Abstract
Toxoplasma gondii tachyzoites and bradyzoites are studied extensively in the laboratory due to the ease with which they can be cultured. In contrast oocysts and the sporozoites within them are more difficult to work with, in that cat infections are required for their generation and isolating sporozoites requires a laborious excystation procedure. More over some parasite species such as Hammondia hammondi are obligately heteroxenous and require passage through a cat for completion of the life cycle. There is no debate that there is great value in studying this important life cycle stage, and so we present here a detailed description of the current protocols used in our laboratories to generate and isolate T. gondii and H. hammondi oocysts, and to excyst and purify the sporozoites within them for use in downstream experimental applications.
Introduction
Toxoplasma gondii is an exceptional model of intracellular parasitism, and a large body of research has been generated using in vitro cultivated parasites, including both tachyzoites and bradyzoites (1). In contrast, the sporozoite stage is less studied, most certainly due at least in part to the logistical difficulties, costs and hazards associated with producing and working with oocysts. To date there are no means of producing oocysts in tissue or cell culture, and therefore production of these life stages requires cat infections. Work with cat life cycle stages have had an enormous impact on the field.
Most notably, sexual crosses can only be carried out in cats, and co-infections with phenotypically distinct strains has resulted in the generation of recombinant progeny that have been crucial for the identification of key virulence determinants (e.g., (2–11)). Moreover, for parasites such as Hammondia hammondi for which no methods of long term in vitro cultivation exist, cat infections are essential for its propagation (12–14) and are key to work aimed at understanding the dramatic developmental and virulence differences between T. gondii and H. hammondi (15–17). Moreover, oocyst shedding provides a remarkably sensitive bioassay for determining the presence of viable tissue cysts in meat, lending great importance to such methods in establishing that curing, freezing, cooking and irradiation are important measures to safeguard food safety and public health (18–21). By identifying antigens exclusively expressed by the sporozoites encased by oocysts, it has been further established that oocysts represent the principal route by which people become exposed to T. gondii (22) which ranks among the most costly foodborne infections (23).
Before describing the means to purify oocysts of Toxoplasma gondii from cat feces, we hasten to underscore the significant health risks inherent in this activity. Conducting this important work requires the highest level of personal and institutional commitment to biosafety and occupational health, and must only be conducted under rigorous regimes of testing, training, oversight, and documentation regarding occupational health, environmental safety, and animal welfare.
A brief review of clinical toxoplasmosis is therefore warranted. Infections are widely prevalent in humans and animals. All 3 stages of Toxoplasma (oocysts/sporozoites, tachyzoites, and bradyzoites) are infective to humans. In human hosts with a competent immune system, T. gondii infection generally develops into an asymptomatic chronic infection, with the organism sequestered in dormant tissue cysts, often for the lifetime of the host. However, in immunocompromised hosts, such as AIDS patients, cancer patients receiving chemotherapy, or organ transplant recipients receiving immunosuppressive therapy, infection can lead to toxoplasmosis, with serious consequences including potential damage to the central nervous system. This can result from a new infection or more commonly from reactivation of a latent infection. In pregnant women who first become infected during pregnancy, the newborn may develop congenital toxoplasmosis, which can result in blindness or mental retardation. In addition, T. gondii is now recognized as a cause of ocular disease in both immunocompetent and immunosuppressed persons. Unlike tachyzoites and bradyzoites, oocysts are environmentally resistant and highly infectious for humans (24). Therefore, extra precautions are needed while working with oocysts. It is highly recommended that immunocompromised individuals or pregnant women do not work directly with sporulated oocysts. However in our experience this does not require that these individuals stop working on the project in question as long as non-pregnant or non-immunocompromised lab members can manipulate the infectious stages and then pass them off once they are no longer in their most infectious form.
Our experience with generating and excysting oocysts of both T. gondii and H. hammondi has led to some recent improvements in sporozoite isolation and purification, including a new method to directly generate transgenic H. hammondi using sporozoites as input (17). The mouse infection and oocyst infection protocols described have been used for decades in the Dubey Lab, while the sporozoite purification protocols are in use in the Boyle Lab and are based on methods that were originally developed by others, most notably those in the laboratory of Michael White (now at the University of South Florida; e.g., (25–27)). For completeness we have included the entire process, starting with mouse infections, cat infections and feces collection, and oocyst purification, sporulation and excystation. We also include general comments on the biosafety precautions that we practice when working with oocysts.
Materials
General Lab Equipment and Personal Protective Equipment
Centrifuge with biocertified, transparent lids
Shaker
Vortexer
Biosafety Level 2 cabinet
N95 facemask
Foot covers
General use needles and syringes for mouse infections
Cat infections and oocyst isolation
Tongue depressors
Disposable cups with lids (Specimen cups such as McKesson-569)
Laxatone (optional)
Sucrose solution in water (1.1 M)
Plastic pasteur pipettes
Sporozoite excystation
Hank’s Balanced Salt Solution (Corning; 21–021-CV)
Phosphate Buffered Saline (Lonza; 17–516)
Clorox® Regular-Bleach
50mL Corning™ Polyethylene Terephthalate (PET) Centrifuge Tubes
15mL Polystrene Centrifuge Tubes
15mL Polyproplyene Centrifuge Tubes
1M Sodium Hydroxide Solution (NaOH)
Disposable hemocytometers (Incyto; DHC-N01–5)
Nail polish
Sterile Disposable serological pipettes (5, 10, and 25mLs)
Glass Beads, acid-washed: 710–1,180μm (Sigma; G1152)
Parafilm
Dulbecco’s Modified Eagle Medium with 100U/mL penicillin, 100μg/mL streptomycin, 2mM L-glutamine, 10% FBS, 3.7g NaH2CO3/L, pH 7.2 (cDMEM)
3mL syringe
25 ⅝ gauge needles
Trypsin from porcine pancreas (Sigma; T4799)
Taurocholic acid sodium salt hydrate (Sigma; T4009)
0.22μm 50mL Steriflip® filter (Millipore; SCGP00525)
Sporozoite purification
Autoclavable (or disposable) cell scrapers
Sealable autoclave bags
Disposable transfer pipettes
5μm filters (Millex®; SLSV025LS)
10mL syringes
PD-10 desalting column with Sephadex G-25 resin (GE Healthcare; 17085101).
Methods
Safety Precautions when working with oocysts
During Cat infections:
During the period of oocyst shedding by cats, T. gondii- and H. hammondi-infected cats are housed individually in stainless steel cages in a room with limited access. In our facility, each cage has a 2-kg stainless steel litter pan with a heavy bottom so that cats cannot tip it and a bottom tray to catch the spilled material. Feces are collected daily from each litter pan using disposable gloves and disposable fecal scoop. The litter pan is filled to about ½ the depth (~1 inch) with crushed corn cobs as bedding. During the period of oocyst shedding, the floor under, and in front of, the cages is covered with plastic sheets to catch any material that may be shed by cats; the plastic sheets are replaced daily and used sheets are incinerated. Care should be exercised to avoid aerosolization. Masks (N95) are to be worn at all times during work with infected materials. During the period of oocyst shedding, the litter pans and bottom trays are not washed to avoid the spread of oocysts. When cats have stopped shedding oocysts, they are euthanized or transferred to clean cages.
There is a public debate concerning whether cats that have been experimentally infected, and which have resolved their initial episode of oocyst shedding, should be euthanized or could instead be candidates for adoption. The historical case for euthanasia derives from the fact that asymptomatic cats may resume oocyst shedding. Persistent antibody titers attest to lifelong latent infections in cats. Such asymptomatic cats have been shown prone to re-shedding millions of oocysts once exposed to another common coccidian infection of cats, Cystoisospora (Isospora) felis (28). It is not known whether natural immunosuppressive conditions (i.e. those mediated by nutritional status, viral infection, or chemotherapy) might similarly provoke renewed oocyst shedding, although in one study experimental immunsuppression of cats that had stopped shedding oocysts did result in the resumption of oocyst shedding (29).. Immunity to secondary challenge with T. gondii, especially to heterologous strains, does wane over time (30,31) and it remains to be determined whether such waning immunity predisposes a cat to undergo subsequent rounds of oocyst shedding in the absence of secondary exposure. At the very least, newfound susceptibility to new infections creates liability for a donor of such cats, because it may be difficult or impossible to establish whether infections derived from an older cat derived from new or prior (experimental) exposure. Any prospective adoptive home must be made aware of risks that doing so may result in serious harms to health.
The contaminated cages, including bottom trays, food and water dishes, and litter pans, are first run through a steam cage washer (internal temperature of >70°C) to kill oocysts before cages are scrubbed. The cage washer must have a final rinse temperature of at least 82°C. All bedding and waste from the room housing infected cats are incinerated. Feces are stored in a refrigerator (4°C) to prevent sporulation prior to being processed in a separate building.
During fecal flotation:
Special precautions are taken to properly dispose of liquid feces or fecal wash. All liquids are poured in plastic containers, tightly capped, and sealed in 12 × 9.5 inches, heat sealable special pouches (Kapak/scotchpack) designed for the electric sealer. The sealed pouches are enclosed in a biohazard bag, and resealed in another biohazard bag for extra security. The biohazard bag is enclosed in a plastic bucket with absorbent material, and labelled.
All contaminated materials from the fecal room are incinerated or boiled. Large bottles, and metal cups used in the centrifuge are boiled in water to kill any oocysts present. Boiling water is poured on the sink surface to kill any infectious Toxoplasma oocysts—precautions should be taken while handling boiled water to prevent burns. All protective coverings/PPE and plastic sheets used to cover the sink are sealed in a red-biohazard bag, enclosed in a cardboard box with a biohazard sign, and properly labelled. All waste is disposed in accordance with the SOP requirements for Special (Regulated) Medical waste disposal. These boxes are transported to the incinerator. Glass slides, coverslips, and pipettes used for fecal examination are placed in an OSHA-approved sharps container, enclosed in red bag, and finally enclosed in a box, labeled biohazard, and incinerated.
During excystation and sporozoite isolation:
Sporulated oocysts are highly infectious and environmentally stable, even when treated with common laboratory disinfectants. Therefore, anyone working with oocysts should be properly trained and should exhibit extreme caution to prevent accidental exposure. To minimize the exposure risk, all individuals working with oocysts should wear proper personal protection equipment, including a lab coat and disposable gloves. Gloves should be changed often and immediately if contamination occurs. To minimize contamination of laboratory equipment, all oocyst work should be conducted in a biological safety cabinet that contains its own biohazard waste disposable bag and liquid oocyst waste container (Figure 1A). Any time a vessel containing oocysts is manipulated outside the hood, the lid should be sealed with parafilm and the vessel should be transported in a secondary container. When centrifuging vessels containing oocysts, transparent biocertified covers should be used to prevent contamination in case vessel rupture occurs. When vessels containing oocyst material are opened, their caps should be visually inspected for any liquid contamination. If contamination occurs, the cap should be immediately discarded in the biohazard waste bag within the biosafety cabinet. Anything that comes into contact with oocyst material should be discarded in this way after use (serological pipette tips, transfer pipettes, etc). After oocyst manipulation has been completed, the biosafety cabinet should be first cleaned liberally with a 10% bleach solution (no more than 1 month in age). Allow bleach to sit for 10–30 minutes before wiping away. After cleaning with bleach, the biosafety cabinet should be cleaned with a hospital grade disinfectant, such as cavacide, followed by 70% ethanol. The UV light source of the biosafety cabinet should be turned on for at least 1 hour prior to additional work. Gloves should be changed frequently during this cleaning process. All liquid waste and any reusable supplies, such as autoclavable cell scrapers, must be autoclaved prior to being discard or reused. If a spill occurs, first alert all personal of the spill and clean the area liberally with a freshly prepared 10% bleach solution, being sure to allow the bleach to sit for 30 minutes. The spill area should then be liberally cleaned with cavacide followed by 70% ethanol. All waste products (paper towel, kimwipe, etc) used in the cleaning process should be discarded in the biohazard waste bag inside the biosafety cabinet since none of these treatments will completely inactivate the oocysts.
Figure 1. Safety precautions to utilize when working with oocysts.

A) Biosafety cabinet set up with biohazard waste bag and oocyst waste containers. B) A piece of plastic is placed between oocyst stock container and cap to shield cap from oocyst exposure and to prevent leaks. C) Transparent biocertified covers for centrifuge buckets are used to contain spills that may occur as a result of container damage. This can also be accomplished by vacuum sealing tubes in plastic bags prior to centrifugation.
Infection of mice for use in oocyst production
General notes:
In our own work we have found that T. gondii strains grown extensively in vitro as tachyzoites lose their ability to produce oocysts in cats despite being very competent at forming bradyzoite cysts in vitro and in mice (Boyle, Boothroyd and White, Unpublished observations). While the reasons for this are unclear, given the costs of cat infections it is important that “cat competent” strains are used to produce oocysts. We recommend “resetting” any strains of interest by infecting mice with low passage samples and feeding those mice to cats. Oocysts collected from these cats can be used as a seed strains to perform further experiments. Mice can be infected parenterally with tachyzoites or tissues cysts or using oocysts (oral or subcutaneous). If oocysts are the starting material, we prefer the subcutaneous route for inoculating mice with T. gondii and H. hammondi if the main goal is the production of oocysts for use in downstream experiments.
Subcutaneous infection of mice with oocysts:
Using oocysts isolated as described below, quantify and take an appropriate volume and neutralize the 2% H2SO4 using 3.3% NaOH (add drops until neutral red is the appropriate hue).
Place the animal on top of a screen or wire-top cage lid and manually restrain it by grasping the base of the tail and slightly lifting the hind limbs off the surface.
As the animal pulls forward with the front feet, insert a 0.5 ml syringe with a 1‑inch, 21-gauge needle into the loose skin located over the back of the neck at a 15° to 30°angle on either side of the spine.
Inject up to 0.5 mL and return the animal to the cage.
Oral inoculation with oocysts:
Pre-measure and mark a ball-tipped oral gavage needle (8–10 mm) to indicate where shaft will be when the ball is near the stomach (just beneath the ribcage).
Manually restrain animal by grasping the loose skin on the back of the neck with the tail secured between the fourth and fifth fingers of the same hand.
Hold the mouse in an upright position with the head tilted slightly back to straighten the esophagus as much as possible.
Introduce an 8 to 10-mm ball-tipped gavage needle attached to a 1 to 3-ml syringe into the side of the mouse’s mouth.
Pass the needle over the tongue and into the esophagus to the mark on the shaft. Slowly expel the contents (0.5 mL to 1 mL buffered oocyst prep) into the mouse using the syringe plunger. Carefully withdraw the gavage needle.
-
Return the animal to the home cage and observe frequently for at least five minutes for signs of complications.
Note: When mice are orally inoculated with T. gondii or H. hammondi oocysts special instructions are posted on each cage because some of the oocysts may pass “undigested” in feces for the first 2 to 3 days. In addition, bedding and feces are incinerated and the caretakers are instructed to use gloves and facemasks while changing or cleaning these cages for the first 7 days after oral oocyst challenge.
Note: It is sometimes necessary to treat these mice with anti-toxoplasma therapy following challenge (1–4 mg/mL sulfadiazine per ml of drinking water) until they have recovered from the initial stages of infection.
Collection of tissues for infection of cats
T. gondii and H. hammondi begin to encyst in mouse tissues within a week of inoculation irrespective of the stage inoculated or the route. T. gondii cysts are formed in many tissues but are concentrated in the brain after 4 weeks post infection. In contrast H. hammondi cysts are not found in significant numbers in the brain. For infection of cats with T. gondii, mice are euthanized and brain is collected. The brain can be fed to cats by placing the brain tissue on the back of the tongue with mouth open by firmly holding the cat. If necessary the entire carcass can be fed to the cat afterwards. For this the mouse is skinned, the feet and intestines are removed, and the rest of the mouse is chopped up with scissors. The chopped mouse tissues can be mixed with canned cat food and offered to the cats. Adding fish or watermelon flavors increases palatability. For H. hammondi infections, the entire carcass is fed to the cat in this manner.
Cat infections and feces collection
Acquire specific pathogen-free (SPF) cats, raised in biosecurity facilities with no exposure to raw meat. These cats should have available data with respect to date born, sex and their parents (queen, tom). Each cat should have a unique identification (ID), preferably, microchipped. The cats should be Toxoplasma-free, based on antibody titers determined by modified agglutination test (MAT) in 1:25 dilution of serum.
Infect Toxoplasma-free weaned cats; optimal oocyst excretion occurs in 10–20 week old cats. Allow cats to consume infected animal tissues (up to 500 g) ad libitum over a period of 2–4 days.
All personnel should wear protective gear including hood covers, a coverall and a ventilation mask (N95). When collecting feces, change gloves between each cage to prevent cross-contamination.
Collect all feces in plastic cups from the litter box daily on day 3–14 post-feeding and refrigerate them immediately to prevent the sporulation of oocysts.
Label each cup with the cat identification number and the date
Use a double gloved hand (or disposable fecal scoop) to collect ALL the fecal material from the litter box
Add 50 ml of DI water to each fecal cup before closing
Cups are to be placed in the designated refrigerator after collection
- If a cat does not defecate, do the following:
- Administer LAXATONE (a petroleum based lubricant) by smearing a 1 inch long streak on either their face or front paws – do not put it in the food dish because they will not eat it.
-
Record the date, number and problem in the animal health log bookNote: It is important that once the collection period has begun, DO NOT empty the bottom cage pans until the cat is euthanized to minimize spread of oocysts in the feces that might have fallen in the bottom tray.
Empty all bedding into biohazard boxes
Determine infection by the identification of oocysts in the feces (see “Fecal Examination” below). During the period of oocyst shedding (between 3 and 14 days after feeding test tissue), take special care in handling cages and cats to avoid inadvertent exposure of personnel.
Euthanize cats at the end of oocyst shedding period (usually 2 weeks after infection) using the following AVMA approved methods of euthanasia. Administer Ketamine at 10 mg/kg body weight in combination with Dexdomitor (dexmedetomidine hydrochloride, 0.5mg/1ml) intramuscularly or subcutaneously to anesthetize cats before intracardiac administration of Beuthanasia (Schering, Plough Animal Health) at 1 mg/10 lb body weight.
Incinerate all cat remains to prevent the spread of Toxoplasma gondii or Hammondia hammondi.
Fecal examination for oocysts
Wear protective disposable coveralls (covering the whole body), a face mask, face shield, boot covers, N95 mask and gloves.
Put fecal cups (securely lidded) with enough water to just cover the feces on the shaker to soften feces and partly emulsify them.
For initial examination (screening), mix approximately 10g (10ml) of feces from each cat with 5 volumes of sucrose solution (specific gravity 1.15 or higher).
Filter by pouring the liquid through two layers of gauze in a cup (layers can be made by folding large pieces of gauze).
Squeeze the feces retained on the gauze to extract as many oocysts and then dispose of the gauze/fecal material in a biohazard bag (to be incinerated).
-
Pour the filtered liquid/feces mixture into a 50-ml conical tube, close cap, and centrifuge at 1180×g for 10 minutes.
Note: Enclose tubes in a sealable bag or use approved centrifuge bucket covers to avoid contamination of the centrifuge in the rare event of tube breakage or cap leakage.
Using a disposable Pasteur pipette (we prefer plastic since they are sealed and easier to dispose of than glass), take a drop of the fecal float from the top of the tube and examine microscopically for T. gondii oocysts. If no oocysts are found, incinerate the remaining cat feces from that cat and that day of collection and discard by incineration. For this, enclose feces in a plastic container, seal in a biohazard bag, and enclose in a plastic disposable hard plastic bucket for incineration.
For positive samples, mix the remainder of each feces/water mixture with a few drops of detergent (household soap) and place on rotary shaker until feces are completely broken.
Filter the mixture through gauze as above and centrifuge in 250-ml bottles at low speed (300×g, 15 min). Discard the supernatant.
-
Mix the sediment with water (up to 150 mL) and centrifuge 2–3 times, decanting the supernatant each time and replacing with clean water. Remove the water from the final wash.
Note: it is important to collect all waste from these procedures into autoclaveable glass or plastic containers rather than pouring down the drain. Autoclave all liquid waste.
Mix the sediment with 5 volumes of sucrose solution of 1.15 specific gravity (530 g/liter) and centrifuge for 10 min at 1180×g in 50-ml tubes with a conical bottom. Most oocysts float to the top of the tube and can be aspirated using a Pasteur pipette.
Pellet these oocysts by spinning for 10 min at 1180×g, and resuspend in 2% H2S04 precautions should be taken while handling sulfuric acid—never add water to acid while making dilution.
Since not all oocysts rise to the top, take the entire supernatant (40–45 ml) and centrifuge it at 1180×gfor 15 min. Wash the sediment containing the oocysts one more time with water and then resuspend it in 2% H2SO4.
Pour the isolated oocysts from steps 12 and 13 above into a 500 ml flat bottom bottle with a sturdy cap (e.g., a HyClone RPMI-1640 Medium bottle). Aerate them on a shaker (Labnet shaker, 150 RPM) for 7 days at room temperature (20–22°C) to induce sporulation.
Sporulation efficiency can be assessed using a hemacytometer.
Sporozoite excystation
Fill appropriately sized beaker with DI water and place beaker in 37°C incubator to serve as a water bath at least 4 hours prior to excystation incubation.
Set up by placing a biohazard bag and liquid oocyst waste bottle in biosafety cabinet (Figure 1A).
Calculate volume of oocysts needed based on the number of sporozoites desired. Assume about a 5% yield unless additional data for a given preparation is available. Calculate volume of HBSS needed for first wash and the volume of 1M NaOH needed for neutralization (Total volume can range between 25 and 45mLs. Neutralization step is optional.)
Cut approximately a 5×5cm square out of a biohazard bag (prepare one for each stock of oocysts used)
Remove the cap from the oocyst/cat feces storage container and immediately discard plastic between cap and container (Figure 1B).
Pipette to mix oocyst/cat feces prep to obtain a homogenous mixture. Transfer the calculated volume of the oocyst stock into a 50 mL PET tube.
Place the square of biohazard bag prepared in step 4 on the opening of the oocyst storage container before replacing the original cap (Figure 1B).
-
Add 10 μL of each oocyst sample to a disposable hemocytometer. Seal the opening with nail polish. Allow the nail polish to drip off of the applicator (e.g., do not touch the applicator to the opening).
Note: Allow the hemocytometer to settle for 3 to 5 minutes before observation.
Add appropriate volume of HBSS to the PET tube, pipette up and down to mix. The solution should be yellow in color.
-
If neutralization is desired, add appropriate volume of 1M NaOH (⅗ volume of oocyst sample) to neutralize the solution. Pipette up and down to mix. The solution should be red/pink color.
Note: The ⅗ volume is an approximation. The exact amount needed for neutralization will vary.
Pellet oocysts by centrifuging at 1,000×g for 8 minutes at room temperature. Be sure to use transparent biocertified lids when spinning oocyst material (Figure 1C).
Count the number of sporulated and non-sporulated oocysts. Record results and verify that you have added enough of the stock oocyst preparation in order to achieve the desired sporozoite yield (Figure 2A).
Remove supernatant with disposable 25mL pipette. Dispose of supernatant is appropriately labeled oocyst waste container (1000mL glass bottle works well).
Resuspend pelleted material in 25mL of HBSS.
Pellet oocysts by centrifuging at 1,000×g for 8 minutes at room temperature.
If the supernatant remains a neutral red/pink color, discard supernatant as directed in step 13. If the supernatant remains an acidic yellow color, repeat steps 14–17 until a neutral red/pink color is obtained.
-
Prepare a 10% bleach solution in PBS.
Note: Prepare 10mLs of 10% bleach solution for each 50mL PET tube used.
Resuspend oocyst solution in 10mLs of 10% bleach in PBS solution.
Secure cap on the 50mL conical tube by wrapping in parafilm. Transfer tube into a secondary container and place on shaker for 30 minutes at room temperature with gentle shaking.
After 30 minutes, remove the tubes from the shaker and verify that the tubes are not leaking.
Remove the parafilm from the caps of the tubes.
-
Pellet the oocyst by centrifuging at 1,000×g for 8 minutes.
Note: Use caution when manipulating the tubes after the bleach step because the pellet is fragile.
Prepare excystation media by adding 0.1g Trypsin (Sigma; T4799) and 2g Taurocholic acid (Sigma; T4009) to 40mLs of PBS. Adjust the pH to 7.5 with 1M NaOH. Sterilize using a 0.22μm 50mL Steriflip® filter. Aliquot ~6mLs of sterile excystation media into 15 mL polyproplyene centrifuge tubes. Place in the 37°C water bath in the incubator to pre-warm.
After centrifugation, remove the supernatant and discard it in the liquid oocyst waste container.
Resuspend the pellet in 25mLs of HBSS. The solution will turn purple.
Pellet the oocyst by centrifuging at 1,000×g for 8 minutes.
Repeat steps 24–26 until solution turns to a neutral red/pink color.
Add 4g of sterile glass beads (pre-aliquoted into glass tubes and autoclaved) to a 15mL polystyrene centrifuge tube.
-
Resuspend the final pellet in 3mLs of HBSS and transfer into the 15mL polystyrene centrifuge tube containing the glass beads.
Note: When working with large numbers of oocysts (~10 million or more) it is best to divide oocyst mixture into multiple tubes. (Example: For 60 million oocysts, resuspend in 9mLs of HBSS and transfer 3mLs each to 3 polystyrene tubes containing 4g of sterile glass beads.)
Cap the tube and seal it by wrapping parafilm around the tube and cap.
Vortex the tube on high for 15 seconds on and 15 seconds off for a total vortex time of 1 minute.
Transfer the supernatant into a new 15mL conical polystyrene tube.
Add 10μL of the supernatant to a disposable hemocytometer and seal with nail polish as done in step (Figure 2B).
Wash remaining beads with 3mLs of HBSS and transfer into the new conical tube. Conduct a total of 2 washes for a total volume of 9mLs.
Pellet the oocysts by centrifuging at 800 ×g for 10 minutes at room temperature.
Replace cap on tube containing glass beads and discard in biohazard waste.
Count number of freed sporocysts using the hemocytometer prepared in step 34.
Remove supernatant and discard in oocyst waste container.
Resuspend pellet in 5mLs of pre-warmed excystation media prepared in step 24.
-
Place tubes in water bath in 37°C incubator. Untwist the cap to allow airflow and incubate for 45 minutes.
Note: Be sure that tubes will not be disturbed or tipped during incubation.
Optional: To verify that sporozoites are emerging from sporocysts, after 30 minutes, remove tubes from incubator, tighten caps, invert once to mix. Remove and discard cap in biohazard waste. Place 10 μL on a hemocytometer and seal it with nail polish. Add a new cap and return tubes to the water bath in incubator. Untwist caps slightly to allow for airflow. Incubate for the remaining 45 minutes.
-
After a 45 minute incubation, transfer the parasite preparation to an empty T25 flask, syringe lyse the parasite preparation 5 times with 25 5/8 gauge needle and 3mL syringe, and place 10 μL on hemocytometer, seal with nail polish, and count (Figure 2C).
Note: Change gloves after each round of syringe lysis. Be extremely cautious with needles, especially when discarding in biohazard sharps container.
Add 7mLs of cDMEM to the tube and gently pipette to mix and quench the reaction.
Pellet sporozoites by centrifuging at 800×g for 10 minutes at room temperature.
Remove supernatant and resuspend pellet in 5mL of cDMEM to wash the sporozoites.
Pellet sporozoites by centrifuging at 800×g for 10 minutes at room temperature
Resuspend sporozoites in an appropriate amount of cDMEM. Quantify using a hemacytometer (Figure 2C). Expected yields from this protocol are shown below in Figures 3 and 4.
Figure 2. Progression of excystation process.
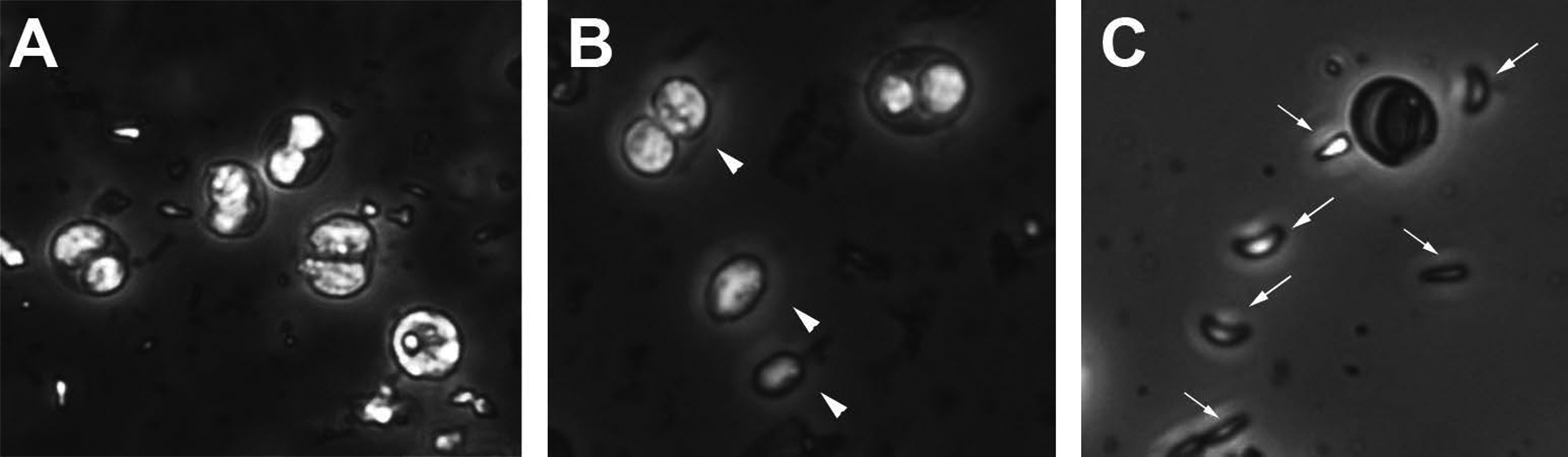
A) Image of oocyst sucrose float containing both sporulated and unsporulated oocysts. B) Image of sporocysts (arrowheads) released from oocysts after vortexing with glass beads. C) Image of sporozoites (arrows) following 45 minute incubation in excystation media and needle passage.
Figure 3.
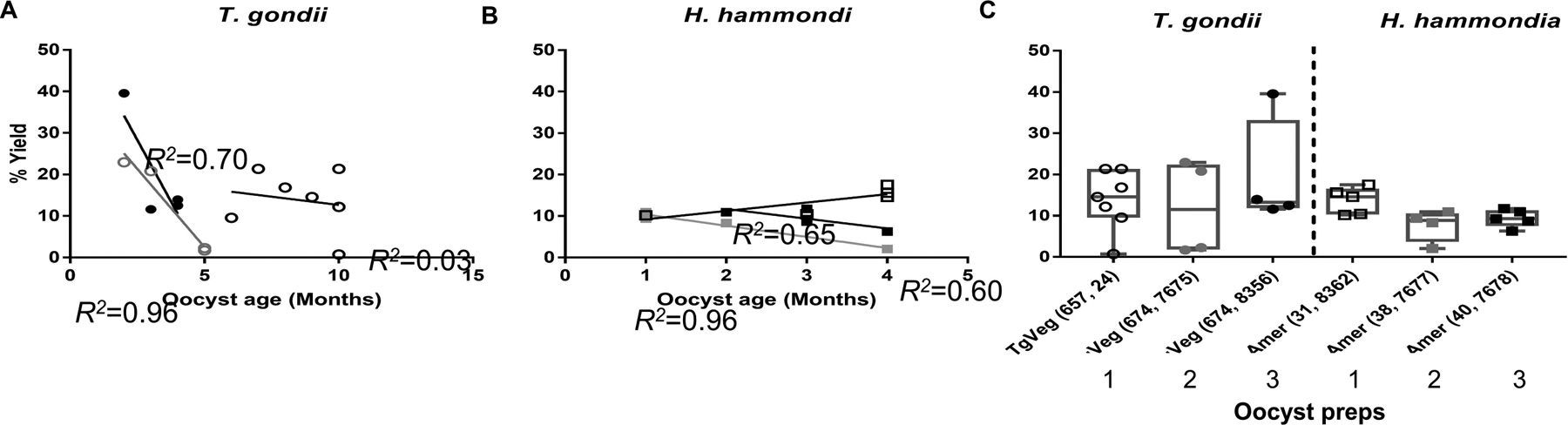
Excystation rates (% yield) of T. gondii and Hammondia hammondi oocysts according to age and oocyst batches. A,B) Scatter plots of sporozoite yields (in percentage) of T. gondii (A) and H. hammondi (B) from oocysts of different ages (in months). Each shade/shape represents a different oocyst preparation and each data point represents an independent excystation experiment. R2 values for each oocyst batch were calculated separately. C) Box and whisker plots show min to max of sporozoite yields from three different batches of T. gondii and H. hammondi oocysts. Each point represents the percent yield of sporozoites from one excystation experiment. Sporozoite yields were not significantly different between T. gondii and H. hammondi (One-way ANOVA, Tukey’s multiple comparison post-test, P>0.05).
Figure 4. Syringe lysis in excystation media may slightly improve sporozoite yield.
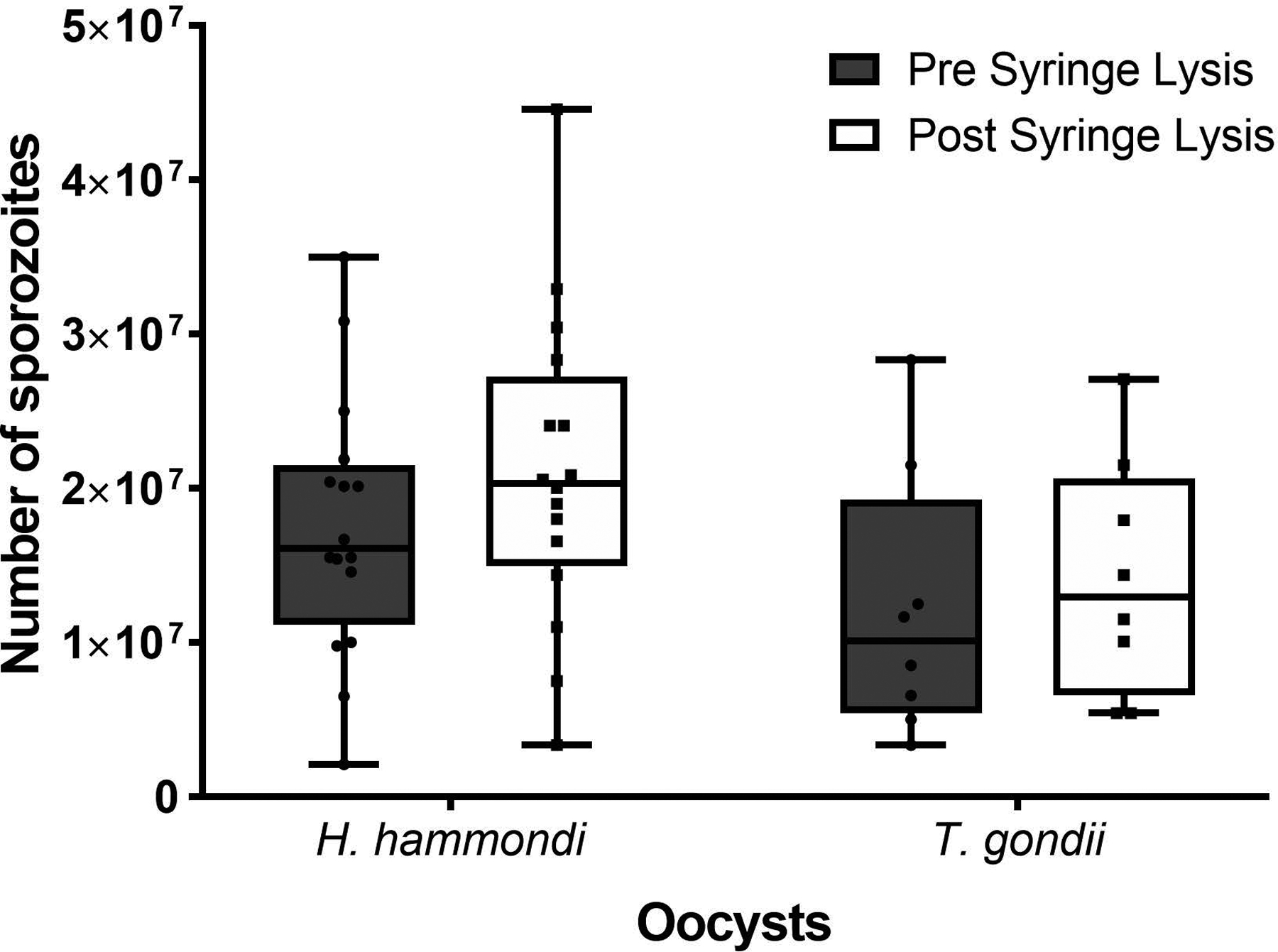
The box and whisker plots show the min to max number of sporozoites excysted prior to and after syringe lysis in excystation media for H. hammondi and T. gondii. Each point represents the number of sporozoites excysted for a given experiment. This data shows a general, but not significant, increase in the number of sporozoites excysted after syringe lysis. (Two-way ANOVA, Sidak’s multiple comparison test, H. hammondi P=0.47, T. gondii P=0.88).
Optional Overnight Infection
-
Infect a confluent monolayer of HFF’s grown in a T-25 with sporozoites.
Note: It is best to allocate the sporozoites between multiple flasks. We find splitting dirtier preps into 3 or more T-25’s improves our parasite yield following filtering.
Incubate overnight at 37 degrees C, 5% CO2.
After overnight incubation, set up biosafety cabinet with biohazard bag and oocyst waste bottle (Figure 1A).
-
Dislodge cells from the T-25 using a cell scraper.
Note: for reusable cell scrapers, place used scrapers that have come in contact with oocysts in a sealable autoclave bag. Seal and autoclave prior to reuse.
-
Syringe lyse the media in the T-25 (5 mLs) 5 times with 25 gauge needle and 3mL syringe and place 10 μL on hemocytometer, seal with nail polish, and count.
Note: Change gloves after each round of syringe lysis. Be extremely cautious with needles, especially when discarding in biohazard sharps container.
Remove oocyst debris using 5 μm syringe-driven filter or PD-10 desalting column. (See below).
Parasite purification with 5 μm syringe-driven filter
Pre-equilibrate a 5 μM filter with 2 ml of cDMEM.
To prepare parasites for purification, rinse monolayer with PBS. Syringe lyse HFF monolayer by five passages through a 25-gauge needle.
Centrifuge at 100 × g for 5 min to remove large debris.
Transfer supernatant to a new Eppendorf tube and centrifuge at 800 × g for 10 min at RT.
Resuspend the pellet (contains parasites) with 5 ml of cDMEM and load onto the pre-equilibrated 5 μM syringe-driven filter (10 ml syringe) and filter.
Replace the syringe with a new syringe (5 ml syringe), add 5 ml of cDMEM and re-elute through the same filter to collect any trapped parasites.
Parasite purification with PD-10 desalting column
-
Pre-equilibrate PD-10 column with chilled cDMEM. The PD10-column hold ~5ml of media, equilibrate column with a total of 30 ml chilled cDMEM. Discard the flow through.
Note: It typically takes ~20 min for the equilibration step. Keep column at 4°C if not used immediately
While column is equilibrating, prepare parasites for purification. Rinse monolayer with PBS, scrape, and then syringe lyse the HFF monolayer by five passages through a 25-gauge needle.
Centrifuge at 100 × g for 5 min at 10°C to remove large debris.
Transfer supernatant to a new eppendorf tube and centrifuge at 800 × g for 10 min at RT. Discard supernatant.
Resuspend the pellet (which contains parasites) with 2.5 ml cDMEM and load onto a pre-equilibrated PD-10 column.
-
Add an additional 3.5 ml of cDMEM to the column. Collect the eluate (~6 ml).
Note: If the initial suspension was particularly dark in color, it may be necessary to add another 3 ml of cDMEM and collect a second eluate. Combine with first eluate.
References cited:
- 1.Weiss LM, Kim K (2014) Toxoplasma gondii : the model apicomplexan - perspectives and methods. 2nd edition edn. Elsevier/AP, Amsterdam [Google Scholar]
- 2.Behnke MS, Khan A, Sibley LD (2015) Genetic mapping reveals that sinefungin resistance in Toxoplasma gondii is controlled by a putative amino acid transporter locus that can be used as a negative selectable marker. Eukaryot Cell 14 (2):140–148. doi: 10.1128/EC.00229-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD (2011) Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci U S A 108 (23):9631–9636. doi:1015338108 [pii] 10.1073/pnas.1015338108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 (5806):1776–1780 [DOI] [PubMed] [Google Scholar]
- 5.Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, Boyle JP, Boothroyd JC (2014) Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol 12 (4):e1001845. doi: 10.1371/journal.pbio.1001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP (2011) Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A 108 (23):9625–9630. doi: 10.1073/pnas.1015980108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bontell IL, Hall N, Ashelford KE, Dubey JP, Boyle JP, Lindh J, Smith JE (2009) Whole genome sequencing of a natural recombinant Toxoplasma gondii strain reveals chromosome sorting and local allelic variants. Genome Biol 10 (5):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle JP, Saeij JP, Harada SY, Ajioka JW, Boothroyd JC (2008) Expression quantitative trait locus mapping of Toxoplasma genes reveals multiple mechanisms for strain-specific differences in gene expression. Eukaryot Cell 7 (8):1403–1414. doi: 10.1128/EC.00073-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445 (7125):324–327. doi: 10.1038/nature05395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314 (5806):1780–1783. doi: 10.1126/science.1133690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP (2011) Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. The Journal of experimental medicine 208 (1):195–212. doi:jem.20100717 [pii] 10.1084/jem.20100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riahi H, Darde ML, Bouteille B, Leboutet MJ, Pestre-Alexandre M (1995) Hammondia hammondi cysts in cell cultures. J Parasitol 81 (5):821–824 [PubMed] [Google Scholar]
- 13.Dubey JP, Sreekumar C (2003) Redescription of Hammondia hammondi and its differentiation from Toxoplasma gondii. Int J Parasitol 33 (13):1437–1453. doi:S0020751903001413 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Heydorn AO, Mehlhorn H (2001) Further remarks on Hammondia hammondi and the taxonomic importance of obligate heteroxeny. Parasitol Res 87 (7):573–577 [DOI] [PubMed] [Google Scholar]
- 15.Walzer KA, Wier GM, Dam RA, Srinivasan AR, Borges AL, English ED, Herrmann DC, Schares G, Dubey JP, Boyle JP (2014) Hammondia hammondi harbors functional orthologs of the host-modulating effectors GRA15 and ROP16 but is distinguished from Toxoplasma gondii by a unique transcriptional profile. Eukaryot Cell 13 (12):1507–1518. doi: 10.1128/EC.00215-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walzer KA, Adomako-Ankomah Y, Dam RA, Herrmann DC, Schares G, Dubey JP, Boyle JP (2013) Hammondia hammondi, an avirulent relative of Toxoplasma gondii, has functional orthologs of known T. gondii virulence genes. Proc Natl Acad Sci U S A 110 (18):7446–7451. doi: 10.1073/pnas.1304322110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokol SL, Primack AS, Nair SC, Wong ZS, Tembo M, Verma SK, Cerqueira-Cezar CK, Dubey JP, Boyle JP (2018) Dissection of the in vitro developmental program of Hammondia hammondi reveals a link between stress sensitivity and life cycle flexibility in Toxoplasma gondii. eLife 7. doi: 10.7554/eLife.36491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey JP, Brake RJ, Murrell KD, Fayer R (1986) Effect of irradiation on the viability of Toxoplasma gondii cysts in tissues of mice and pigs. Am J Vet Res 47 (3):518–522 [PubMed] [Google Scholar]
- 19.Dubey JP, Kotula AW, Sharar A, Andrews CD, Lindsay DS (1990) Effect of high temperature on infectivity of Toxoplasma gondii tissue cysts in pork. J Parasitol 76 (2):201–204 [PubMed] [Google Scholar]
- 20.Kotula AW, Dubey JP, Sharar AK, Andrews CD, Shen SK, Lindsay DS (1991) Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J Food Protection 54:687–690 [DOI] [PubMed] [Google Scholar]
- 21.Hill DE, Benedetto SM, Coss C, McCrary JL, Fournet VM, Dubey JP (2006) Effects of time and temperature on the viability of Toxoplasma gondii tissue cysts in enhanced pork loin. J Food Prot 69 (8):1961–1965 [DOI] [PubMed] [Google Scholar]
- 22.Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P, McLeod R (2011) Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis 53 (11):1081–1089. doi: 10.1093/cid/cir667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann S, Batz MB, Morris JG Jr. (2012) Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75 (7):1292–1302. doi: 10.4315/0362-028X.JFP-11-417 [DOI] [PubMed] [Google Scholar]
- 24.Dubey JP, Dubey JP (2010) Toxoplasmosis of animals and humans. 2nd edn. CRC Press, Boca Raton [Google Scholar]
- 25.Radke JR, Gubbels MJ, Jerome ME, Radke JB, Striepen B, White MW (2004) Identification of a sporozoite-specific member of the Toxoplasma SAG superfamily via genetic complementation. Mol Microbiol 52 (1):93–105 [DOI] [PubMed] [Google Scholar]
- 26.Jerome ME, Radke JR, Bohne W, Roos DS, White MW, Veterinary Molecular Biology MSUBMUSA (1998) Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infection and immunity 66(10):4838–4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilley M, Fichera ME, Jerome ME, Roos DS, White MW (1997) Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains only a subset of dense-granule proteins. Infect Immun 65 (11):4598–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubey JP (1976) Reshedding of Toxoplasma oocysts by chronically infected cats. Nature 262 (5565):213–214 [DOI] [PubMed] [Google Scholar]
- 29.Malmasi A, Mosallanejad B, Mohebali M, Sharifian Fard M, Taheri M (2009) Prevention of shedding and re-shedding of Toxoplasma gondii oocysts in experimentally infected cats treated with oral Clindamycin: a preliminary study. Zoonoses Public Health 56 (2):102–104. doi: 10.1111/j.1863-2378.2008.01174.x [DOI] [PubMed] [Google Scholar]
- 30.Dubey JP (1995) Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J Parasitol 81 (3):410–415 [PubMed] [Google Scholar]
- 31.Zulpo DL, Sammi AS, Dos Santos JR, Sasse JP, Martins TA, Minutti AF, Cardim ST, de Barros LD, Navarro IT, Garcia JL (2018) Toxoplasma gondii: A study of oocyst re-shedding in domestic cats. Veterinary parasitology 249:17–20. doi: 10.1016/j.vetpar.2017.10.021 [DOI] [PubMed] [Google Scholar]


