Abstract
Immunotherapy using immune checkpoint blockade has revolutionized the treatment of many types of cancer. Radiation therapy (RT)-particularly when delivered at high doses using newer techniques-may be capable of generating systemic antitumor effects when combined with immunotherapy in breast cancer. These systemic effects might be due to the local immune-priming effects of RT resulting in the expansion and circulation of effector immune cells to distant sites. Although this concept merits further exploration, several challenges need to be overcome. One is an understanding of how the heterogeneity of breast cancers may relate to tumor immunogenicity. Another concerns the need to develop knowledge and expertise in delivery, sequencing, and timing of RT with immunotherapy. Clinical trials addressing these issues are under way. We here review and discuss the particular opportunities and issues regarding this topic, including the design of informative clinical and translational studies.
Introduction
Advances in systemic therapy and molecular classification of breast cancer subsets have facilitated the identification of patients with breast cancer who may potentially benefit from therapy escalation or de-escalation. In breast radiation oncology, most modern clinical trials have focused on identifying the patients whose tumor characteristics are most appropriate for strategies to minimize overtreatment by exploring the omission of regional nodal irradiation, boost, or radiation therapy (RT) altogether in low-risk breast cancer.1 A smaller set of trials in women at high risk of locoregional recurrence is combining radiosensitizing agents such as PARP inhibitors with RT in an attempt to improve outcomes (NCT03945721, NCT03542175, NCT03598257, and NCT01618357). A critical goal, however, is the accurate identification of patients with breast cancer who remain at high risk of disease or progression, despite aggressive standard-of-care therapies. Furthermore, with multiple systemic therapies being rapidly incorporated into breast cancer treatment, critical questions arise regarding the optimal leveraging of RT to effectively synergize with DNA repair-based therapies, kinase inhibitors, endocrine therapies, or immunotherapies.
Among these novel emerging therapies, immunotherapy in particular is a promising therapeutic strategy in breast cancer, given established clinical activity, growing recognition of the role of immunosuppression in the tumor microenvironment (TME), and the association of more robust immune responses with favorable prognosis across many tumor types.2,3 A variety of local, ablative strategies are under investigation for their potential to overcome intrinsic resistance to immune checkpoint inhibitors (ICI), including cryotherapy and RT.4–6 RT is a well-established method of inducing localized tumor cell death. However, RT can modulate the TME by both stimulation and suppression of antitumor immune responses.5–7 Furthermore, the development of sophisticated technologies that enable precise delivery of high RT doses to the tumor may also make RT a practical partner for immune-based therapies; however, much remains to be understood regarding the impact of dose and scheduling of RT on immunotherapy efficacy.
Clinical Opportunities for Immunotherapy/Radiation Therapy Strategies in Breast Cancer
Breast cancer is a heterogenous disease that can be grouped into clinically relevant subtypes defined by expression patterns of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Each subtype displays distinct genomic alterations and clinical behaviors and is treated differently.8,9 The majority of patients with stage II-III ER-negative, PR-negative, and HER2-negative breast cancer (referred to as triple negative breast cancer [TNBC]) and HER2-positive (HER2+) breast cancer receive preoperative (neoadjuvant) chemotherapy.10 This preoperative administration of systemic therapy has several advantages, including improved vasculature for drug delivery, the potential to allow for less radical surgery, an ability to assess the tumor response in vivo before surgery, and avoiding delay in starting systemically active agents in patients at elevated risk of distant disease progression. Despite favorable pathologic complete response (pCR) rates, overall response rates (ORRs) ranging from 40% to 70% with standard taxane-based chemotherapy, and the addition of dual HER2-targeted agents for HER2+ breast cancer,11–13 patients with TNBC and HER2+ breast cancer with residual disease in the breast and particularly in the lymph nodes after neoadjuvant chemotherapy remain at extremely high risk of recurrence.14 Patients with hormone receptor-positive (ER- and/or PR-positive)/HER2-negative breast cancer have historically fared better, but select subsets, including those with locoregionally advanced disease at presentation and high-grade or high genomic assay scores, remain at elevated risk of recurrence, despite standard hormone therapy.15
The emergence of ICI over the past decade has transformed the therapeutic landscape for diseases such as melanoma and lung cancer. Higher tumor mutation burden in these tumor types has been associated with a greater likelihood of ICI response.16 In contrast, the relatively lower tumor mutation burden of breast cancer has been perceived as a potential barrier to immune recognition.17 Furthermore, although the majority of breast cancers contain tumor-infiltrating lymphocytes (TILs), only about 11% are considered lymphocyte predominant.18 Breast cancer-associated TILs may also affect response and efficacy of not just ICI, but radiation as well. These properties may explain in part the modest ORRs of ICI monotherapy in unselected metastatic breast cancer, ranging from 5% to 23% across breast cancer subtypes.19–22
Biomarker analyses of the responding patients within these trials led to the identification of pretreatment PD-L1 expression as a candidate predictive biomarker of ICI response.19,21,23 For example, in the Impassion130 trial, patients with untreated metastatic TNBC were randomized to receive atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel. Among patients with PD-L1 immune cell-positive TNBC (41% of all patients), the median overall survival was 25 months and 18 months for the atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel groups, respectively (hazard ratio, 0.71; 95% CI, 0.54–0.94).24 The results of this study led to the accelerated approval of this combination in PD-L1-positive metastatic TNBC. To date, clinical benefit of ICI in the metastatic setting has been greatest when administered earlier in a patient’s disease course. These results highlight the potential opportunity for improved outcomes by introducing immunotherapeutic combinations earlier in the disease course.
In the phase 3 KEYNOTE 522 trial, the addition of pembrolizumab to standard-of-care neoadjuvant chemotherapy and adjuvant treatment of operable stage II or III TNBC led to higher rates of pCR (51.2% vs 64%) representing an absolute difference of 13.6% (95% CI, 5.4%−21.8%; P = .00055).25 The magnitude of pCR benefit with addition of pembrolizumab to chemotherapy was subsequently reported to be higher among patients with node-positive disease, compared with the node-negative group.26 This raises the question of whether this differential effect could have been secondary to an immune-priming phase in the involved lymph nodes and whether the addition of RT or other novel agents could further augment responses (Table 1). An early read-out of improved event-free survival was also reported in the pembrolizumab arm. If the exciting results from KEYNOTE 522 are sustained with prolonged follow-up, neoadjuvant ICI will be incorporated into the routine management of early-stage TNBC. In turn, this will provide an efficient platform to test whether the addition of RT can further amplify immune responses in TNBC. Of note, PD-L1 has remained an imperfect biomarker; pCR rate was also higher in PD-L1-negative tumors in KEYNOTE 522, and some PD-L1-negative patients appeared to also benefit from treatment in the metastatic setting.25 Moreover, controversy still exists in terms of the optimal assay to define PD-L1 status. The lack of validated predictive biomarkers of response to ICI in breast cancer remains a major gap in our understanding of how to best use these agents, and these findings highlight the importance of incorporating biomarker discovery correlative studies in clinical trials to guide patient selection and improve long-term cure rates.
Table 1.
Design elements of preoperative IO/RT clinical trials in breast cancer
| Key elements | Pros | Cons |
|---|---|---|
| Inclusion of lymph node–positive disease only |
|
|
| Inclusion of TN and high-risk, HR+/HER2− breast cancer |
|
|
| Testing multiple dose levels including a control (no RT) |
|
|
| Conducting trial within a cooperative group setting |
|
|
| Inclusion of SBRT and proton radiation therapy |
|
|
Abbreviations: HR+/HER2− = hormone receptor positive/human estrogen receptor 2 negative; IO = immune oncology; NAC = neoadjuvant chemotherapy; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
Dense lymphocytic infiltration has been observed in a significant proportion of TNBCs and HER2+ enriched tumors, indicating a complex interplay between the tumor and the immune system.27,28 ER- and/or PR-positive breast cancers are less likely to be infiltrated by CD8+ cytotoxic TILs compared with TNBC and HER2+ tumors, and it has been suggested that these HR+ tumors are associated with decreased immunogenicity.29,30 Consistently, response rates of ICI have been lowest in patients with HR+ disease.20 That said, elevated mRNA expression of immune checkpoint molecules, including PD-L1, CTLA4, B7H-3, and IDO1, has been noted in the HR+ luminal population. Furthermore, a subset of HR+/HER2− tumors has increased lymphocytic infiltrate that is not as strongly driven by estrogen and belongs to the luminal B subtype.31,32 Whether this subset will be more responsive to ICI and radiation combinations is currently being tested (NCT03875573) and highlights the potential for further optimization of ICI combinations to augment the antitumor immune response beyond TNBC.
The Abscopal Effect of RT in Breast Cancer
The goal of immunotherapy/RT (IO/RT) is to induce prolonged local and distant antitumor effects through the induction of systemic immunity. This is clinically manifested through the so-called abscopal effect, in which systemic regression of tumor occurs outside the irradiated portal, after focal irradiation of a single site.6 Although rarely observed after RT alone,33 it has been increasingly reported in IO/RT regimens in breast cancer (Fig. 1).
Fig. 1.
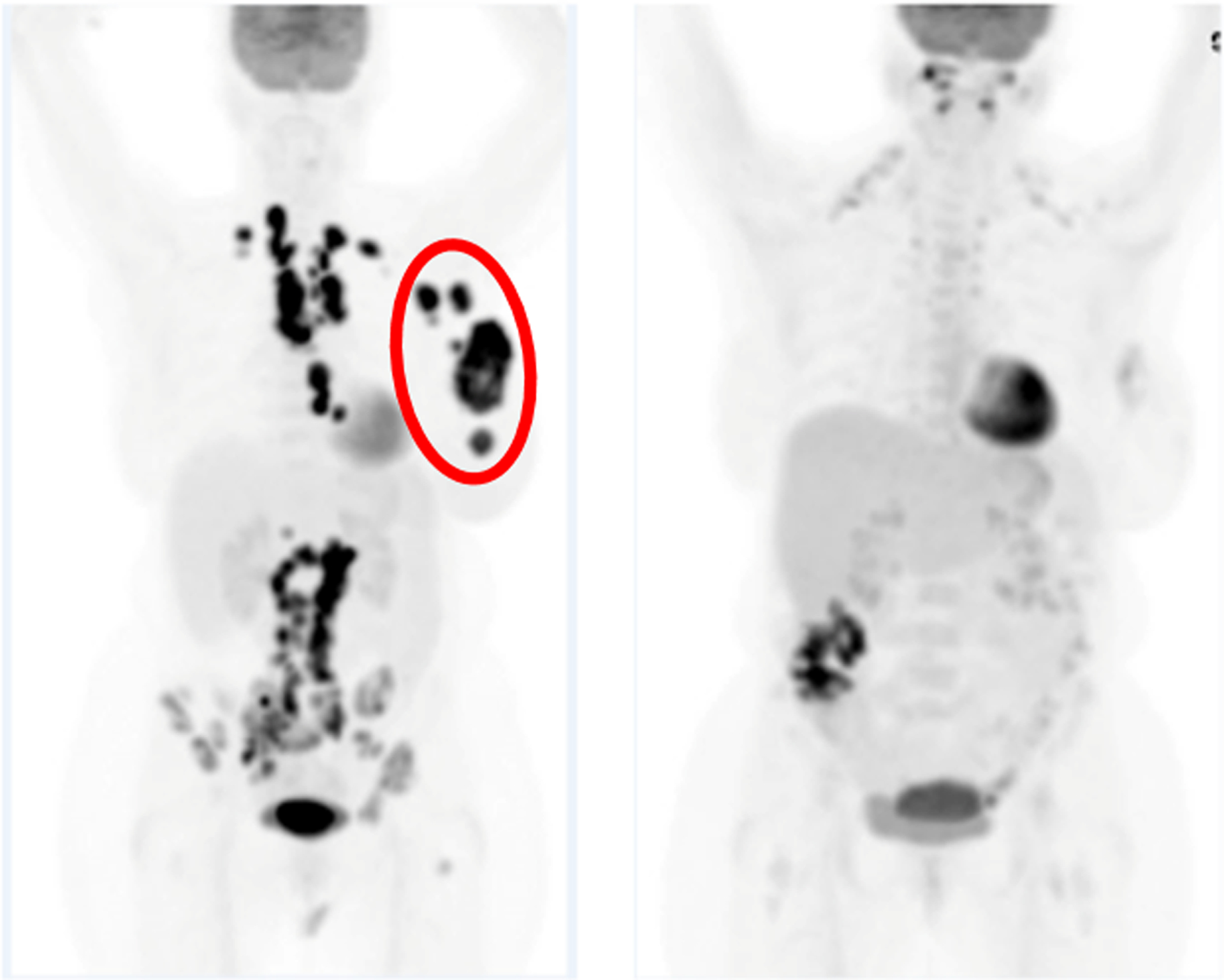
An example of an abscopal effect observed on PET/CT scanning in a patient with metastatic TNBC treated with pembrolizumab and hypofractionated RT (30 Gy/5 fractions) to a breast mass. The red circle denotes the RT field. At 19 weeks, regression of tumor in the mediastinal lymph nodes outside the RT portal is observed.37 Abbreviations: CT = computed tomography; PET = positron emission tomography; RT = radiation therapy; TNBC = triple negative breast cancer.
Little is understood about systemic modifiers of abscopal responses beyond the activities within the TME or regional lymph nodes, resulting in a critical need to understand the potential rate-limiting steps involved in the conversion of a localized immune response to a systemic immune response. Additionally, heterogeneity or lack of reporting of this abscopal response in trials using IO/RT in breast cancer further hinders our ability to define populations most likely to experience out-of-field responses. Progress in these areas of research and more careful and consistent reporting of out-of-field response rates will refine our selection of patients for IO/RT combination therapies, guide biomarkers of treatment response and efficacy, and help select additional therapeutic combinations to overcome resistance to IO/RT. A key barrier is the development and establishment of clinically relevant preclinical models for immunotherapy research and focused clinical translational studies to understand the biological mechanisms that may help develop predictive biomarkers of response and resistance and inform rational, immunotherapy-based combinations.
Early Evidence for IO/RT Combinations in Metastatic Breast Cancer
To date, IO/RT combinations constitute only 13 (7%) of 185 trials involving ICI, compared with 64 trials (35%) that combine chemotherapy with ICI.34 An early experience with IO/RT in breast cancer was reported in the TONIC trial, an adaptive phase 2 trial of 67 women with metastatic TNBC that explored a variety of induction strategies in combination with nivolumab, an anti-PD-1 antibody.35 In the cohort of patients (n = 12) who received palliative RT (24 Gy in 3 fractions) followed 3 weeks later by nivolumab, the ORR was disappointingly low (8%) relative to the ORR in the overall cohort (20%), precluding any further investigation into the combination. A limitation of this study is the relatively long interval between completion of RT and initiation of ICI, which may reduce the likelihood of therapeutic synergy.36
Subsequently, more encouraging experiences with IO/RT in breast cancer were reported in a multicenter, phase 2, single-arm study of pembrolizumab, a humanized, monoclonal anti-PD-1 antibody, and hypofractionated stereotactic body RT (SBRT) (30 Gy in 5 fractions) in 17 patients with metastatic TNBC who were unselected for PD-L1 status.37 Pembrolizumab was administered within 3 days of the first fraction of RT and continued every 3 weeks until tumor progression. The definition of ORR was radiographic response outside of the irradiation portal as per Response Evaluation Criteria in Solid Tumors (RECIST). The ORR in the entire cohort was 18% (3 of 17); however, among the 9 women who were radiographically evaluable at week 17, 3 demonstrated a complete response, with 100% reduction in tumor volume outside the irradiated field. All 3 responders were PD-L1 positive, suggesting that PD-L1 status might serve as a biomarker of response in patients receiving IO/RT, as it has for other immunotherapy trials in breast cancer.13,19,22,38 Although the results from this signal-seeking study were positive, the small sample size and inability to differentiate the effect of RT and ICI on the primary end point limit immediate application among metastatic TNBC patients. Nevertheless, this study demonstrated the tolerability of SBRT using a hypofractionated approach concurrently with ICI in heavily pretreated patients with metastatic TNBC, paving the path for clinical trials using other combinatorial approaches with IO/RT.
Finally, the combination of pembrolizumab and RT was also well tolerated in another phase 2 trial of metastatic HR+/HER2− breast cancer performed at the Dana Farber Cancer Institute.39 In contrast to earlier trials, the RT dose was lower in this trial (20 Gy in 5 fractions) and directed to a bone metastatic lesion. Owing to the lack of any objective responses, the trial was closed after accruing 8 patients, leading to the speculation that the lower doses of RT used in the trial may have been insufficient to invigorate a response. Similar to the pembrolizumab/RT trial in metastatic TNBC, limitations were the inability to associate biomarkers with response owing to lack of evaluation and no responses.
Collectively, these early experiences signal the potential for IO/RT combinations to be effective in breast cancer. Further study is essential to elucidate the optimal dose, fractionation, and timing of RT delivery with respect to ICI. Accurate patient selection is critical to ensure benefit with IO/RT combinations and may potentially be informed by PD-L1 status, quantification of TILs, or a combination of biomarkers. Insights gained from these early trials have inevitably guided the next generation of IO/RT clinical trials in breast cancer, as will be further described.
Opportunity and Practical Challenges of Combining Breast Radiation and Immunotherapy in Preoperative Settings
The incorporation of immunotherapy agents into the preoperative (ie, neoadjuvant) setting presents unique advantages for priming antitumor immune responses and potential eradication of disseminated micrometastatic disease.40 Additionally, the ability to accurately and definitely localize primary disease and target it with radiation is an advantage of preoperative administration of radiation. Despite these advantages, however, integration of RT with immunotherapy in the preoperative setting for patients with localized breast cancer poses several pragmatic challenges (Table 1). Foremost is the issue of identifying the optimal dose of RT required to elicit a productive immune response with the least risk of toxicity. It is possible that the dose can be escalated to elicit a productive immune response when administered preoperatively because much of the irradiated tissue will be resected. Nevertheless, many patients will require standard-of-care adjuvant RT after surgical resection, which could pose additional acute and late toxicity risks to reirradiated tissue that is not removed at surgery.
Vanpouille-Box et al have demonstrated that RT doses higher than 10 Gy per fraction can stimulate expression of the exonuclease Trex1, which abrogates the synergy between RT and ICI by suppressing activation of cytosolic DNA damage-sensing pathways.41 When combined with anti-CTLA-4, hypofractionated RT (8 Gy × 3) led to increased accumulation of cytosolic DNA damage, activation of cGAS/STING, and increased type I interferon signaling that was necessary for CD8+ T-cell mediated antitumor immune responses and regression of nonirradiated lesions, compared with single-fraction high-dose RT. It is important to note that the widely used dose of 24 Gy in 3 fractions was developed in murine model systems and not yet validated as the preferred RT dose in human clinical trials.42 Thus, the optimal immunostimulatory preoperative RT dose in patients poses a genuine dilemma when translating preclinical findings into a clinical setting. Insufficient dose might not induce an immune response, whereas excessive dose could suppress the immune response or lead to excess toxicity, inferior cosmesis, and diminished quality of life for patients.
Limited knowledge of and expertise in developing consistent techniques of preoperative high-dose RT delivery in breast cancer IO/RT trials across centers also represents a practical barrier to developing large, multicenter trials of breast radiation and immunotherapy. Thus, there is a need for development of standardized contouring guidelines and dose constraints for normal tissues and tumor in the context of IO/RT. Furthermore, the optimal scheduling of RT with ICI continues to be hotly debated, with early clinical trials such as the aforementioned TONIC trial administering nivolumab sequentially, following hypofractionated RT (24 Gy in 3 fractions) and other data from the GEPARNuevo study suggesting that IO given before chemotherapy may prime T-cell responses.43 In contrast, all current trials of preoperative RT and ICI (Table 2) are delivering RT concurrently with ICI, based on preclinical data suggesting concomitant RT/ICI is more effective for generating a robust antitumor immune response than sequential administration.44,45
Table 2.
Preoperative IO/RT clinical trials* in breast cancer
| Sponsor/study name | Phase | N | Tumor type | Intervention |
|---|---|---|---|---|
| Jules Bordet Institute, Institut Curie (NCT03875573) | 2 | 147 | HR+/HER2− | SBRT ± durvalumab and oleclumab (24 Gy in 3 fractions) |
| Weill Medical College of Cornell University (NCT03804944) | 2 | 100 | HR+/HER2− | HT + RT (24 Gy in 3 fractions) ± FLT-3, pembrolizumab or both |
| Cedars-Sinai Medical Center (NCT03366844) | 1 | 60 | HR+/HER2− or TNBC | Pembrolizumab + RT (24 Gy in 3 fractions) |
| Columbia University (NCT02977468) | 1 | 15 | TNBC | Pembrolizumab + IORT |
Abbreviations: HR+/HER2− = hormone receptor positive/human estrogen receptor 2 negative; HT = hormone therapy; IO/RT = immunotherapy/radiation therapy; IORT = intraoperative radiation therapy; RT = radiation therapy; SBRT = stereotactic body radiation therapy; TNBC = triple negative breast cancer.
Registered on clinicaltrials.gov as of April 1, 2020.
Finally, establishing reliable end points and reproducible biomarkers to assess response to neoadjuvant IO/RT treatments is critical for uniform assessment of a systemic, immune-mediated response. Irradiation of an intact tumor may confound assessment by the classical definition of pCR, which includes response in the primary tumor and/or lymph nodes.46 pCR rates of 25% to 67% after preoperative RT in breast cancer have been reported.47,48 A phase 1 dose-escalation study examined the effect of 5 different dose levels of SBRT delivered concomitantly with neoadjuvant chemotherapy (NAC) in 25 evaluable patients with HER2-negative breast cancer.39 The pCR rate was 36% in the entire cohort, with no pCR observed at the first 2 dose levels and the maximum response of 67% observed at dose level 3 (25.5 Gy delivered in 3 fractions). pCR rates did not increase with dose escalation beyond dose level 3, suggesting 25.5 Gy is the optimal dose for preoperative SBRT in the development of future phase 2 trials.
A key goal of current studies of IO/RT in development is to ultimately build a standardized approach for RT delivery and response assessment that balances all of the previously mentioned considerations. A treatment paradigm that permits systemic evaluation of preoperative RT by assessing response in the lymph nodes (rather than the primary irradiated breast tumor, which would confound interpretation of pCR) is illustrated in Figure 2. Adopting this preoperative RT paradigm in node-positive TNBC would obviate concerns about depriving patients of the potential benefit of adjuvant capecitabine for residual disease that could be obfuscated by a radiation-induced pCR.
Fig. 2.
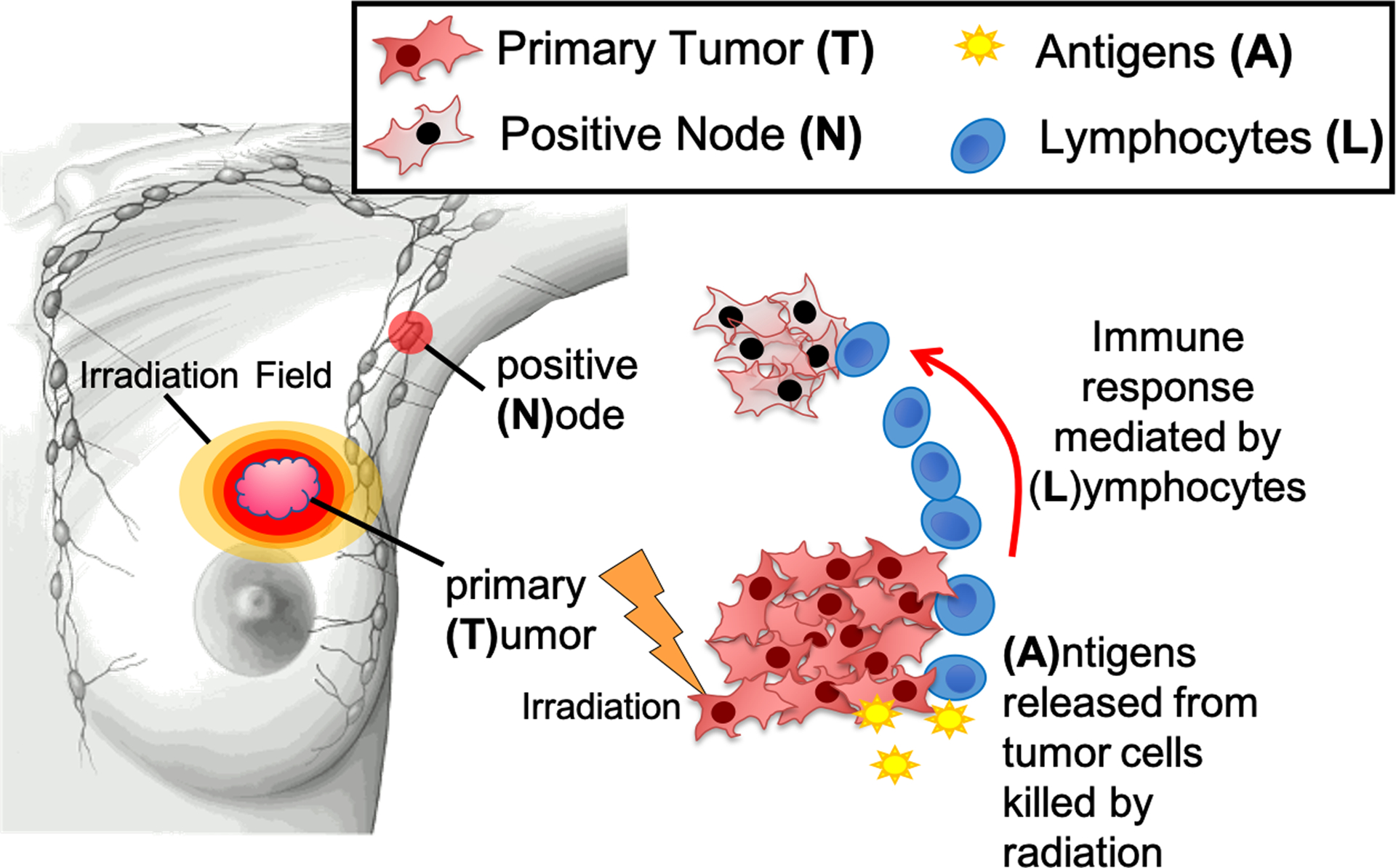
Model overview presenting the relationship between the primary tumor (T), antigen released (A), circulating lymphocytes (L), and the tumor cells in the positive node (N). Image created by Clemens Grassberger, PhD and Corey Speers, MD, PhD.
The Biologic Complexity of Combining Radiation With Immunotherapy
The biological complexity that underlies immunotherapy response remains an active area of investigation. Increasingly, evidence points to a complex interplay of tumor genomics, TME, host germline genetics, host immune status, and host microbiome that determines the likelihood of eliciting an antitumor immune response to immunotherapy. Furthermore, understanding the unique immunobiology of RT response is critical for establishing when, where, and how RT should be applied to overcome immunotherapy resistance (Fig. 3). This knowledge will also provide a distinction between RT and other ablative modalities, which may be associated with distinct biological effects on the TME and systemic level, despite inducing similar levels of localized cell kill. We will summarize 4 key knowledge gaps in the fundamental biology of RT-induced immune modulation. Progress in these areas will help facilitate scientifically guided translation of IO/RT combinations for clinical investigation.
Fig. 3.
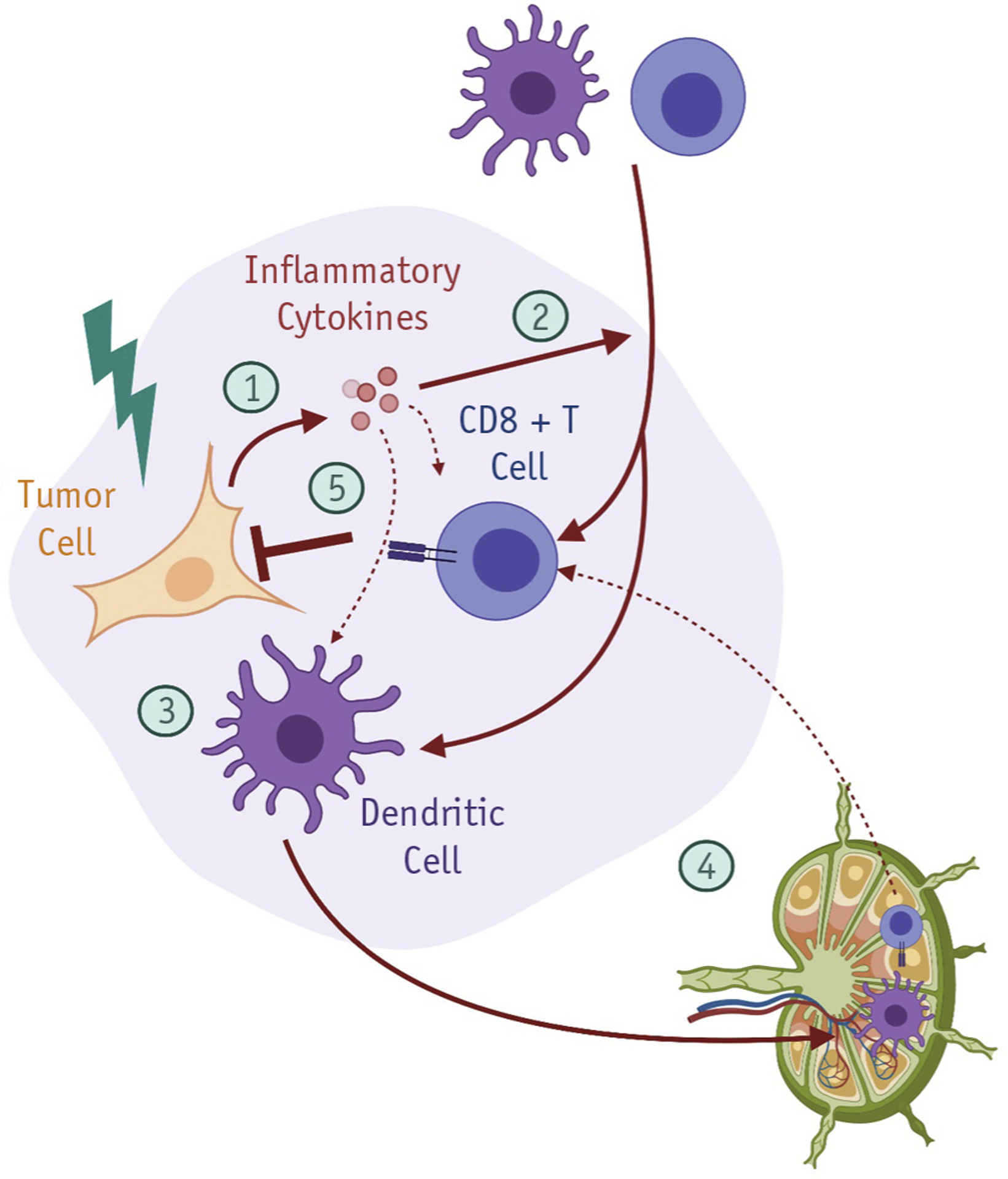
RT modulation of antitumor immune responses. RT exerts pleiotropic immunomodulatory effects on the tumor microenvironment. (1) RT-induced DNA damage in tumor cells stimulates innate immune signaling, in part through expression of type I interferon genes. (2) RT-induced chemokines promote infiltration of circulating lymphocytes and innate immune cells into the tumor microenvironment. (3) RT/IO can stimulate tumor cell phagocytosis by professional APCs, and (4) cross-presentation of tumor neoantigens in tumor-draining lymph nodes (TDLNs). (5) RT/IO can facilitate activation of primed tumor-reactive T cells to eradicate tumor cells at both the primary site and distant sites. Figure created with Biorender.com. Abbreviations: APC = antigen-presenting cells; RT = radiation therapy; RT/IO = radiation therapy/immunotherapy; TDLN = tumor-draining lymph nodes.
Radiation and innate immune signaling
A critical interplay has emerged between RT-induced damage and immune-sensing pathways that determine response to both RT and immunotherapy.5,49,50 One such mechanism involves the transmission of nuclear DNA damage to the cytoplasm, where it can engage cytoplasmic DNA sensors (eg, cGas/STING) and stimulate innate immune signaling, such as the type I interferon gene response.51–53 Abrogation of the cGas/STING pathway blunts abscopal responses induced by RT and ICI in preclinical cancer models.51,54,55 Alternative immune-sensing pathways are also operative after RT-induced DNA damage. Recently, DNA-PK was shown to act in a STING-independent pathway for cytosolic DNA damage sensing.56 RT also induces expression of endogenous retroviral genes and other immunogenic RNAs that can activate cytoplasmic RNA sensor pathways such as RIG-I.57,58 RT-induced DNA damage responses (DDRs) also induce expression of ligands for the activating NK cell receptor, NKG2D, which can potentiate responses to RT.59,60 Leveraging this RT effect in combination with emerging NK cell-based immunotherapy is a promising area for future research.61
The relative contributions of these RT-induced immune-sensing pathways likely depend on a variety of tumor-specific factors. Some tumors may silence or otherwise inactivate the cGas/STING pathway during tumorigenesis,62 whereas others may co-opt the pathway in a chronically activated form to facilitate metastatic potential.63 How functional perturbations in the STING pathway affect responsiveness to IO/RT combinations remains an outstanding question.41,53 Chronic activation of interferon-mediated JAK/STAT signaling has been shown to induce broad resistance to ICI and may represent targets for combination therapy in some tumors.64 Investigation of IO/RT response determinants will clarify how RT-induced innate immune signaling may be optimized to engender productive antitumor immune responses in patients.
Radiation and tumor neoantigen presentation
Another key step in the induction of a productive antitumor immune response is the processing and presentation of tumor-specific neoantigens to the adaptive immune system. RT has been shown to stimulate antigen presentation in a variety of tumor model systems, including breast cancer.65 RT can facilitate cross-presentation of HLA class I-restricted tumor neoantigens by professional antigen presenting cells (APCs) in tumor-draining lymph nodes (TDLNs), which promotes CD8+ T cell-mediated cytotoxicity.65 Conversely, irradiation of TLDNs can also negatively affect the balance between tumoricidal and immunosuppressive TILs and attenuate the combinatorial efficacy of IO/RT strategies.66
In breast cancer, there is a growing appreciation for the role of a more complex antigen-directed immune response, namely, one that entails follicular-type T helper cells and antibody-producing B cells that can infiltrate tumors and potentially generate tertiary lymphoid structures within the TME.67–69 This phenomenon may explain why antibody genes and B cells are strongly associated with favorable outcomes in breast cancer data sets.70 It is plausible that RT may also be able to stimulate the processing of HLA class II-restricted antigens by professional APCs-an activity that may be even more crucial in neoantigen-poor breast cancers. Improved research tools to predict, monitor, and assess HLA class II-restricted tumor neoantigens are in development.71–73 Understanding the barriers to effective tumor neoantigen presentation in the TME and in TDLNs will help guide future, rational IO/RT strategies.
Effects of RT on the immune tumor microenvironment
RT can also have direct effects on stromal and immune cells in the TME. For example, it is known that PD-1/PD-L1 expression by tumor-associated macrophages can inhibit their phagocytic activity and confer sensitivity to anti-PD-1/PD-L1 blockade.74–76 Thus, RT-induced expression of PD-1/PD-L1 may counteract RT-induced stimulation of HLA class I genes and impede productive antigen cross-presentation. This may, in part, explain the observed synergy between RT and anti-PD1/L1 therapy in stimulating productive antitumor immune responses.65 Additionally, RT induces expression of transforming growth factor β, which is a potent immunosuppressive growth factor77 and thus a pathway of great interest in IO/RT combination trials.78 Transforming growth factor β is a key upstream regulator of T-cell reprogramming and contributes to intratumoral T-cell radioresistance.79 Recruitment of immunosuppressive myeloid cell types can also mediate radioresistance, including by inhibiting tumor neoantigen priming to the adaptive immune system.80,81 Investigations into the impact of scheduling of different immunotherapeutic strategies with RT has shown that the best choice of sequence can depend on the exact mechanisms of action,45 an important aspect to consider when translating current insight to more effective IO approaches. Elucidation of additional immunosuppressive effects of RT will help to identify novel therapeutic combinations to augment IO/RT therapy.
Biological heterogeneity of RT-induced responses
As previously discussed, breast cancer is not a singular disease but rather a complex organ-specific malignancy with molecularly distinct subtypes.8,9 Based on this understanding, molecular subtyping in a variety of forms has contributed to many of the clinical advances in personalized breast cancer therapy during the past 2 decades, including radiation response.82,83 Emerging evidence indicates that breast cancers differ substantially in their biological response to DNA damage. Biomarkers of differential DDR in breast cancer are in development and may help guide the clinical implementation of RT in the standard-of-care management of breast cancer.83–87 The observed heterogeneity in DDR across breast cancers will likely also affect tumor immunogenicity and the efficacy of IO/RT combinations. A more precise understanding of perturbed DDR in breast cancer may help to identify patients who are most likely to benefit from IO/RT combinations. Characterization of synthetic lethality vulnerabilities in the DDR pathway may also suggest targets for overcoming therapeutic resistance to IO/RT, such as PARP inhibitors, ATR inhibitors, or other DNA repair-directed therapies.88,89 Thus, the development of biomarkers of DDR heterogeneity in breast cancer should remain a priority to facilitate the optimal clinical implementation of IO/RT combinations.
Exploiting Novel Breast Radiation Therapy Techniques With Immunotherapy
A pragmatic issue associated with the delivery of high doses of preoperative RT with immunotherapy is concern about the development of downstream toxicities, given that the vast majority of patients with breast cancer who are eligible will have high-risk disease characteristics requiring postoperative RT to the breast and lymph nodes. The impact of the sum of these therapies on local side effects such as treatment-induced fibrosis, breast cosmesis, lymphedema, and surgical and reconstruction outcomes is largely unknown. These concerns have led to the exploration of newer radiation techniques such as SBRT, particle therapy, and magnetic resonance imaging (MRI) guidance to improve coverage of the intact breast tumor while minimizing exposure of radiation to uninvolved breast tissue and surrounding organs.
This has also raised the intriguing question of whether irradiating the entire tumor is necessary in the IO/RT paradigm, in which the goals are to stimulate an antitumor response while minimizing toxicities in women who may receive further RT to the breast/chest wall and lymph nodes in the adjuvant setting. This concept has been compellingly illustrated in a preclinical study by Markovsky et al, in which 67NR murine orthotopic breast tumors received irradiation to either 50% (partial irradiation) or 100% (full irradiation) of the tumor volume. In immunocompetent mice, partial irradiation resulted in tumor responses similar to full-volume radiation, in contrast to immune-deficient nude mice, in which this effect was not observed.90 Investigators at the National Cancer Institute demonstrated similar findings in an allogenic bilateral Lewis lung carcinoma in which they irradiated varying tumor volumes on one side.91 These concepts were explored in a phase 1 trial of SBRT and pembrolizumab in multiple tumor types, in which safety and response to SBRT in combination with pembrolizumab was evaluated in 79 patients, including 6 patients with breast cancer. SBRT was delivered using 1 of 3 dose fractionation regimens, depending on the anatomic location of the tumor. The in-field ORR in the entire cohort was 13.2%. The out-of-field response rate was 13.5%, using the aggregate diameter of nonirradiated RECIST target metastases. Interestingly, the response rate was 26.9% using response defined as 30% reduction in any single nonirradiated RECIST target metastasis. Of note, in 25% of patients, metastases measuring >65 mL were partially irradiated. There was no difference in response reported between partially versus fully irradiated tumors.92 Additional information on techniques for introducing intentional dose heterogeneity into tumors (GRID and lattice RT) and their potential mechanisms for local and distant responses can be found in a recent review.93
Although there are consensus guidelines on SBRT normal tissue dose constraints, these approaches have been little studied in the intact breast. Furthermore, it is uncertain whether these need to be adjusted in the setting of delivering immunotherapy and adjuvant RT. Similarly, as previously detailed, target volume coverage is also debated because the proportion of the target volume that needs to receive the prescription dose to achieve the desired immunostimulatory effect is unknown.
Alternative radiation modalities for IO/RT
Particle therapies, such as proton therapy and carbon ion therapy, are being investigated as part of multimodality therapy for breast cancer and as alternatives to photon therapy.94–96 Particle therapies have distinct physical and biological properties that may be particularly advantageous for the delivery of IO/RT combinations, with the goal of augmenting the antitumor immune response (Table 1). Unlike photons, which slowly attenuate through tissue, charged particles deposit most of their energy at the Bragg peak, with reduced dose deposition proximal to the target volume and none distal to the target volume. Therefore, depending on target location and anatomy, particle therapy often enables less exposure to uninvolved normal tissue, such as the heart and lungs, which may reduce the risk of acute, subacute, and late adverse events of combination therapy.97–99 Moreover, for partial breast irradiation, in which only the target area of the breast is treated, particle therapy would be expected to reduce the volume of irradiated breast tissue outside the target and thereby reduce the risk of adverse cosmetic outcomes.100,101 This may lead to less exposure of infiltrating effector immune cells not only in the surrounding breast tissue but also the circulating blood cells, which could improve outcomes. The reduced risk of local lymphocyte depletion with proton therapy has been shown in large patient cohorts,102–104 and lymphopenia is strongly associated with survival of metastatic patients treated with immunotherapy and IO/RT combinations.105
Initially, partial breast irradiation with proton therapy used aperture- and compensator-based double scattering delivery methods, which provided limited skin-sparing ability.106,107 However, pencil-beam scanning has emerged in recent years and enables spot-by-spot intensity control within the proton field. This technology provides for the routine use of skin sparing when desired, which may lead to improved cosmetic outcomes.100 Skin sparing may be particularly attractive in the setting of the large fraction sizes, which some preclinical data suggest may be most immunogenic.41,108,109
Recent findings have highlighted the interconnectedness of the cellular DNA damage and immune responses.110 Photons and protons are both considered low linear energy transfer (LET) therapies. However, although a relative biological effectiveness (RBE) of 1.1 is used in the clinic for proton therapy, preclinical and clinical data have clearly established that the RBE of protons varies along the proton beam profile.111 The RBE is highest at the Bragg peak and distal fall-off where the LET reaches its peak, and the DNA damage induced is more clustered and difficult to repair. These areas of high LET can be manipulated such that they localize to areas of gross disease during proton planning.112,113 Therefore, proton therapy has recently attracted interest for IO/RT combination therapy, given the potentially distinct immunologic effects resulting from the unique spectra of DNA damage induced by photons and protons.114 An important question facing the field is whether the more clustered and difficult-to-repair damage resulting from protons deposited at the distal track or from higher-LET heavy particle therapy (eg, carbon ions) may be more immunogenic than DNA damage induced by photon therapy, alone or in combination with ICI therapy.114,115
FLASH RT is another emerging technology that has generated significant interest with regard to combination with immunotherapy. FLASH RT consists of ultrahigh dose rates (>40 Gy/s), and preliminary data suggest markedly reduced radiation toxicity to normal healthy tissues while inhibiting tumor growth, with similar efficiency compared with conventional-dose-rate RT.116,117 Data suggest that FLASH RT also modulates inflammatory cytokines (TGF-β and others) and differentially activates immunologic responses within tumor and normal tissues.116–119 Development of a medical linear accelerator system that is capable of treating large-volume targets at FLASH dose rates is currently under way.120 However, our understanding of FLASH RT is limited to preclinical studies, with no published studies reporting on human translation to date. Although the concept of FLASH RT holds promise, further development of the technology and a deeper understanding of the radiobiology underpinning FLASH RT is necessary before it is ready for primetime in patients receiving immunotherapy.
Identifying Biomarkers of Immune Response With IO/RT
Integration of immune and tumor biomarkers into clinical trials of IO/RT combinations is essential for the optimal development of this strategy. Evaluation of pretreatment tumor biopsies may help identify biomarkers that predict responses to IO/RT, which would be critical for optimal patient selection. Several baseline tumor and TME features are important to query before treatment. Morphologic analysis of TILs, although prognostic across many breast cancer subtypes,121 may not provide adequate information regarding the molecular status of TILs. For example, quantification of TCF7-positive CD8+ T lymphocytes predicted clinical immunotherapy response more accurately than density of total CD8+ T cells.122 In breast cancer, CD4+ follicular type T cells and antibody-producing B cell subsets are associated with favorable outcomes.67,68,70 The co-occurrence of these proimmunogenic cell subsets is reminiscent of the recently identified correlation between immunotherapy response and tertiary lymphoid structures in the TME.69 Tumor mutational burden and biomarkers of a T cell-inflamed TME represent independent features that correlate with ICI response rates.123 Consideration of intrinsic biological subtype, as well as biomarkers of DDR status,86,124 may also be critical for predicting responses to IO/RT combination therapy in breast cancer.
Whenever possible, serial posttreatment biopsies should also be obtained, within and outside the irradiation field. These samples enable assessment of treatment effects, which can inform mechanisms of action and resistance in responders and nonresponders, respectively. Establishing differences in biological response will be critical when comparing IO/RT versus IO monotherapy or when comparing different RT doses or fractionation regimens for immune-stimulatory effects. Methods to assess the immune response to radiation range from lymphocyte subsets, humoral markers, and cytokines to a variety of imaging methods based on magnetic resonance imaging and positron emission tomography.125
Blood-circulating biomarkers provide unique opportunities for monitoring treatment effects, systemic immune status, and disease response. Owing to their accessible nature, circulating biomarkers can be assessed at various timepoints, including pretreatment, during treatment, posttreatment, and at follow-up. These include analyses of peripheral blood mononuclear cells, plasma protein biomarkers, and circulating tumor nucleic acids. Microbiome representation has also been correlated with ICI response126 and may provide useful information regarding diet, metabolic state, and host immune status that is not well represented by classical biomarkers. In addition to traditional biomarkers, artificial intelligence approaches based on imaging are being used to predict the response to IO,127,128 demonstrating that standard diagnostic imaging may contain information that can help to improve patient stratification.
Technological advances have resulted in a wealth of opportunities when it comes to biomarkers that can potentially be integrated into clinical trials. A distinction should be made between pragmatic biomarkers that can be broadly applied to larger patient populations across many different institutions and discovery biomarkers that require specialized sampling procedures or complex molecular assays and thus can only be performed in a research setting. Pragmatic biomarkers should be selected to evaluate a prespecified hypothesis formulated on pre-existing clinical or preclinical data. In contrast, discovery biomarkers can be broad and exploratory in nature. Both types of biomarkers provide unique opportunities; however, pragmatic biomarkers have a greater potential for clinical utility in future settings.
Thus, careful design of research biospecimens and correlative biomarkers is essential for the success of IO/RT clinical trials. By stratifying patients based on the likelihood of response to IO monotherapy, response rates can be more meaningfully interpreted. Furthermore, biomarkers assessed both pre- and posttreatment may validate the suspected engagement of a productive immune response in responders and potentially identify mechanisms of treatment resistance in nonresponders to guide future combination therapy trials.
Framework for Progress: The P-RAD Study
To answer some of these key questions, a clinical trial of neoadjuvant “breast boost” RT, immunotherapy, and chemotherapy in biopsy-proven, node-positive TNBC or high-risk, HR+/HER− breast cancer has been proposed through the Translational Breast Cancer Research Consortium (TBCRC). The P-RAD trial (NCT04443348), a randomized study of preoperative chemotherapy, pembrolizumab, and no-, low-, or high-dose RT in node-positive, HER2− breast cancer, will randomize eligible patients in 1:1:1 fashion to 1 of 3 arms: no RT, conventional boost dose (9 Gy), or high-dose (24 Gy) RT, given concurrently with pembrolizumab (Fig. 4). The RT boost will be delivered in 3 consecutive, daily fractions preoperatively to the radiographically evident primary breast cancers. The immunologic effects induced by the different doses of RT will be assessed by quantifying peritumoral and stromal TILs analyzed from a biopsy of the primary tumor, which will be performed approximately 10 days after completion of the RT boost and first cycle of pembrolizumab. This approach is designed to reveal where and which immune cell subsets become activated after varying doses of RT.
Fig. 4.
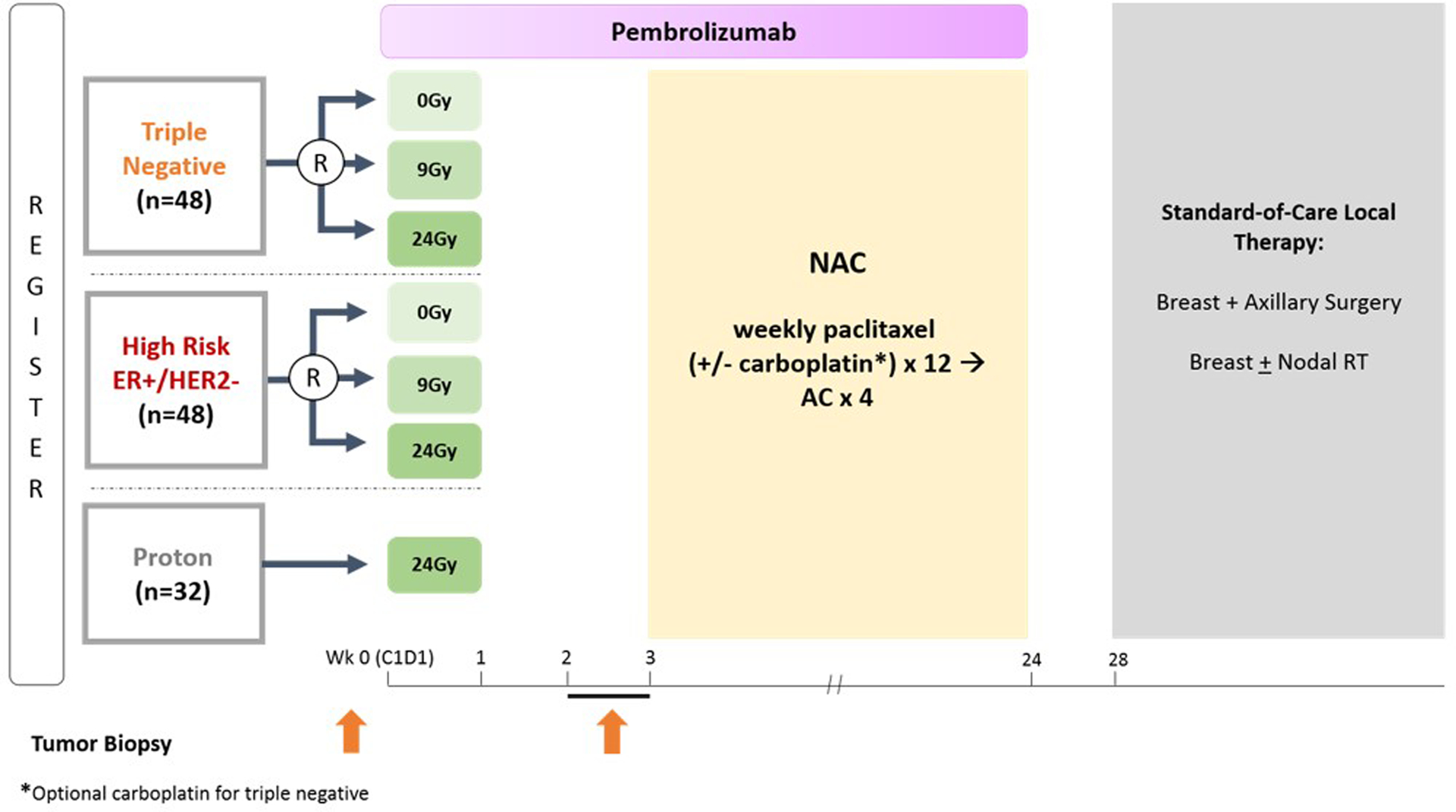
Trial schema for P-RAD, a randomized study of preoperative chemotherapy, pembrolizumab, and no-, low-, or high-dose radiation in node-positive, HER2− breast cancer. Abbreviations: AC = doxorubicin and cyclophosphamide; NAC = neoadjuvant chemotherapy; RT = radiation therapy.
Response in the lymph nodes, which were biopsy proven for malignancy and clipped before the initiation of neoadjuvant therapy, will serve as a surrogate for the abscopal effect. Response of the irradiated tumor will be assessed as a secondary end point but is specifically not included within the definition of the primary end point, given that focal irradiation of the tumor would confound the interpretation of pCR. Additional caveats are that the subsequent neoadjuvant administration of concurrent chemotherapy and pembrolizumab may also affect pathologic response in the nodes or disease-free survival. Toxicities, cosmesis, and patient-reported outcomes will also be assessed in all RT dose cohorts, adding important correlative data in relation to RT dose and immunotherapy.
To explore the hypothesis that targeting the intact breast tumor with proton therapy may result in better cosmesis compared with photon therapy, an exploratory, unrandomized cohort of patients with breast cancer receiving high-dose (24-Gy) RT has been incorporated into the trial, in which the RT boost will be delivered with proton therapy. Treatment, eligibility criteria, and end points remain identical to the randomized photon cohorts. Results will be compared descriptively to the photon cohort. Although the power of potential findings may be limited by the small sample size, this appears to be the most pragmatic approach to investigating the question of whether proton therapy may provide immunologic clinical advantages over conventional photon therapy when high doses of RT are used for the boost.
The deluge of interest in the synergy of IO/RT in breast cancer is evidenced by the number of new trials being conducted both in the metastatic and preoperative settings. A search on clinicaltrials.gov with the terms “breast cancer,” “radiation,” and “immunotherapy” returned 38 clinical trials, 15 of which were not IO/RT trials and were subsequently excluded. As of April 1, 2020, there are 19 registered IO/RT trials in the metastatic setting with 10 actively recruiting clinical trials (Table 3) and 4 registered trials in the preoperative setting with 3 recruiting clinical trials (Table 2). Notable trials in metastatic breast cancer include the TROG AZTEC trial (n = 52), a randomized trial of SBRT doses (20 Gy in 1 fraction vs 24 Gy in 3 fractions) in patients with metastatic TNBC with brain metastases. Few trials include immunotherapy and RT in the post-neoadjuvant setting; however, trials in this category include SWOGS1418/NRGBr006, a phase 3 randomized trial of TNBC patients with ≥1 cm residual disease after NAC who will be randomized to pembrolizumab versus placebo. Adjuvant RT may be delivered either before or concurrently with pembrolizumab.129 The BreastImmune03 trial is investigating adjuvant RT concurrent with ipilimumab and nivolumab versus RT concurrent with capecitabine.130
Table 3.
IO/RT clinical trials* in metastatic breast cancer
| Sponsor | Phase | N | Tumor type | Intervention |
|---|---|---|---|---|
| Abramson Cancer Center of the University of Pennsylvania (NCT02639026) | 1 | 30 | Multiple tumor types, including BC | MEDI4736 and tremelimumab + hypofractionated RT (24 Gy in 3 fractions or 17 Gy in 1 fraction) |
| Kyoto University Hospital, Japan (NCT03430479) | 1, 2 | 32 | HR+/HER2− mBC | Nivolumab and hormone therapy + RT |
| RACHEL1: MD Anderson Cancer Center (NCT03524170) | 1 | 20 | HR+/HER2− mBC | M7824 (Anti-PDL1/TGF-beta trap) + RT |
| Houston Methodist Cancer Center (NCT03004183) | 2 | 57 | mTNBC or lung cancer | ADV/HSV-tk and valacyclovir and pembrolizumab + SBRT (30 Gy in 5 fractions) |
| Peter MacCallum Cancer Centre, Australia, Trans-Tasman Radiation Oncology Group (TROG) (NCT03464942) | 2 | 52 | mTNBC | Atezolizumab + SBRT (20 Gy in 1 fraction or 24 Gy in 3 fractions) |
| Institut Bergoni, Roche Pharma AG, National Cancer Institute, France (NCT03915678) | 2 | 247 | Multiple tumor types, including mTNBC | Atezolizumab and G100 + short-course RT (4 Gy in 2 fractions) or SBRT (27 Gy to 60 Gy in 3–5 fractions) |
| Memorial Sloan Kettering Cancer Center (NCT02563925) | 1 | 28 | mBC with CNS metastases | Tremelimumab and HER2-directed therapy and durvalumab + WBRT or SRS |
| Dana-Farber Cancer Institute (NCT03483012) | 2 | 45 | TNBC with CNS metastases | Atezolizumab + SRS |
| Weill Cornell (NCT03449238) | 1, 2 | 41 | mBC with CNS metastases | Pembrolizumab + SRS |
| H. Lee Moffitt Cancer Center and Research Institute (NCT03807765) | 1 | 12 | mBC with CNS metastases | Nivolumab + SRS |
Abbreviations: CNS = central nervous system; HR+/HER2− = hormone receptor positive/human estrogen receptor 2 negative; IMRT = intensity modulated radiation therapy; mBC = metastatic breast cancer; mTNBC = metastatic triple negative breast cancer; RT = radiation therapy; SBRT = stereotactic body radiation therapy; SRS = stereotactic radiosurgery; WBRT = whole brain radiation therapy.
Registered on clinicaltrials.gov of April 1, 2020.
Future Directions/Conclusion
Understanding the heterogeneity of breast cancer is pivotal to designing studies that will effectively leverage RT to boost antitumor responses to immunotherapy. The paradigm of combining IO/RT is supported by compelling preclinical dose-response studies and clinical trial data demonstrating safety and tolerability in metastatic breast cancer. The risk-benefit ratio of IO/RT combinations may be most productive in high-risk patients with innate or acquired resistance to conventional therapies. In the non-metastatic setting, introducing IO/RT combinations early in the disease course will optimize responses by enabling treatment during the window in which tumor burden is lowest and the potential for eradicating micrometastatic disease is greatest. Alignment of expertise among breast radiation oncologists is essential for standardizing high-dose RT delivery with immunotherapy. Future success in conducting clinical trials with end points and biomarkers relevant to IO/RT calls for intensified collaboration with our medical oncology and surgeon colleagues. Ongoing awareness of long-term toxicities with IO/RT combinations continues to be critically important, particularly for patients with breast cancer with curable disease.
Acknowledgments
We would like to thank all of our colleagues and patient advocates in the Translational Breast Cancer Research Consortium for their support and careful query of the concepts presented in this article.
Disclosures: A.Y.H. reports grants from Merck & Co, GSK Inc, and the Breast Cancer Research Foundation and personal fees from Amgen, outside the submitted work. C.A.S.-M. reports grants from Pfizer, AstraZeneca, Tesaro, and Novartis and other from BMS, Halozyme, Polyphor, Athenex, and Genomic Health, outside the submitted work. A.B. reports grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics, and Biothernostics Inc and personal fees from Biothernostics Inc, Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Taiho, Sanofi, Diiachi Pharma/AstraZeneca, Puma, Phillips, Eli Lilly, and Foundation Medicine, outside the submitted work. A.T. reports other from Pfizer, outside the submitted work. L.S. reports other from Novartis, Puma, and Lumicell and grants and personal fees from Merck and Tesaro, outside the submitted work. C.S. reports nonfinancial support and other from PFS Genomics, outside the submitted work. E.M. reports grants from AstraZeneca, EMD Serono, Genentech, and GSK and personal fees from Merck, Genomic Health, and SELLAS Lifesciences, outside the submitted work. T.A.K. reports personal fees from Genomic Health, outside the submitted work. S.J.I. reports other from Immunomedics, Mylan, Myriad, Puma, Oncopep, Abbvie, and NCCN and grants from AstraZeneca, Oncopep, Abbvie Genentech, Merck, and Pharmamar, outside the submitted work. D.G.D. reports grants from Exelixis, Bayer, and BMS and personal fees from Simcere, Bayer, and BMS, outside the submitted work. J.H.C. reports nonfinancial support and other from Nanostring Technologies, outside the submitted work. S.B. reports other from Varian and Via Oncology, outside the submitted work. I.E.K. reports grants and other from Genentech/Roche, Pfizer, and Daiichi-Sankyo and other from Macrogenics, Context Therapeutics, Taiho Oncology, Merck, Novartis, and Bristol-Myers Squibb, outside the submitted work. No other disclosures are reported.
References
- 1.Horton JK, Jagsi R, Woodward WA, et al. Breast cancer biology: Clinical implications for breast radiation therapy. Int J Radiat Oncol Biol Phys 2018;100:23–37. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286–4293. [DOI] [PubMed] [Google Scholar]
- 5.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 2011;71:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 9.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN guidelines for patients: Invasive breast cancer. Available at: https://www.nccn.org/patients/guidelines/breast-invasive/44/index.html. Accessed April 20, 2020.
- 11.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol 2017;35(suppl 15):506.28029304 [Google Scholar]
- 12.Loibl S, Untch M, Burchardi N, et al. A randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab, a PD-L1 antibody, to a taxane-anthracycline containing chemotherapy in triple negative breast cancer (TNBC). J Clin Oncol 2017;35(suppl 15):3062. [Google Scholar]
- 13.Schmid P, Cortes J, Bergh JCS, et al. KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple-negative breast cancer (TNBC). J Clin Oncol 2018;36(suppl 15):TPS602. [Google Scholar]
- 14.Mougalian SS, Hernandez M, Lei X, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol 2016;2:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prat A, Fan C, Fernandez A, et al. Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 2015;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016;29:241–250. [DOI] [PubMed] [Google Scholar]
- 18.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol 2016;2:1354–1360. [DOI] [PubMed] [Google Scholar]
- 19.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res 2018;24:2804–2811. [DOI] [PubMed] [Google Scholar]
- 21.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol 2019;5:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat 2018;167:671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 24.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:44–59. [DOI] [PubMed] [Google Scholar]
- 25.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 26.Schmid PP, Ferreira M, Mouret-Reynier M, et al. KEYNOTE-522 study of neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early triple-negative breast cancer: Pathologic complete response in key subgroups and by treatment exposure, residual cancer burden, and breast-conserving surgery. Paper presented at: SABCS December 12, 2019; San Antonio, TX. [Google Scholar]
- 27.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: A retrospective multicenter study. Ann Oncol 2014;25:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Ellison SJ, Alarid ET, et al. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene 2007;26: 4106–4114. [DOI] [PubMed] [Google Scholar]
- 30.Mostafa AA, Codner D, Hirasawa K, et al. Activation of ERalpha signaling differentially modulates IFN-gamma induced HLA-class II expression in breast cancer cells. PLoS One 2014;9:e87377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 2013;31:860–867. [DOI] [PubMed] [Google Scholar]
- 32.Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat 2010;120:293–308. [DOI] [PubMed] [Google Scholar]
- 33.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- 34.Esteva FJ, Hubbard-Lucey VM, Tang J, et al. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol 2019;20:e175–e186. [DOI] [PubMed] [Google Scholar]
- 35.Kok M, Voorwerk L, Horlings H, et al. Adaptive phase II randomized trial of nivolumab after induction treatment in triple negative breast cancer (TONIC trial): Final response data stage I and first translational data. J Clin Oncol 2018;36(suppl 15):1012. [Google Scholar]
- 36.Buchwald ZS, Wynne J, Nasti TH, et al. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front Oncol 2018;8:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020;126:850–860. [DOI] [PubMed] [Google Scholar]
- 38.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397–404. [DOI] [PubMed] [Google Scholar]
- 39.Barroso-Sousa R, Krop IE, Trippa L, et al. A phase II study of pembrolizumab in combination with palliative radiotherapy (RT) for hormone receptor-positive (HR+) metastatic breast cancer (MBC). J Clin Oncol 2019;37(suppl 15):1047. [DOI] [PubMed] [Google Scholar]
- 40.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367: eaax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279–1288. [DOI] [PubMed] [Google Scholar]
- 44.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 45.Young KH, Baird JR, Savage T, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 47.Bondiau PY, Courdi A, Bahadoran P, et al. Phase 1 clinical trial of stereotactic body radiation therapy concomitant with neoadjuvant chemotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2013; 85:1193–1199. [DOI] [PubMed] [Google Scholar]
- 48.Riet FG, Fayard F, Arriagada R, et al. Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. Eur J Cancer 2017;76: 45–51. [DOI] [PubMed] [Google Scholar]
- 49.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol 2017;14: 365–379. [DOI] [PubMed] [Google Scholar]
- 50.Wilkins AC, Patin EC, Harrington KJ, et al. The immunological consequences of radiation-induced DNA damage. J Pathol 2019;247: 606–614. [DOI] [PubMed] [Google Scholar]
- 51.Harding SM, Benci JL, Irianto J, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017;548:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017;548:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov 2020;10:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia T, Konno H, Ahn J, et al. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep 2016;14:282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konno H, Yamauchi S, Berglund A, et al. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 2018; 37:2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burleigh K, Maltbaek JH, Cambier S, et al. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci Immunol 2020;5:eaba4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JR, Ahn K, Kim YJ, et al. Radiation-induced human endogenous retrovirus (HERV)-R Env gene expression by epigenetic control. Radiat Res 2012;178:379–384. [DOI] [PubMed] [Google Scholar]
- 58.Nabet BY, Qiu Y, Shabason JE, et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 2017;170:352–366.e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss T, Schneider H, Silginer M, et al. NKG2D-dependent anti-tumor effects of chemotherapy and radiotherapy against glioblastoma. Clin Cancer Res 2018;24:882–895. [DOI] [PubMed] [Google Scholar]
- 60.Gasser S, Orsulic S, Brown EJ, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005;436:1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Hermanson DL, Moriarity BS, et al. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 2018;23:181–192.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang H, Wang H, Ren J, et al. cGAS is essential for cellular senescence. Proc Natl Acad Sci U S A 2017;114:E4612–E4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018; 553:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benci JL, Xu B, Qiu Y, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016;167:1540–1554.e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 2015;3:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res 2018;24:5058–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu-Trantien C, Loi S, Garaud S, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hollern DP, Xu N, Thennavan A, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 2019;179:1191–1206. e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iglesia MD, Vincent BG, Parker JS, et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res 2014;20:3818–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao XM, Bhattacharya R, Huang J, et al. High-throughput prediction of MHC class I and class II neoantigens with MHCnuggets. Cancer Immunol Res 2020;8:396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen B, Khodadoust MS, Olsson N, et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol 2019;37:1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith CC, Chai S, Washington AR, et al. Machine-learning prediction of tumor antigen immunogenicity in the selection of therapeutic epitopes. Cancer Immunol Res 2019;7:1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018;128:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 2018; 128:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity 2019;50:924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravi R, Noonan KA, Pham V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun 2018;9:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arina A, Beckett M, Fernandez C, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun 2019; 10:3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones KI, Tiersma J, Yuzhalin AE, et al. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol Med 2018;10:e9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kozin SV, Kamoun WS, Huang Y, et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res 2010;70:5679–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sjostrom M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol 2017;35:3222–3229. [DOI] [PubMed] [Google Scholar]
- 83.Sjostrom M, Chang SL, Fishbane N, et al. Clinicogenomic radiotherapy classifier predicting the need for intensified locoregional treatment after breast-conserving surgery for early-stage breast cancer. J Clin Oncol 2019;37:3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mulligan JM, Hill LA, Deharo S, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst 2014;106:djt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst 2017;109:djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma P, Barlow WE, Godwin AK, et al. Validation of the DNA damage immune response signature in patients with triple-negative breast cancer from the SWOG 9313c trial. J Clin Oncol 2019;37: 3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Reports 2018;23:239–254.e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vendetti FP, Karukonda P, Clump DA, et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest 2018;128:3926–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tu X, Kahila MM, Zhou Q, et al. ATR inhibition is a promising radiosensitizing strategy for triple-negative breast cancer. Mol Cancer Ther 2018;17:2462–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markovsky E, Budhu S, Samstein RM, et al. An antitumor immune response is evoked by partial-volume single-dose radiation in 2 murine models. Int J Radiat Oncol Biol Phys 2019;103:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanagavelu S, Gupta S, Wu X, et al. In vivo effects of lattice radiation therapy on local and distant lung cancer: Potential role of immunomodulation. Radiat Res 2014;182:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Billena C, Khan AJ. A current review of spatial fractionation: Back to the future? Int J Radiat Oncol Biol Phys 2019;104:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corbin KS, Mutter RW. Proton therapy for breast cancer: Progress & pitfalls. Breast Cancer Manag 2018;7. [Google Scholar]
- 95.Bekelman JE, Lu H, Pugh S, et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: The Radiotherapy Comparative Effectiveness (Rad-Comp) consortium trial protocol. BMJ Open 2019;9:e025556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karasawa K, Omatsu T, Arakawa A, et al. A phase I clinical trial of carbon ion radiotherapy for stage I breast cancer: Clinical and pathological evaluation. J Radiat Res 2019;60:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bradley JA, Dagan R, Ho MW, et al. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast cancer compared with photons. Int J Radiat Oncol Biol Phys 2016;95:411–421. [DOI] [PubMed] [Google Scholar]
- 98.Jimenez RB, Hickey S, DePauw N, et al. Phase II study of proton beam radiation therapy for patients with breast cancer requiring regional nodal irradiation. J Clin Oncol 2019;37:2778–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith NL, Jethwa KR, Viehman JK, et al. Post-mastectomy intensity modulated proton therapy after immediate breast reconstruction: Initial report of reconstruction outcomes and predictors of complications. Radiother Oncol 2019;140:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mutter RW, Jethwa KR, Gonuguntla K, et al. 3 Fraction pencil-beam scanning proton accelerated partial breast irradiation: Early provider and patient reported outcomes of a novel regimen. Radiat Oncol 2019;14:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys 2010;76:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther 2018;4:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuan C, Wang Q. Comparative analysis of the effect of different radiotherapy regimes on lymphocyte and its sub-populations in breast cancer patients. Clin Transl Oncol 2018; 20:1219–1225. [DOI] [PubMed] [Google Scholar]
- 104.Venkatesulu BP, Mallick S, Lin SH, et al. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 105.Cho Y, Park S, Byun HK, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2019;105:1065–1073. [DOI] [PubMed] [Google Scholar]
- 106.Cuaron JJ, Chon B, Tsai H, et al. Early toxicity in patients treated with postoperative proton therapy for locally advanced breast cancer. Int J Radiat Oncol Biol Phys 2015;92:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Macdonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: Early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2013;86:484–490. [DOI] [PubMed] [Google Scholar]
- 108.Mutter RW, Remmes NB, Kahila MM, et al. Initial clinical experience of postmastectomy intensity modulated proton therapy in patients with breast expanders with metallic ports. Pract Radiat Oncol 2017;7:e243–e252. [DOI] [PubMed] [Google Scholar]
- 109.Depauw N, Batin E, Daartz J, et al. A novel approach to post-mastectomy radiation therapy using scanned proton beams. Int J Radiat Oncol Biol Phys 2015;91:427–434. [DOI] [PubMed] [Google Scholar]
- 110.Mouw KW, Goldberg MS, Konstantinopoulos PA, et al. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov 2017;7:675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paganetti H Proton relative biological effectiveness-Uncertainties and opportunities. Int J Part Ther 2018;5:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grassberger C, Trofimov A, Lomax A, et al. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys 2011; 80:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan Chan Tseung HS, Ma J, Kreofsky CR, et al. Clinically applicable Monte Carlo-based biological dose optimization for the treatment of head and neck cancers with spot-scanning proton therapy. Int J Radiat Oncol Biol Phys 2016;95:1535–1543. [DOI] [PubMed] [Google Scholar]
- 114.Ebner DK, Tinganelli W, Helm A, et al. The immunoregulatory potential of particle radiation in cancer therapy. Front Immunol 2017; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimokawa T, Ma L, Ando K, et al. The future of combining carbonion radiotherapy with immunotherapy: Evidence and progress in mouse models. Int J Part Ther 2016;3:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014;6:245ra293. [DOI] [PubMed] [Google Scholar]
- 117.Bourhis J, Montay-Gruel P, Gonçalves Jorge P, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol 2019;139:11–17. [DOI] [PubMed] [Google Scholar]
- 118.Vozenin MC, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res 2019;25:35–42. [DOI] [PubMed] [Google Scholar]
- 119.Loo BW, Schuler E, Lartey FM, et al. Delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys 2017;98: E16. [Google Scholar]
- 120.Maxim PG, Tantawi SG, Loo BW Jr. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol 2019;139:28–33. [DOI] [PubMed] [Google Scholar]
- 121.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. [DOI] [PubMed] [Google Scholar]
- 122.Sade-Feldman M, Yizhak K, Bjorgaard SL, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018;175:998–1013.e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018; 362:eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cejalvo JM, Martinez de Duenas E, Galvan P, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res 2017;77:2213–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grassberger C, Ellsworth SG, Wilks MQ, et al. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol 2019;16:729–745. [DOI] [PubMed] [Google Scholar]
- 126.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol 2018;19:1180–1191. [DOI] [PubMed] [Google Scholar]
- 128.Trebeschi S, Drago SG, Birkbak NJ, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol 2019;30:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.US National Library of Medicine. Testing MK-3475 (Pembrolizumab) as adjuvant therapy for triple receptor-negative breast cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT02954874. Accessed June 4, 2020.
- 130.US National Library of Medicine. Evaluate the clinical benefit of a post-operative treatment associating radiotherapy + nivolumab + ipilimumab versus radiotherapy + capecitabine for triple negative breast cancer patients with residual disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03818685. Accessed June 4, 2020.


