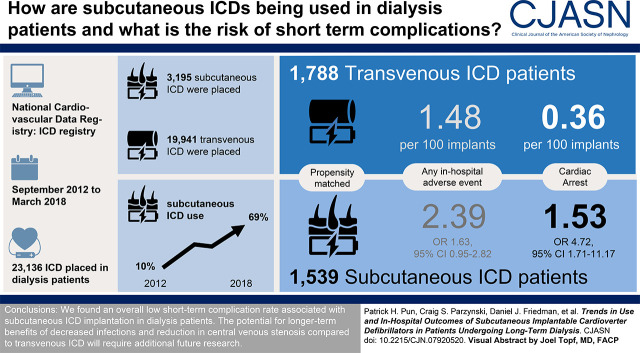
Visual Abstract
Keywords: arteriovenous access, cardiovascular, cardiovascular disease, dialysis, dialysis access, end stage kidney disease, epidemiology and outcomes, hospitals, defibrillators, implantable
Abstract
Background and objectives
Patients on dialysis are at high risk of complications related to implantable cardioverter defibrillator (ICD) implantation; use of subcutaneous ICDs may be preferred over transvenous devices due to lower risk of bloodstream infection and interference with vascular access sites. We evaluated trends in use and in-hospital outcomes of subcutaneous compared with transvenous ICDs among patients on dialysis in the United States.
Design, setting, participants, & measurements
Retrospective analysis of ICD implants from 2012 to 2018 among patients on dialysis reported to the National Cardiovascular Data Registry ICD Registry, a nationally representative US ICD Registry. We examined overall trends in subcutaneous ICD adoption as a proportion of all eligible ICD implants among patients on dialysis and then compared in-hospital outcomes between eligible subcutaneous ICD and transvenous ICD recipients using inverse probability of treatment weighting.
Results
Of the 23,136 total ICD implants in patients on dialysis during the study period, 3195 (14%) were subcutaneous ICDs. Among eligible first-time ICD recipients on dialysis, the proportion of subcutaneous ICDs used increased yearly from 10% in 2012 to 69% in 2018. In propensity score–weighted analysis of 3327 patients, compared with transvenous ICDs, patients on dialysis receiving subcutaneous ICDs had a higher rate of in-hospital cardiac arrest (2% versus 0.4%, P=0.002), but there was no significant difference in total in-hospital complications (2% versus 1%, P=0.08), all-cause death, or length of hospital stay.
Conclusions
The utilization of subcutaneous ICDs among US patients on dialysis has been steadily increasing. The overall risk of short-term complications is low and comparable with transvenous ICDs, but higher risks of in-hospital cardiac arrest merits closer monitoring and further investigation.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_09_23_CJN07920520.mp3
Introduction
For patients with kidney failure who are maintained on dialysis, sudden cardiac death is the largest contributor to mortality, accounting for a quarter of deaths of patients on hemodialysis and an annual risk of about 5%–7% per year, exceeding the rate in the general population by 20- to 30-fold (1,2). Implantable cardioverter defibrillators (ICDs) have been shown to reduce the risk of sudden cardiac death and mortality for cardiac arrest survivors, patients with sustained ventricular arrhythmias in the presence of structural heart disease, and for patients with significant left ventricular dysfunction with an ejection fraction ≤35% due to ischemic or nonischemic cardiomyopathy (3). Unfortunately, patients with concurrent kidney failure have been excluded from pivotal randomized clinical trials of ICDs, and observational studies (4) have failed to demonstrate any association with better survival in patients on maintenance dialysis. Albeit among patients with preserved ejection fraction, a recent randomized study among patients on maintenance dialysis also failed to demonstrate a survival benefit associated with ICDs (5). High competing risks of infection and vascular injury associated with the indwelling intravascular leads utilized by traditional transvenous ICDs may reduce the effectiveness of ICDs in improving survival in patients on dialysis (6,7).
To overcome the limitations of transvenous ICDs, the subcutaneous ICD was developed as an entirely subcutaneous system that does not require vascular access or intravascular defibrillator leads. The subcutaneous ICD was approved in 2012 for primary and secondary prevention of sudden cardiac death among patients meeting conventional ICD implantation criteria but who do not have an indication for permanent pacing, antitachycardiac pacing for treatment of ventricular tachycardia, or preexisting unipolar pacemaker leads (3). Some have advocated for the preferential use of subcutaneous ICDs over transvenous ICDs among eligible patients on dialysis to reduce high risks of device-related complications (8,9); however, to date, no large-scale studies have been performed to examine prevalent use of subcutaneous ICD among patients on dialysis and associated outcomes.
Therefore, the purpose of this study was to (1) describe the overall use of subcutaneous ICD among eligible US patients on dialysis since approval, and (2) compare in-hospital outcomes of those patients on dialysis eligible for subcutaneous ICD who received either a subcutaneous ICD or a single chamber transvenous ICD. Our a priori hypotheses were that use of subcutaneous ICD among patients on dialysis is rapidly increasing, and that there are significant differences in the risks of adverse in-hospital outcomes between recipients of subcutaneous ICDs and those of transvenous ICDs.
Materials and Methods
Data Source
Study patients were identified from the National Cardiovascular Data Registry (NCDR) ICD Registry, which includes approximately 90% of all ICD implantations in the United States (10). Until February 2018, all Medicare beneficiaries receiving a primary prevention ICD were required to be enrolled in the ICD Registry, and many centers that perform the procedure submitted information on all patients undergoing ICD implantation regardless of insurance provider. The registry includes extensive information on baseline patient characteristics and in-hospital outcomes. Data abstraction processes and standards are mandated and include standardized variable definitions, electronic quality checks, electronic data submission, and annual on-site audits of selected enrolling sites. Receipt of maintenance dialysis (including either hemodialysis or peritoneal dialysis) for kidney failure is recorded as a standardized variable. These rigorous standards have led to >90% accuracy for data elements (11). The Yale University Human Investigation Committee approved this analysis with waiver of informed consent.
Study Population
We had two study cohorts for this study. For our descriptive analysis of subcutaneous ICD usage trends, we included all unique patients on dialysis who received a new subcutaneous ICD between September 28, 2012 (when the subcutaneous ICD first became available) through March 31, 2018. For our comparative outcome analysis between recipients of subcutaneous ICDs and recipients of transvenous ICDs, we restricted the population to only patients on maintenance dialysis who met guideline criteria to receive a subcutaneous ICD; therefore, we excluded all patients with an indication for either bradycardia or cardiac resynchronization therapy pacing (3). For both study cohorts, we included both primary prevention and secondary prevention ICDs. We defined an indication for bradycardia pacing as a history of bradycardic arrest; prior or current pacemaker; or one or more of the following electrocardiogram findings: pacing, idioventricular rhythm, second- or third-degree heart block, or sinus arrest. We defined an indication for resynchronization therapy pacing as the inclusive combination of a QRS interval of >120 ms, New York Heart Association class 2–4 heart failure, and an ejection fraction ≤35%. Finally, we included only patients who had not received a previous ICD and were hospitalized electively for ICD implantation to reduce difficulties in propensity matching patients who were hospitalized due to acute illness.
Patient Characteristics
Baseline characteristics were obtained from the ICD Registry versions 2.1 and 2.2. We used a set of registry variables common to both versions that included patient demographics, baseline medical history and risk factors, diagnostic studies, and hospital characteristics. Before the analysis, implausible values were set to missing.
Treatment and Outcomes
For the comparative analysis, the treatment of interest was ICD type (subcutaneous versus transvenous). Treatment groups were defined based on the first attempted procedure during index hospitalizations with more than one procedure.
The primary outcome for the comparative analysis was a composite outcome of any recorded in-hospital adverse event: death, cardiac arrest, cardiac perforation, valve injury, hematoma, hemothorax, infection requiring antibiotics, lead dislodgement, myocardial infarction, pericardial tamponade, pneumothorax, transient ischemic attack or stroke, urgent cardiac surgery, and arterial vessel dissection. Individual adverse events, including in-hospital death and hospital length of stay, were also compared. Length of stay was defined as the number of overnight stays after the index procedure was performed.
Statistical Analyses
For the comparative analysis, characteristics of the overall population of patients eligible for an ICD were reported according to the type of device received (subcutaneous versus transvenous ICD). We used inverse probability of treatment weighting (IPTW) to balance differences across the devices. Briefly, we calculated the propensity score of receiving a subcutaneous versus a transvenous ICD by calculating the predicted probability as a function of patient comorbidities, census region, teaching status, hospital size, and implant year in a multivariable logistic regression. We then generated stabilized IPTWs from propensity scores using the observed probability of subcutaneous ICD divided by the predicted probability for the subcutaneous ICD group, and the observed probability of receiving transvenous ICD divided by 1 minus the propensity score for patients receiving transvenous ICD. We then used weighted logistic regression (with a robust variance estimator to account for clustering within facilities) to estimate the odds of complication between subcutaneous and transvenous ICDs and each of its components. To further characterize the composite outcome, we also calculated weighted event rates for the overall composite and each of its components.
To assess the balance of covariates after propensity weighting, we calculated preweighted and postweighted standardized differences. Values <10% were considered to be sufficiently balanced (12). Before modeling the propensity score, we performed single imputation for missing values using fully conditional specification (13). Missing values were generally rare with the large majority of variables having <0.5% missing and three variables having <2.5% missing. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Statistical significance was considered at P<0.05; all tests accounted for multiple comparisons.
Results
Overall Subcutaneous ICD Use among Patients on Dialysis
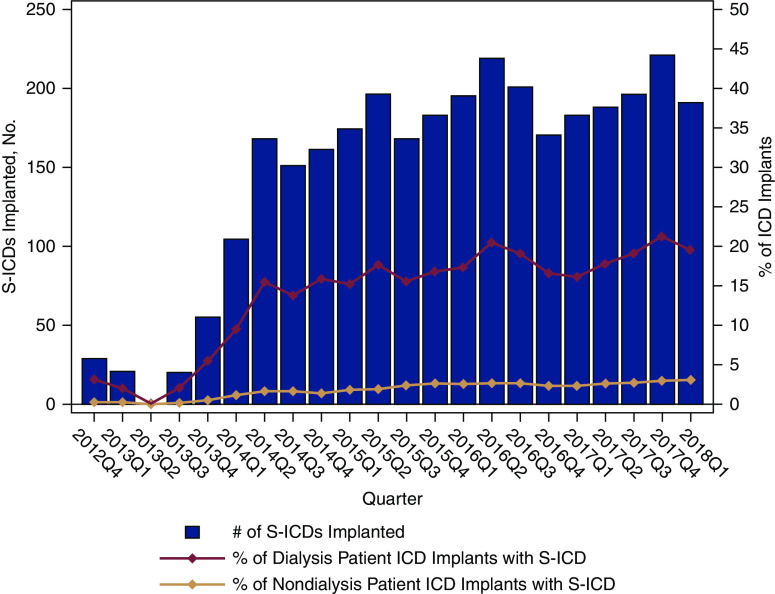
Between September 28, 2012 and March 31, 2018, a total of 23,136 ICD implant procedures were recorded in the ICD Registry among patients on maintenance dialysis. Subcutaneous ICD implants comprised 3195 (14%) of these devices. Figure 1 shows the absolute number of subcutaneous ICD implants and the relative proportion of all ICD implants in both the dialysis and nondialysis populations. There was a four-fold increase in subcutaneous ICD utilization during the study time period within the dialysis population, from around 5% of all ICD implants in 2012–2013 to 20% of all ICD implants in 2018. Subcutaneous ICDs comprised a greater proportion of all ICDs among the patients on maintenance dialysis compared with the nondialysis population, where subcutaneous ICDs accounted for only about approximately 3% of all ICD implants.
Figure 1.
Increasing proportion of subcutaneous ICD implants among patients on maintenance dialysis compared with nondialysis patients, from September 2012 to March 2018. Red trend line represents the percentage of patients on dialysis implanted with a subcutaneous ICD, and pink trend line represents the percentage of patients who were not on dialysis and were implanted with a subcutaneous ICD. A supply chain disruption occurred during early 2013, corresponding to the observed drop in subcutaneous ICD implantation during 2013, in quarter 2 (Q2). ICD, implantable cardioverter defibrillator; S-ICD, subcutaneous ICD.
Comparison of Characteristics and Outcomes of Recipients of Subcutaneous ICDs and Recipients of Transvenous ICDs
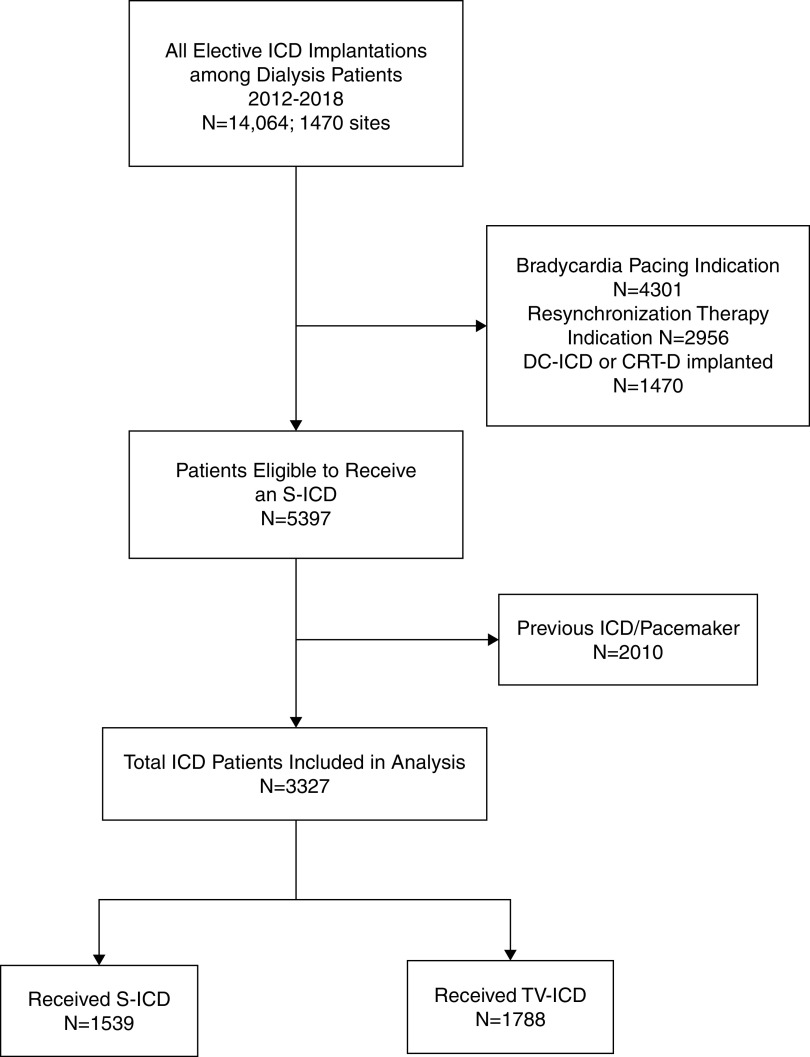
Figure 2 illustrates the assembly of our comparative study cohort. After limiting the cohort to only patients on dialysis who met guideline criteria for subcutaneous ICD implantation (i.e., without a pacing or cardiac resynchronization therapy indication) and who were hospitalized electively for first-time ICD implantation, there were 1539 recipients of subcutaneous ICDs and 1788 recipients of transvenous ICDs during the study period. Among this cohort of patients eligible for subcutaneous ICDs, the utilization of subcutaneous ICD increased dramatically between 2012 and 2018, from 10% of all ICD implants in 2012 to 69% of all implants in 2018 (Figure 3). The characteristics of the comparative subcutaneous ICD and transvenous ICD cohorts before and after propensity weighting are shown in Table 1. Before propensity score weighting, recipients of subcutaneous ICDs were younger, more likely to be Black, and less likely to be from the southern US states. Hospitals implanting subcutaneous ICDs in patients on dialysis were more likely to be teaching hospitals with >500 hospital beds. Apart from these characteristics, there were no major differences between the two groups in patient demographics and comorbid conditions. After IPTW weighting, all standardized differences between groups were <5%.
Figure 2.
Flow chart depicting comparative study cohort assembly. CRT-D, cardiac resynchronization therapy device; DC-ICD, dual-chamber ICD; S-ICD, subcutaneous ICD; TV-ICD, transvenous ICD.
Figure 3.
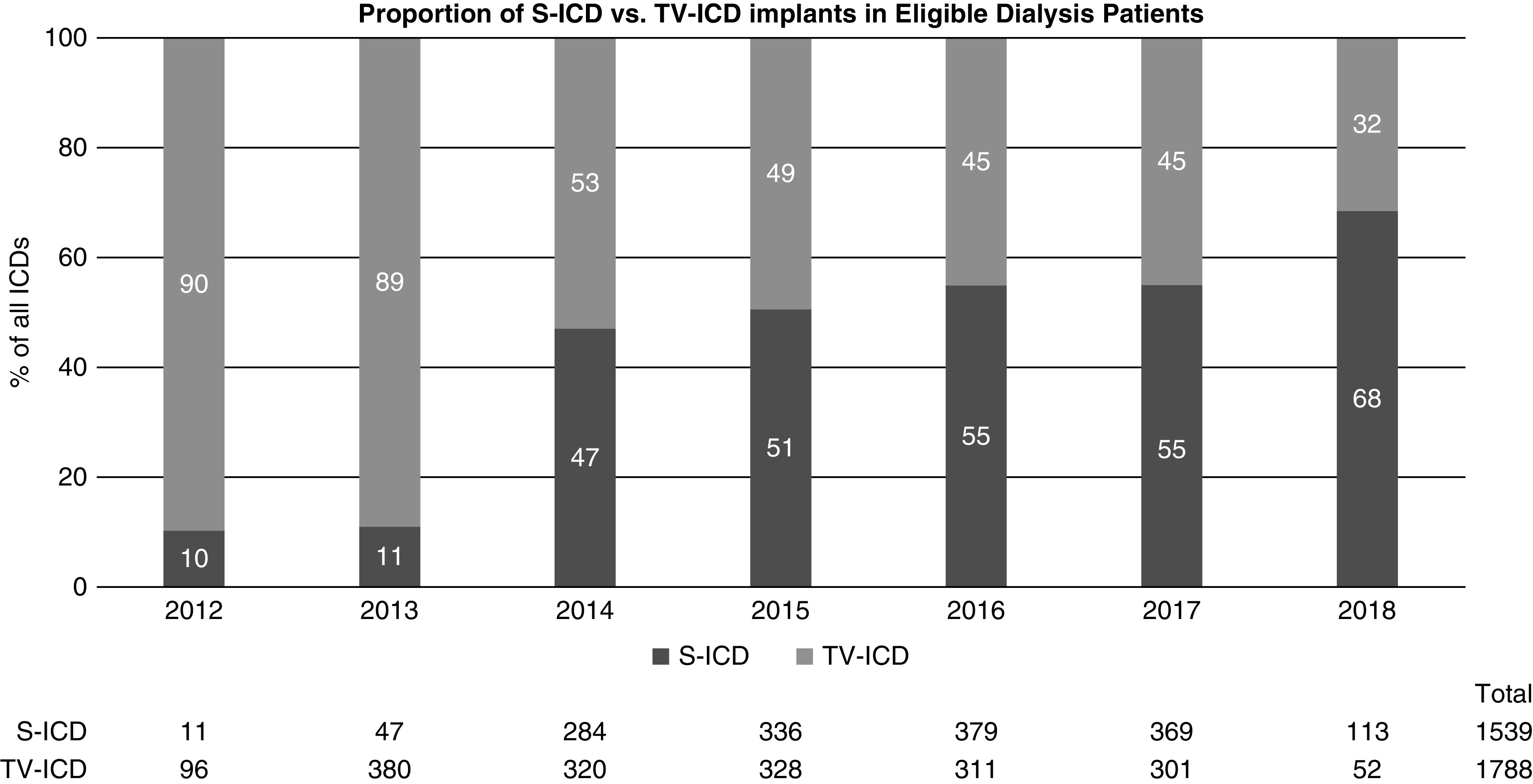
The proportion of subcutaneous ICD versus transvenous ICD implants increased from 10% to 68% among patients on maintenance dialysis who were eligible for a subcutaneous ICD in the comparative study cohort during the study period. S-ICD, subcutaneous ICD; TV-ICD, transvenous ICD.
Table 1.
Characteristics of study patients receiving subcutaneous versus transvenous implantable cardioverter defibrillator, before and after inverse probability of treatment weighting and missing value imputation
| Characteristics | Unweighted | IPT Weighted, Missing Value Imputation | ||||
|---|---|---|---|---|---|---|
| Subcutaneous ICD (n=1539) | Transvenous ICD (n=1788) | Std. Diff. (%) | Subcutaneous ICD | Transvenous ICD | Std. Diff. (%) | |
| Age in years, mean | 58 | 60 | 0.13 | 59 | 59 | 0.01 |
| Female | 491 (32%) | 560 (31%) | 0.01 | 33% | 32% | 0.02 |
| Race | ||||||
| White | 779 (51%) | 1038 (58%) | 0.15 | 55% | 54% | 0.02 |
| Black | 656 (43%) | 614 (34%) | 0.17 | 38% | 39% | 0.02 |
| Other | 104 (7%) | 136 (7%) | 0.03 | 8% | 8% | 0.00 |
| Insurance payer | ||||||
| Private | 715 (47%) | 912 (51%) | 0.09 | 49% | 49% | 0.00 |
| Medicare | 1210 (79%) | 1339 (75%) | 0.09 | 77% | 76% | 0.01 |
| Medicaid | 430 (28%) | 437 (24%) | 0.08 | 27% | 26% | 0.01 |
| Other | 50 (3%) | 73 (4%) | 0.04 | 4% | 4% | 0.02 |
| No insurance | 9 (0.6%) | 20 (1%) | 0.06 | 0.9% | 0.9% | 0.00 |
| ICD indication | ||||||
| Primary prevention | 1326 (86%) | 1580 (88%) | 0.07 | 86% | 87% | 0.02 |
| Secondary prevention | 213 (14%) | 208 (12%) | 0.07 | 14% | 13% | 0.02 |
| Nonischemic dilated cardiomyopathy | 708 (46%) | 748 (42%) | 0.08 | 44% | 44% | 0.01 |
| Ischemic heart disease | 890 (58%) | 1085 (61%) | 0.06 | 60% | 59% | 0.01 |
| Atrial fibrillation/flutter | 326 (21%) | 359 (20%) | 0.03 | 22% | 21% | 0.02 |
| Ventricular tachycardia | 267 (17%) | 298 (17%) | 0.02 | 18% | 17% | 0.01 |
| NYHA class | ||||||
| I | 52 (4%) | 82 (5%) | 0.05 | 5% | 4% | 0.02 |
| II | 641 (42%) | 752 (42%) | 0.01 | 41% | 42% | 0.01 |
| III | 678 (44%) | 767 (43%) | 0.02 | 44% | 44% | 0.01 |
| IV | 12 (0.8%) | 23 (1%) | 0.04 | 1% | 1% | 0.00 |
| No HF | 149 (10%) | 159 (9%) | 0.03 | 9% | 9% | 0.00 |
| Prior PCI | 551 (36%) | 663 (37%) | 0.03 | 36% | 36% | 0.01 |
| Prior CABG | 362 (24%) | 474 (27%) | 0.07 | 24% | 25% | 0.02 |
| Diabetes mellitus | 955 (62%) | 1091 (61%) | 0.02 | 61% | 61% | 0.01 |
| Prior MI | 718 (47%) | 870 (49%) | 0.04 | 48% | 48% | 0.01 |
| Syndromes associated with sudden cardiac death | 72 (5%) | 69 (4%) | 0.04 | 4% | 4% | 0.02 |
| Chronic lung disease | 274 (18%) | 341 (19%) | 0.03 | 19% | 18% | 0.02 |
| Cerebrovascular disease | 289 (19%) | 312 (18%) | 0.03 | 19% | 18% | 0.02 |
| Cardiac arrest | 192 (13%) | 177 (10%) | 0.08 | 12% | 12% | 0.01 |
| Syncope | 182 (12%) | 201 (11%) | 0.02 | 11% | 12% | 0.03 |
| Left ejection fraction, mean | 27% | 27% | 0.02 | 28% | 27% | 0.01 |
| Sodium (mEq/L) | ||||||
| ≤135 | 337 (22%) | 344 (20%) | 0.06 | 21% | 21% | 0.01 |
| 135–145 | 1176 (78%) | 1403 (80%) | 0.05 | 78% | 79% | 0.03 |
| >145 | 8 (0.5%) | 14 (0.8%) | 0.03 | 1% | 0.7% | 0.06 |
| Hemoglobin (g/dl) | ||||||
| ≤10.1 | 349 (23%) | 348 (20%) | 0.07 | 23% | 22% | 0.02 |
| 10.1–11.6 | 586 (39%) | 643 (37%) | 0.05 | 39% | 38% | 0.01 |
| >11.6 | 573 (38%) | 755 (43%) | 0.11 | 39% | 40% | 0.03 |
| Census region | ||||||
| Midwest region | 358 (23%) | 351 (20%) | 0.09 | 22% | 21% | 0.01 |
| Northeast region | 314 (20%) | 322 (18%) | 0.06 | 19% | 19% | 0.01 |
| South region | 666 (43%) | 881 (49%) | 0.12 | 45% | 46% | 0.03 |
| West region | 192 (13%) | 230 (13%) | 0.01 | 0.4% | 0.4% | 0.00 |
| US territories | 9 (0.6%) | 4 (0.2%) | 0.06 | 14% | 13% | 0.03 |
| Hospital characteristics | ||||||
| Teaching hospital | 967 (63%) | 951 (53%) | 0.20 | 60% | 59% | 0.03 |
| No. of patient beds | ||||||
| ≤100 | 29 (2%) | 74 (4%) | 0.13 | 3% | 3% | 0.02 |
| 101 to ≤500 | 713 (46%) | 1011 (57%) | 0.21 | 50% | 51% | 0.03 |
| ≥501 | 797 (52%) | 703 (39%) | 0.25 | 48% | 46% | 0.03 |
| Implant year | ||||||
| 2012 | 11 (0.7%) | 96 (5%) | 0.27 | 3% | 3% | 0.01 |
| 2013 | 47 (3%) | 380 (21%) | 0.58 | 11% | 13% | 0.04 |
| 2014 | 284 (19%) | 320 (18%) | 0.01 | 18% | 18% | 0.01 |
| 2015 | 336 (22%) | 328 (18%) | 0.09 | 20% | 20% | 0.01 |
| 2016 | 379 (25%) | 311 (17%) | 0.18 | 21% | 21% | 0.00 |
| 2017 | 369 (24%) | 301 (17%) | 0.18 | 21% | 20% | 0.02 |
| 2018 | 113 (7%) | 52 (3%) | 0.20 | 5% | 6% | 0.02 |
ICD, implantable cardioverter defibrillator; IPT, inverse probability of treatment; Std. Diff., standardized difference; NYHA, New York Heart Association; HF, heart failure; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; MI, myocardial infarction.
Table 2 compares the rate of all in-hospital outcomes between the propensity weighted groups. For the primary composite outcome of any adverse in-hospital event, the event rate did not differ significantly between patients on dialysis receiving subcutaneous ICDs (2.39 events per 100 implants) and those receiving transvenous ICDs (1.48 events per 100 implants; odds ratio [OR], 1.63; 95% confidence interval [95% CI], 0.95 to 2.82; P=0.08, subcutaneous versus transvenous ICD). However, examination of specific adverse events revealed a higher rate of cardiac arrest among recipients of subcutaneous ICDs (1.53 events per 100 implants versus 0.36 per 100 implants; OR, 4.72; 95% CI, 1.71 to 11.17; P=0.0002). Overall, there were 20 in-hospital cardiac arrests among recipients of subcutaneous ICDs compared with seven among recipients of transvenous ICDs. Information on intraprocedural defibrillation threshold testing was available for 12 of the recipients of subcutaneous ICDs and five of the recipients of transvenous ICDs who experienced cardiac arrest. Seven of the recipients of subcutaneous ICDs (58%) underwent defibrillation threshold testing compared with only one patient (20%) of the recipients of transvenous ICDs who had cardiac arrest. There were no other significant differences in other adverse events, including the risk of in-hospital death.
Table 2.
Comparison of in-hospital adverse events between patients on dialysis who received subcutaneous implantable cardioverter defibrillators and those who received transvenous implantable cardioverter defibrillators
| Adverse Event | No. of Events per 100 Implants (95% CI) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Subcutaneous ICD | Transvenous ICD | Subcutaneous versus Transvenous ICD | ||
| Any adverse event | 2.39 (1.62 to 3.51) | 1.48 (1.02 to 2.14) | 1.63 (0.95 to 2.82) | 0.08 |
| Death | 0.82 (0.4 to 1.68) | 0.31 (0.15 to 0.67) | 2.62 (0.92 to 7.47) | |
| Cardiac arrest | 1.53 (0.9 to 2.61) | 0.36 (0.17 to 0.76) | 4.72 (1.71 to 11.17) | 0.002 |
| Hematoma | 0.56 (0.3 to 1.05) | 0.25 (0.11 to 0.57) | 2.25 (0.80 to 6.32) | 0.1 |
| Hemothorax | — | 0.07 (0.01 to 0.47) | NA | NA |
| Infection requiring antibiotics | 0.10 (0.03 to 0.42) | 0.03 (0.00 to 0.22) | 3.29 (0.30 to 36.57) | 0.3 |
| Lead dislodgement | 0.22 (0.08 to 0.59) | 0.62 (0.34 to 1.11) | 0.35 (0.11 to 1.13) | 0.08 |
| Tamponade | — | 0.03 (0.00 to 0.22) | NA | NA |
| Pneumothorax | — | 0.10 (0.03 to 0.42) | NA | NA |
| Stroke/TIA | 0.08 (0.01 to 0.60) | — | NA | NA |
| Urgent cardiac surgery | — | 0.07 (0.01 to 0.46) | NA | NA |
| AMI | — | — | NA | NA |
| Cardiac perforation | — | — | NA | NA |
95% CI, 95% confidence interval; ICD, implantable cardioverter defibrillator; NA, not applicable; TIA, transient ischemic attack.
Finally, we examined overall hospital length of stay between recipients of subcutaneous ICDs and recipients of transvenous ICDs. There was no significant difference between the two groups (mean length of stay 1.57 days [95% CI, 1.22 to 1.93] for subcutaneous ICD group; 1.24 days [95% CI, 1.12 to 1.36] for transvenous ICD group; mean difference 0.34 days [95% CI, −0.04 to 0.70 days]; P=0.08).
Discussion
Despite the high risk of sudden cardiac death that patients on dialysis experience, ICDs have not been shown to reduce sudden cardiac death or better long-term survival in these patients. Traditional transvenous ICDs have been associated with markedly higher device-associated complications among patients on dialysis, including higher risks of device infection, bacteremia, central venous thrombosis and stenosis, and higher immediate postimplantation complications (7,14). Due to the extravascular design, subcutaneous ICDs offer potential benefits to patients on dialysis by reducing the risk of endovascular infection and injury, and they also may help preserve central veins for hemodialysis vascular access. Additionally, the simplified implant procedure, with no need for fluoroscopy or intravascular manipulation, potentially lowers the risk of procedural complications, such as infection, and eliminates the risks of pneumothorax, hemothorax, and cardiac tamponade. Because of these potential benefits, professional guidelines appear to recommend subcutaneous ICD as the device of choice among eligible patients with kidney failure, citing the high risk of complications of inserting transvenous leads and those at high risk of infection (3). Despite the lack of definitive data supporting the safety of subcutaneous ICDs in patients on dialysis, our study reports a steady increase in subcutaneous ICD use in the United States, accounting for nearly 20% of all ICD implants and nearly 70% of patients on dialysis who met eligibility criteria for subcutaneous ICDs in 2018.
To our knowledge, our study is the first to compare the risks of peri-procedural outcomes between subcutaneous ICD and traditional transvenous ICDs in a large national cohort of patients on dialysis. We found no significant difference in the overall in-hospital complication rate between recipients of the two ICD types. This finding is consistent with preliminary results from a recent randomized trial in the general population of subcutaneous versus transvenous ICD (15). Although the overall complication rate of 2% is low in patients on dialysis, it is more than two-fold greater than in-hospital subcutaneous ICD complication rates in the general population (0.9% in an earlier report from the ICD Registry [16]), consistent with previous reports of higher peri-procedural complications of transvenous ICDs among patients on maintenance dialysis compared with those patients not on dialysis (7). In contrast, a recently published postapproval study of recipients of subcutaneous ICDs compared intermediate-term outcomes of 220 patients on dialysis with 1637 patients who were not on dialysis, and did not find significant differences in the overall complication rate between the two groups out to 1 year (17).
Although there was no difference in the composite outcome of all in-hospital complications, there was a higher rate of in-hospital cardiac arrest events among recipients of subcutaneous ICDs. A higher rate of peri-procedural cardiac arrests among all patients with a subcutaneous ICD was also observed in a prior comparative analysis (16). Although we used propensity score weighting to decrease confounding, this observation could be explained by unmeasured confounding related to the greater burden of comorbidity among patients selected to receive a subcutaneous ICD versus a transvenous ICD. Other possible explanations should be considered. Due to a higher energy requirement to successfully convert ventricular fibrillation to normal sinus rhythm, defibrillation threshold testing at the time of implant is recommended by guidelines for all subcutaneous ICDs, whereas it is not routinely performed for transvenous ICDs (18). Defibrillation threshold testing typically requires deeper sedation and could predispose the patient to hemodynamic compromise and cardiac arrest, even in the setting of successful ventricular fibrillation conversion (19). In our study, a greater proportion of patients with a subcutaneous ICD who experienced in-hospital cardiac arrest underwent defibrillation threshold testing (58%) compared with patients with a transvenous ICD who experienced cardiac arrest (20%). Out of 33 cardiac arrests in a recent study of subcutaneous ICD complications reported to the Food and Drug Administration, ten were temporally tied to defibrillation threshold testing (20). On the other hand, appropriate use of defibrillation threshold testing might mitigate future cardiac arrest events that would be inadequately treated without testing, which has greater relevance among patients on dialysis who often have higher defibrillation thresholds compared with patients who are not on dialysis (6). Unfortunately, in our study, we cannot confirm the timing of cardiac arrest events in relationship to defibrillation threshold testing and the implant procedure, or the specific type of arrhythmia preceding cardiac arrest, because these data are not recorded in the ICD Registry. Although the overall rate was low, the higher risks of peri-procedural cardiac arrest associated with subcutaneous ICD in patients on dialysis, and whether defibrillation threshold testing contributes to this risk, merits closer monitoring and further investigation. More data are also needed on whether defibrillation threshold testing differs between subcutaneous ICD and transvenous ICD among patients on dialysis.
Consistent with the subcutaneous design, among 1519 subcutaneous ICD implants, there were no reported intravascular complications—including hemothorax, cardiac perforation, and cardiac tamponade—associated with subcutaneous ICD, whereas a few events were reported among patients who received a transvenous ICD. The reduction in these complications and the potential to also reduce longer-term complications (9,17) may offset the higher implantation costs of subcutaneous ICDs compared with transvenous devices; however, a full cost-benefit analysis would require further knowledge of downstream costs related to longer-term outcomes.
Although our study is the largest national study to examine trends of use of subcutaneous ICDs in patients with kidney failure, and to directly compare in-hospital outcomes of subcutaneous ICD with transvenous ICD, several key limitations should be noted. First, despite the use of propensity weighting to eliminate significant differences between groups, this is an observational study and the results could be influenced by residual confounding. Although we adjusted for many important clinical factors and cardiovascular comorbidities, we did not have data on dialysis-specific data, including vascular access type, which could have influenced the study results. Second, only in-hospital outcomes are available in the ICD Registry; therefore, the number of events was small, and we are unable to report on longer-term outcomes. It will be important to examine longer-term outcomes and other complications, such as inappropriate shocks between subcutaneous and transvenous ICDs, to fully understand the risk-benefit ratio of subcutaneous ICDs. Additional data from long-term studies will also be needed to examine the potential benefits of vascular access preservation and avoidance of central venous stenosis among recipients of subcutaneous ICDs. Third, the ICD Registry does not contain data on cardiac arrest heart rhythm and whether the device provided a shock in response to cardiac arrest. We also did not have data on electrolyte levels at the time of cardiac arrest, nor timing of events in relationship to dialysis treatment. Thus, we are unable to comment further on potential contributions of these factors to the cardiac arrest events observed. Finally, although the ICD Registry collects information on the majority of recipients of subcutaneous ICDs in the United States, it is possible that our results may not be generalizable to patients who were not reported to the registry; further, our study results may not be generalizable to patients who underwent the procedure during a nonelective hospitalization.
In conclusion, in this short-term study of in-hospital complications, we found an overall low complication rate associated with subcutaneous ICD implantation in patients on dialysis, which was not significantly higher compared with transvenous ICD implants. Although the low complication rate supports subcutaneous ICD use among eligible patients on dialysis, the potential benefits of lower long-term infection risk and reduction in central venous stenosis, compared with transvenous ICD, have not been proven. Longer-term studies are needed to better define the overall risk-benefit and cost-effectiveness of subcutaneous ICD as a means to prevent cardiac arrest, reduce ICD-associated complications, and lower overall mortality among at-risk patients on dialysis.
Disclosures
S.M. Al-Khatib reports receiving grants and research and speaking fees from Abbott; and grants and research, speaking, and consultancy fees from Medtronic; outside the submitted work. J.P. Curtis receives salary support under contract with the NCDR to provide analytic services. He also has a contract with the American College of Cardiology for his role as Senior Medical Officer, NCDR; receives salary support from the Centers for Medicare and Medicaid Services to develop and maintain performance measures that are used for public reporting; and holds equity interest in Medtronic. D.J. Friedman has received research support from Abbott, Biosense Webster, and Boston Scientific; educational grants from Abbott, Biotronik, Boston Scientific, Johnson and Johnson, and Medtronic; and consulting fees from Abbott and AtriCure. In addition, he has a patent “Method and System for Determination of the Vector of Ventricular Electrical Activation Using Three Extra-cardiac Electrodes for Cardiac Rhythm Discrimination and Localization of the Origin of Ventricular Tachycardias and Premature Ventricular Contractions” pending to Yale. C.S. Parzynski reports receiving other from American College of Cardiology, during the conduct of the study. P.H. Pun has received consulting fees from Astra Zeneca, Fresenius North America, Janssen, and Relypsa; and research support from the National Institutes of Health. The remaining author has nothing to disclose.
Funding
This study was supported by the American College of Cardiology Foundation’s NCDR. The ICD Registry is an initiative of the American College of Cardiology with partnering support from the Heart Rhythm Society. P.H. Pun is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R03DK113324.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.United States Renal Data System (USRDS) : 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 2.Makar MS, Pun PH: Sudden cardiac death among hemodialysis patients. Am J Kidney Dis 69: 684–695, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL: 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society [published correction appears in Heart Phythm 15: e278–e281, 2018 10.1016/j.hrthm.2018.09.026]. Heart Rhythm 15: e190–e252, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh S, Zalucky A, Hemmelgarn BR, Roberts DJ, Ahmed SB, Wilton SB, Jun M: Incidence of sudden cardiac death in adults with end-stage renal disease: A systematic review and meta-analysis. BMC Nephrol 17: 78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Krol-van Straaten MJ, Hommes N, Gabreëls B, van Dorp W, van Dam B, Herzog CA, Schalij MJ, Rabelink TJ; ICD2 Trial Investigators : Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients: The Prospective Randomized Controlled ICD2 Trial. Circulation 139: 2628–2638, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta A, Montalvo J, Medendorp S, Lloyd-Jones DM, Ghossein C, Goldberger J, Passman R: Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis 49: 656–663, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal A, Wang Y, Rumsfeld JS, Curtis JP, Heidenreich PA; National Cardiovascular Data Registry : Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm 6: 1565–1571, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Dhamija RK, Tan H, Philbin E, Mathew RO, Sidhu MS, Wang J, Saour B, Haqqie SS, Beathard G, Yevzlin AS, Salman L, Boden WE, Siskin G, Asif A: Subcutaneous implantable cardioverter defibrillator for dialysis patients: A strategy to reduce central vein stenoses and infections. Am J Kidney Dis 66: 154–158, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Koman E, Gupta A, Subzposh F, Saltzman H, Kutalek SP: Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on hemodialysis. J Interv Card Electrophysiol 45: 219–223, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Hammill SC, Kremers MS, Stevenson LW, Heidenreich PA, Lang CM, Curtis JP, Wang Y, Berul CI, Kadish AH, Al-Khatib SM, Pina IL, Walsh MN, Mirro MJ, Lindsay BD, Reynolds MR, Pontzer K, Blum L, Masoudi F, Rumsfeld J, Brindis RG: Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm 7: 1340–1345, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers M, Reynolds MR, Heidenreich PA, Al-Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J: The national ICD registry report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm 10: e59–e65, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28: 3083–3107, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Buuren S: Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16: 219–242, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins C, McLean R, Cheng A, Brinker JA, Marine JE, Nazarian S, Spragg DD, Sinha S, Halperin H, Tomaselli GF, Berger RD, Calkins H, Henrikson CA: End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 22: 1099–1104, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Heart Rhythm Society : Head-to-head trial data demonstrate impact of subcutaneous ICD therapy compared to TV-ICDs. Available at: https://www.hrsonline.org/news/press-releases/hrs2020science-lbct-praetorian-trial. Accessed July 29, 2020
- 16.Friedman DJ, Parzynski CS, Varosy PD, Prutkin JM, Patton KK, Mithani A, Russo AM, Curtis JP, Al-Khatib SM: Trends and in-hospital outcomes associated with adoption of the subcutaneous implantable cardioverter defibrillator in the United States. JAMA Cardiol 1: 900–911, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Chami MF, Burke MC, Herre JM, Shah MH, Sadhu A, Niebauer MJ, Kutalek SP, Carter N, Gold MR: Outcomes of subcutaneous implantable cardioverter-defibrillator in dialysis patients: Results from the S-ICD post-approval study [published online ahead of print May 4, 2020]. Heart Rhythm 10.1016/j.hrthm.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 18.Stiles MK, Fauchier L, Morillo CA, Wilkoff BL: 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing [published online ahead of print January 21, 2020]. J Interv Card Electrophysiol 10.1007/s10840-019-00662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DJ, Parzynski CS, Heist EK, Russo AM, Akar JG, Freeman JV, Curtis JP, Al-Khatib SM: Ventricular fibrillation conversion testing after implantation of a subcutaneous implantable cardioverter defibrillator: Report from the national cardiovascular data registry. Circulation 137: 2463–2477, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeitler EP, Friedman DJ, Loring Z, Campbell KB, Goldstein SA, Wegermann ZK, Schutz J, Smith N, Black-Maier E, Al-Khatib SM, Piccini JP: Complications involving the subcutaneous implantable cardioverter-defibrillator: Lessons learned from MAUDE. Heart Rhythm 17: 447–454, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]