1‐11. 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995–2002. 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in‐hospital survival among hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(1):122‐124. 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID‐19 patients: a French monocenter retrospective study. Crit Care. 2020;24(1):275. 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russo V, Di Maio M, Attena E, et al. Clinical impact of pre‐admission antithrombotic therapy in hospitalized patients with COVID‐19: a multicenter observational study. Pharmacol Res. 2020;159:104965. 10.1016/j.phrs.2020.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID‐19 era. Eur J Intern Med. 2020;77:158‐160. 10.1016/j.ejim.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tremblay D, van Gerwen M, Alsen M, et al. Impact of anticoagulation prior to COVID‐19 infection: a propensity score–matched cohort study. Blood. 2020;136(1):144‐147. 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148‐150. 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giacomelli A, Ridolfo AL, Milazzo L, et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sivaloganathan H, Ladikou EE, Chevassut T. COVID‐19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. 190, 2020(4):e192–e195. 10.1111/bjh.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li W, Xiong J, Guo Y, Lip GYH. Risk factors for systemic and venous thromboembolism, mortality and bleeding risks in 1125 patients with COVID‐19: relationship to anticoagulation status. 2020. https://search.proquest.com/openview/4142cbb8cb14389b343078ed91f093f8/1?pq-origsite=gscholar&cbl=4361587 [DOI] [PMC free article] [PubMed]
- 40. Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid‐19. J Thromb Thrombolysis. 2020;50(2):298‐301. 10.1007/s11239-020-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bousquet G, Falgarone G, Deutsch D, et al. ADL‐dependency, D‐Dimers, LDH and absence of anticoagulation are independently associated with one‐month mortality in older inpatients with Covid‐19. Aging (Albany NY). 2020;12(12):11306‐11313. 10.18632/aging.103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalez‐Porras JR, Belhassen‐Garcia M, Bernus AL, Vaquero‐Roncero LM. Low molecular weight heparin in adults inpatient COVID‐19. 10.2139/ssrn.3586665. [DOI]
- 43. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trinh M, Chang DR, Govindarajulu US, et al. Therapeutic anticoagulation is associated with decreased mortality in mechanically ventilated COVID‐19 patients. medRxiv;30:20117929. 10.1101/2020.05.30.20117929. [DOI] [Google Scholar]
- 45. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020;18(7):1743‐1746. 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nasiri MJ, Haddadi S, Tahvildari A, et al. COVID‐19 clinical characteristics, and sex‐specific risk of mortality: systematic review and meta‐analysis. Front Med. 2020;7:459. 10.3389/fmed.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rico‐Mesa JS, Rosas D, Ahmadian‐Tehrani A, White A, Anderson AS, Chilton R. The role of anticoagulation in COVID‐19‐induced hypercoagulability. Curr Cardiol Rep. 2020;22(7):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moore HB, Barrett CD, Moore EE, et al. Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID‐19 associated acute respiratory distress syndrome? J Trauma Acute Care Surg. 2020;88(6):713‐714. 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arachchillage DJ, Stacey A, Akor F, Scotz M, Laffan M. Thrombolysis restores perfusion in COVID‐19 hypoxia. Br J Haematol. 2020;190(5):e270–e274. 10.1111/bjh.17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marietta M, Ageno W, Artoni A, et al. COVID‐19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020;18(3):167‐169. 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lucatelli P, De Rubeis G, Citone M, et al. Heparin‐related major bleeding in covid‐19‐positive patient: perspective from the outbreak. Cardiovasc Interv Radiol. 2020;43:1216–1217. 10.1007/s00270-020-02532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Conti CB, Henchi S, Coppeta GP, Testa S, Grassia R. Bleeding in COVID‐19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147‐149. 10.1016/j.ejim.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeng D‐X, Xu J‐L, Mao Q‐X, et al. Association of Padua prediction score with in‐hospital prognosis in COVID‐19 patients. QJM. 2020;(899):1‐20. 10.1093/qjmed/hcaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


