Visual Abstract
Keywords: kidney biopsy, bleeding, Epidemiology and outcomes, nationwide data, score, Diabetic Nephropathies, Thrombocytopenia, Frailty, Hemorrhage, Hematoma, Amyloidosis, Nephrectomy, Thrombotic Microangiopathies, Blood Transfusion, anemia
Abstract
Background and objectives
The risk of major bleeding after percutaneous native kidney biopsy is usually considered low but remains poorly predictable. The aim of the study was to assess the risk of major bleeding and to build a preprocedure bleeding risk score.
Design, setting, participants, & measurements
Our study was a retrospective cohort study in all 52,138 patients who had a percutaneous native kidney biopsy in France in the 2010–2018 period. Measurements included major bleeding (i.e., blood transfusions, hemorrhage/hematoma, angiographic intervention, or nephrectomy) at day 8 after biopsy and risk of death at day 30. Exposures and outcomes were defined by diagnosis codes.
Results
Major bleeding occurred in 2765 of 52,138 (5%) patients (blood transfusions: 5%; angiographic intervention: 0.4%; and nephrectomy: 0.1%). Nineteen diagnoses were associated with major bleeding. A bleeding risk score was calculated (Charlson index [2–4: +1; 5 and 6: +2; >6: +3]; frailty index [1.5–4.4: +1; 4.5–9.5: +2; >9.5: +3]; women: +1; dyslipidemia: −1; obesity: −1; anemia: +8; thrombocytopenia: +2; cancer: +2; abnormal kidney function: +4; glomerular disease: −1; vascular kidney disease: −1; diabetic kidney disease: −1; autoimmune disease: +2; vasculitis: +5; hematologic disease: +2; thrombotic microangiopathy: +4; amyloidosis: −2; other kidney diagnosis: −1) + a constant of 5. The risk of bleeding went from 0.4% (lowest score group =0–4 points) to 33% (highest score group ≥35 points). Major bleeding was an independent risk of death (500 of 52,138 deaths: bleeding: 81 of 2765 [3%]; no bleeding: 419 of 49,373 [0.9%]; odds ratio, 1.95; 95% confidence interval, 1.50 to 2.54; P<0.001).
Conclusions
The risk of major bleeding after percutaneous native kidney biopsy may be higher than generally thought and is associated with a twofold higher risk of death. It varies widely but can be estimated with a score useful for shared decision making and procedure choice.
Introduction
Kidney biopsies are routinely performed in most nephrology centers all over the world. They provide crucial diagnostic information and guide optimal management of patients with kidney abnormalities. Since the early 1980s, advancements have been made in biopsy technique, and guidelines have been published (1). The risk of bleeding is considered low in the most recent meta-analyses (2).
However, the risk of bleeding after percutaneous native kidney biopsy remains unclear, is highly variable in the literature, and is poorly predictable on an individual basis (1). Reported complication rates do not reflect real-life practice for many reasons (1). First, most data come from case series in individual centers, and therefore, selection and reporting biases are likely to occur. Smaller center size (<30 biopsies per year) was identified as a risk factor of bleeding (3). This finding supports the view that the risk of bleeding may be underestimated in the literature because small centers do not usually publish their results. Second, although most bleeding complications occur within the first 24 hours, some may appear later, and they may be missed due to loss of follow-up (3–5). Third, meta-analyses and case series from major centers show that the risk of bleeding varies (0.3%–14.5% for the risk of macroscopic hematuria and 0.9%–15% for the risk of blood transfusion) (1,2,6–8). Although risk factors have been identified, the causes of this variability are not fully understood (1,2,6–9).
Moreover, the risk of bleeding of percutaneous native kidney biopsy should ideally be estimated prior to the procedure. Estimation of the risk of bleeding on an individual basis would allow adequate patient information, shared decision making, and adequate procedure choice (1). A reassessment of the procedure’s safety using very large cohorts of unselected patients is necessary because the sample size of published studies may be inadequate to assess the exact risk factors of bleeding.
In this report, we assessed the rate and risk factors of major bleeding after percutaneous native kidney biopsies from 2010 to 2018 in all French patients using nationwide data; we also estimated the risk of death after bleeding, and we built a bleeding risk score to guide physician and patient decision.
Materials and Methods
Study Design
This longitudinal cohort study was on the basis of the national hospitalization database covering hospital care from the entire French population. The data for all patients admitted who had a native kidney biopsy in France from January 2008 to December 2018 were collected from the national medico-administrative “programme de médicalisation des systèmes d’information” (PMSI) database (i.e., medicalized information system program), which was inspired by the US Medicare system. Through this program, which was implemented in 2004, hospital medical activity is recorded in a database, computed, and rendered anonymous. It includes >98% of the French population (67 million people) from birth (or immigration) to death (or emigration), even if a person changes occupation or retires. This process allows the determination of each hospital’s budget in the 1546 French health care facilities for both public and private hospitals. Each hospitalization is encoded in a standardized dataset, which includes information about the patient (age and sex), hospital, stay (date of admission, date of discharge, and mode of discharge), pathologies, and procedures. Routinely collected medical information includes the principal and secondary diagnoses according to the International Classification of Diseases, Tenth Revision (ICD10). All medical procedures are recorded according to the national nomenclature, Classification Commune des Actes Medicaux. The reliability of PMSI data has already been assessed (10), and this database has previously been used to study patients with cardiovascular conditions (11–15).
The study was conducted retrospectively, and because patients were not involved in its conduct, there was no effect on their care. Ethical approval was not required because all data were anonymized. The French Data Protection Authority granted access to the PMSI data. Procedures for data collection and management were approved by the Commission Nationale de l'Informatique et des Libertés (CNIL), the independent National Ethical Committee protecting human rights in France, which ensures that all information is kept confidential and anonymous in compliance with the Declaration of Helsinki. This study falls within the scope of the French Reference Methodology MR-005 according to 2016–41 law dated January 26, 2016 on the modernization of the French health system, which requires neither information nor nonopposition of the included individuals. Access to linked anonymous file in the PMSI databases was approved by the CNIL (MR-005 registration number 0415141119).
Patient Selection
We restricted the analysis to patients admitted after 2010 because this allowed us to obtain at least 2 years of past events to define comorbidities since 2008. Patients who had a percutaneous native kidney biopsy in France during the 2010–2018 period were considered according to ICD10 codes (Supplemental Table 1, ICD10 codes: JAHB001, JAHJ006, and JAGJ007).
Major Bleeding and Risk of Death after Biopsy
Major bleeding (blood transfusion [ICD10 code: FELF011 and JAFA023], hematoma/hemorrhage [ICD10 code: T810], angiographic intervention [ICD10 code: EDSF003 and EDSF008], and nephrectomy [ICD10 code: JAFA002 and JAFA023]) during an 8-day period after kidney biopsy was ascertained by diagnosis code (Supplemental Table 1). For the risk of death after biopsy, a 30-day period was considered.
Collected Data
Demographic, Cardiovascular, and Metabolic Conditions.
Patient information (demographics, comorbidities, medical history, and events during hospitalization or follow-up) was described using data collected in the hospital records. For each hospital stay, combined diagnoses at discharge were obtained. Each variable was identified using ICD10 codes (Supplemental Table 1). We also used the Charlson comorbidity index and the claims-based frailty indicator to assess patient clinical status (16,17). Because the information was on the basis of codes, there were no missing values. Cardiovascular and metabolic conditions of interest included hypertension, diabetes, obesity, heart failure, valve diseases, coronary artery disease, smoking, dyslipidemia, stroke, vascular disease, and atrial fibrillation.
Kidney Diagnoses Known at the Time of Biopsy.
These parameters included reported history of acute kidney failure, glomerular disease, vascular or hypertensive kidney disease, diabetic kidney disease, autoimmune diseases, vasculitis, tubulointerstitial disease, thrombotic microangiopathy, hematologic-related kidney diseases, amyloidosis, and other kidney diseases (Supplemental Table 1).
Other Relevant Parameters.
We collected information regarding history of alcohol-related diagnoses, lung diseases, liver diseases, cancer within the years preceding the biopsy, thrombocytopenia, and anemia. Of note, medications including antiplatelet agents and anticoagulants were not available (see below).
Statistical Analyses
Data are presented as means and SDs for quantitative parameters and percentages for categorical parameters. Patients who had major bleeding complications (blood transfusion, hematoma/hemorrhage, angiographic intervention, or nephrectomy) during 8 days following the biopsy were compared with other patients using t test or chi-squared test as appropriate. Multivariable logistic regressions were used, and results were expressed as odds ratios and 95% confidence intervals (95% CIs).
Major Bleeding Risk Score
A bleeding risk score was built using multivariable logistic regressions. To create the score points, the regression coefficients with P<0.05 were divided by the smallest coefficient and rounded up to the nearest integer (18,19). For this score, information available at the time of biopsy (history of comorbid conditions and kidney diagnoses known at entry) was used, and significant variables were entered into the models. Receiver operating characteristic curves were constructed, and areas under the curve (c indexes) with 95% CIs were calculated to evaluate the predictive ability of major bleeding events after kidney biopsy of the score and compared using the DeLong test.
As sensitivity analyses, we assessed the validity of this score in the subgroups of patients with atrial fibrillation (condition associated with use of anticoagulants) and without atrial fibrillation (for this analysis, patients with coronary artery disease were excluded). We assessed the validity of this score in the subgroups of patients with coronary artery disease (prone to receive antiplatelet agents) and without coronary artery disease (similarly, patients with atrial fibrillation were excluded from the analysis). We also tested our bleeding risk score when major bleeding was defined as blood transfusion, angiographic intervention, or nephrectomy (but not hematoma/hemorrhage).
Internal validation was obtained by means of 1000 bootstrap replicates. For model calibration, we plotted observed versus predicted risks by decile of predicted risk, and the regression line was drawn against line of equity (intercept =0, slope =1).
We also built a “simplified” risk score: all parameters except specific kidney disease diagnoses were used. The same methodology was applied.
Results
Baseline Characteristics
During the 2010–2018 period, 52,138 patients had a percutaneous native kidney biopsy in France. Two thirds of patients were diagnosed with hypertension, and a quarter were diagnosed with diabetes (Table 1). Diagnosis of cardiovascular comorbid conditions was present in many patients (heart failure: 10%; valve diseases: 4%; coronary artery diseases: 10%; and atrial fibrillation: 9%) (Table 1). Diagnosis of history of anemia was present in 23% of patients, and history of cancer within the preceding years was present in 23% of patients (Table 1).
Table 1.
Characteristics of patients who underwent kidney biopsy in France from 2010 to 2018
| Patient Characteristics | No Major Bleeding | Major Bleeding |
|---|---|---|
| n | 49,373 | 2765 |
| Age, yr | 57±18 | 63±17 |
| Sex (men) | 30,216 (61) | 1489 (54) |
| Charlson comorbidity index | 4.45±2.79 | 5.64±2.76 |
| Frailty index | 6.60±7.25 | 10.95±8.39 |
| History of cardiovascular and metabolic diseases | ||
| Hypertension | 26,271 (53) | 1651 (60) |
| Diabetes mellitus | 10,766 (21) | 682 (25) |
| Obesity | 7501 (15) | 375 (14) |
| Heart failure | 4820 (10) | 563 (20) |
| Valve disease | 2013 (4) | 242 (9) |
| Coronary artery disease | 4784 (10) | 363 (13) |
| Vascular disease | 5064 (10) | 377 (14) |
| Atrial fibrillation | 3979 (8) | 438 (16) |
| Stroke | 885 (2) | 61 (2) |
| Smoker | 4820 (10) | 321 (12) |
| Dyslipidemia | 9371 (19) | 524 (19) |
| History of other comorbid conditions | ||
| Malnutrition | 3758 (8) | 483 (18) |
| Alcohol-related diagnoses | 3199 (7) | 267 (10) |
| Lung disease | 5046 (10) | 418 (15) |
| Liver disease | 2854 (6) | 257 (9) |
| Anemia | 10,290 (21) | 1754 (63) |
| Thrombocytopenia | 3202 (7) | 437 (16) |
| Cancer within preceding 5 yr | 11,192 (23) | 935 (34) |
| Kidney diagnoses | ||
| Abnormal kidney function | 14,217 (29) | 1313 (48) |
| AKI | 13,729 (28) | 1790 (65) |
| Glomerular disease | 16,715 (34) | 702 (25) |
| Vascular or hypertensive disease | 4208 (9) | 205 (7) |
| Diabetic kidney disease | 3448 (7) | 150 (5) |
| Autoimmune disease | 2949 (6) | 228 (8) |
| Vasculitis | 1816 (4) | 343 (12) |
| Hematologic-related kidney disease | 1426 (3) | 207 (8) |
| Thrombotic microangiopathy | 694 (1) | 180 (7) |
| Amyloidosis | 187 (0.4) | 6 (0.2) |
| Other diagnoses | 592 (1) | 41 (20,029) |
Parameters are presented as number (percentage) except for age, Charlson comorbidity index, and frailty index (presented as mean ± SD).
Among the kidney presentations known before biopsy procedure, diagnoses of AKI (30%), glomerular disease (33%), vascular or hypertensive kidney disease (9%), and diabetic kidney disease (7%) were the most frequent ones (Table 1).
Risk of Major Bleeding
Major bleeding was diagnosed in 2765 of 52,138 (5%) patients (blood transfusion: 2415 of 52,138 [5%]; hemorrhage/hematoma: 243 of 52,138 [0.5%]; angiographic intervention: 186 of 52,138 [0.4%]; and nephrectomy: 34 of 52,138 [0.1%]).
Multivariable analyses indicated that several parameters were associated with the risk of major bleeding, including women, Charlson index, frailty index, history of thrombocytopenia, anemia, cancer within the preceding years, and obesity (Table 2). Of note, the calculated score was similar in obese and nonobese patients (12.2±8.0 versus 12.4±8.0 points) when obesity was not taken into account. Because diagnoses of Charlson and frailty indexes were both associated with the risk of major bleeding, we calculated the correlation coefficient between Charlson index and frailty index (r=0.19). Among kidney diagnoses, AKI, thrombotic microangiopathy, autoimmune diseases, vasculitis, and hematologic-related kidney diseases were also associated with the risk of major bleeding (Table 2).
Table 2.
Risk factors for major bleeding after percutaneous native kidney biopsy
| Risk Factors | Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| Unadjusted | Adjusted | |
| Age, per +1 quartile | 1.05 (0.99 to 1.12) | 1.04 (0.99 to 1.09) |
| Charlson comorbidity index, per +1 quartile | 1.08 (1.00 to 1.16) | 1.10 (1.05 to 1.16) |
| Frailty index, per +1 quartile | 1.23 (1.19 to 1.35) | 1.26 (1.21 to 1.32) |
| Sex, men | 0.80 (0.71 to 0.90) | 0.80 (0.73 to 0.87) |
| History of cardiovascular and metabolic disease | ||
| Hypertension | 0.87 (0.76 to 1.00) | 0.91 (0.83 to 1.00) |
| Diabetes mellitus | 0.98 (0.84 to 1.15) | 0.91 (0.81 to 1.02) |
| Heart failure | 1.01 (0.85 to 1.20) | 1.06 (0.95 to 1.20) |
| Valve disease | 1.16 (0.93 to 1.46) | 1.16 (0.99 to 1.36) |
| Coronary artery disease | 0.93 (0.76 to 1.13) | 0.98 (0.86 to 1.13) |
| Vascular disease | 0.85 (0.70 to 1.04) | 0.92 (0.80 to 1.05) |
| Atrial fibrillation | 1.07 (0.89 to 1.28) | 1.10 (0.97 to 1.24) |
| Stroke | 0.96 (0.66 to 1.38) | 0.78 (0.59 to 1.03) |
| Smoker | 0.97 (0.80 to 1.18) | 1.05 (0.92 to 1.21) |
| Dyslipidemia | 0.99 (0.90 to 1.10) | 0.88 (0.78 to 0.98) |
| Obesity | 0.75 (0.63 to 0.89) | 0.77 (0.68 to 0.88) |
| Malnutrition | 1.04 (0.88 to 1.22) | 0.99 (0.88 to 1.11) |
| Alcohol-related diagnoses | 1.05 (0.84 to 1.31) | 0.96 (0.81 to 1.12) |
| Lung disease | 0.87 (0.73 to 1.04) | 0.90 (0.80 to 1.02) |
| Liver disease | 1.01 (0.81 to 1.25) | 0.92 (0.78 to 1.08) |
| Anemia | 3.55 (3.11 to 4.05) | 3.47 (3.16 to 3.80) |
| Thrombocytopenia | 1.32 (1.11 to 1.57) | 1.29 (1.14 to 1.45) |
| Cancer within preceding years | 1.24 (1.07 to 1.44) | 1.21 (1.09 to 1.34) |
| Known kidney diagnosis at the time of biopsy | ||
| Abnormal kidney function | 1.35 (1.19 to 1.53) | 1.31 (1.20 to 1.43) |
| AKI | 2.03 (1.78 to 2.32) | 2.12 (1.93 to 2.32) |
| Glomerular disease | 0.77 (0.67 to 0.88) | 0.74 (0.67 to 0.81) |
| Vascular or hypertensive kidney disease | 0.84 (0.67 to 1.06) | 0.85 (0.72 to 0.99) |
| Diabetic kidney disease | 0.70 (0.53 to 0.91) | 0.67 (0.55 to 0.81) |
| Autoimmune disease | 1.16 (0.92 to 1.46) | 1.29 (1.10 to 1.51) |
| Vasculitis | 2.26 (1.85 to 2.76) | 2.35 (2.05 to 2.70) |
| Hematologic-related kidney disease | 1.36 (1.07 to 1.73) | 1.35 (1.14 to 1.59) |
| Thrombotic microangiopathy | 2.02 (1.56 to 2.62) | 1.92 (1.59 to 2.31) |
| Amyloidosis | 0.28 (0.07 to 1.16) | 0.40 (0.17 to 0.92) |
| Other diagnosis | 0.63 (0.39 to 1.04) | 0.70 (0.50 to 0.98) |
Age quartiles were <45, 45–60, 61–71, and >71. Variables included in the multivariable model were Charlson comorbidity index, frailty index, sex, dyslipidemia, obesity, anemia, thrombocytopenia, cancer, abnormal kidney function, AKI, glomerular disease, vascular or hypertensive kidney disease, vasculitis, hematologic-related kidney disease, thrombotic microangiopathy, amyloidosis, and other diagnosis.
Major Bleeding Risk Score
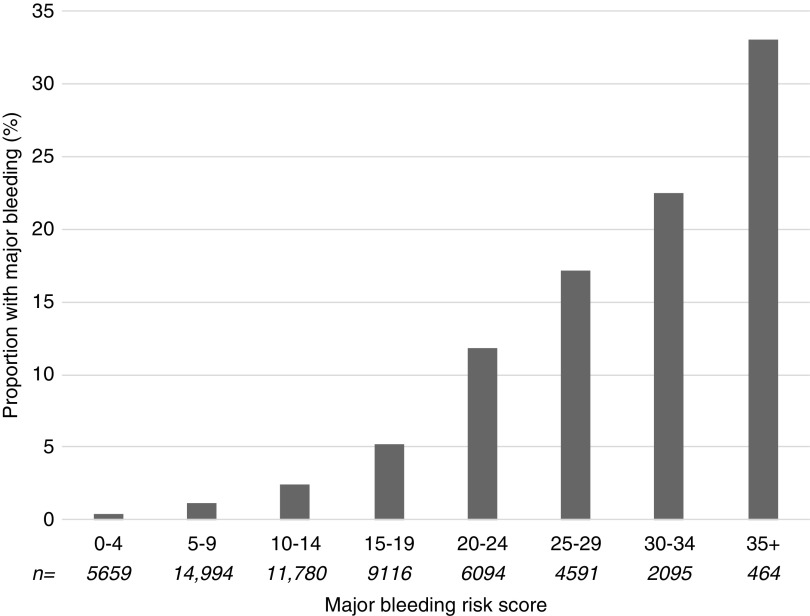
We then built a bleeding risk score (Table 3). In our cohort, minimal score was 0, and maximal score was 41 (Figure 1). There was a 75-fold difference in the proportion of major bleeding between the lowest and highest risk groups: 0.4% for 0–4 points; 1% for 5–9 points; 2% for 10–14 points; 5% for 15–19 points; 12% for 20–24 points; 17% for 25–29 points; 23% for 30–34 points; and 33% for ≥35 points (Figure 1). Area under the receiver operating characteristic curve (AUC) was 0.803 (95% CI, 0.80 to 0.81) in the whole population when the score was used as a continuous variable (Figure 2A) and 0.796 (95% CI, 0.79 to 0.80) when the score used eight levels of risk (Figure 2B). Using a bootstrap sampling procedure for internal validation, the score had an AUC of 0.796 (95% CI, 0.79 to 0.80). Calibration plots of risk model for the overall cohort showed that calibration of the bleeding risk score was relatively good up to a predicted risk of major bleeding of approximately 30% (i.e., in 50,234 of 52,138 [96%] patients) (Supplemental Figure 1).
Table 3.
Major bleeding risk score
| Components of the Score | Points |
|---|---|
| Base scorea | |
| Charlson comorbidity index | |
| 2–4 | 1 |
| 5–6 | 2 |
| >6 | 3 |
| Frailty index | |
| 1.5–4.4 | 1 |
| 4.5–9.5 | 2 |
| >9.5 | 3 |
| Sex (men) | −1 |
| Dyslipidemia | −1 |
| Obesity | −1 |
| Anemia | 8 |
| Thrombocytopenia | 2 |
| Cancer within preceding years | 2 |
| Abnormal kidney function | 2 |
| Acute kidney failure | 4 |
| Glomerular disease | −1 |
| Vascular or hypertensive kidney disease | −1 |
| Diabetic kidney disease | −1 |
| Autoimmune disease | 2 |
| Vasculitis | 5 |
| Hematologic-related kidney disease | 2 |
| Thrombotic microangiopathy | 4 |
| Amyloidosis | −2 |
| Other diagnosis | −1 |
The major bleeding risk score was calculated as follows: base score (i.e., sum of the points for each diagnosis) + a constant of 5 points for all patients. The major bleeding score went from 0 (minimal score) to 41 (maximal score) points.
Figure 1.
Observed proportion of major bleeding according to bleeding risk score. The proportion of major bleeding (blood transfusion, hemorrhage/hematoma, angiography intervention, or nephrectomy) is shown in relation to the number of points of the bleeding risk score levels (from 0–4 to ≥35). The number of biopsies was 52,138 (5659 for score 0–4; 14,994 for score 5–9; 11,780 for score 10–14; 9116 for score 15–19; 6094 for score 20–24; 4591 for score 25–29; 2095 for score 30–34; and 464 for score ≥35).
Figure 2.
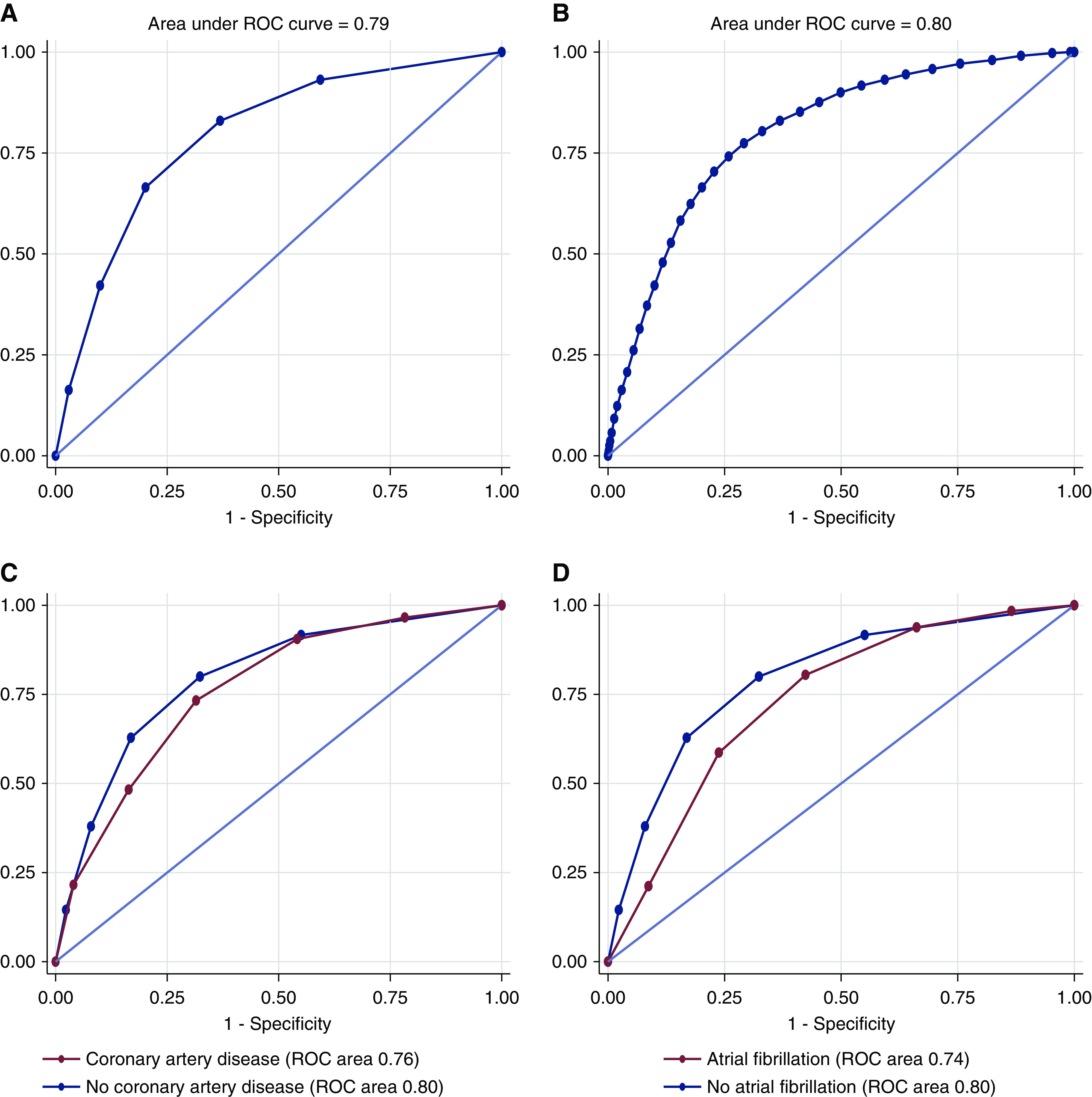
Major bleeding risk score receiver operating characteristic (ROC) curve. ROC curves are presented in the whole population (A and B), in patients with or without coronary artery disease (C), and in patients with or without atrial fibrillation (D).
It was possible to identify the center where the biopsy was performed in 52,035 of 52,138 patients (99.8%). The proportion of major bleeding in relation to the number of biopsies performed annually per center was 722 of 13,114 (5.2%) for the first quartile (<23 biopsies), 723 of 12,996 (5.3%) for the second quartile (23–55 biopsies), 834 of 13,769 (5.7%) for the third quartile (56–106 biopsies), and 712 of 12,156 (5.5%) for the fourth quartile (>106 biopsies). The performance of our score seemed clinically similar, regardless of the quartile (Supplemental Figure 2).
Sensitivity Analyses
We tested our bleeding risk score when major bleeding was defined as blood transfusion, angiographic intervention, or nephrectomy (but not hematoma/hemorrhage); the performance of our score was even better (AUC for continuous score =0.83; AUC for eight-level risk score =0.82).
Receiver operating characteristic curves were also built for patients with atrial fibrillation, patients with coronary artery disease, and patients without any of these conditions (Figure 2, C and D). AUC was 0.76 (95% CI, 0.74 to 0.79) in the 3851 patients with coronary artery disease versus 0.80 (95% CI, 0.79 to 0.81) in the 43,870 patients without coronary artery disease (P=0.02) (Figure 2C). AUC was 0.74 (95% CI, 0.71 to 0.76) in the 3121 patients with atrial fibrillation versus 0.80 (95% CI, 0.79 to 0.81; P<0.001) in the patients without atrial fibrillation (Figure 2D).
Simplified Score
Parameters associated with the risk of bleeding included age, Charlson index, frailty index, sex, hypertension, diabetes mellitus, obesity, dyslipidemia, anemia, thrombocytopenia, cancer, abnormal kidney function, and AKI (Supplemental Table 2). The simplified major bleeding risk score went from 0 to 29 points. There was a 25-fold difference in the proportion of major bleeding between the lowest and highest risk groups: 1% for 0–4 points; 3% for 5–9 points; 6% for 10–14 points; 13% for 15–19 points; 19% for 20–24 points; and 24% for ≥25 points. AUC was 0.78 for the continuous score and 0.77 for the six-level risk score (Supplemental Figure 3, B and C).
Risk of Death after Biopsy: Association with Major Bleeding
There were 500 of 52,138 (0.96%) deaths recorded at day 30 (major bleeding: 81 of 2765 [3%]; no bleeding: 419 of 49,373 [0.85%]). Major bleeding was an independent risk factor for death in multivariable analysis (adjusted odds ratio, 1.95; 95% CI, 1.23 to 2.26; P=0.001) (Supplemental Table 3). Other independent risks of death were older age, malnutrition, heart failure, coronary artery disease, liver disease, cancer, amyloidosis, and acute kidney failure (Supplemental Table 3).
Median time to transfusion was 1 day (interquartile range, 1–1), and median time to death was 20 days (interquartile range, 14–25) (Supplemental Figure 4).
Discussion
Using the French nationwide database, the largest cohort of patients with percutaneous native kidney biopsy was analyzed. We found that the overall risk of bleeding associated with the procedure is not negligible and is highly variable. There was a 75-fold difference in the risk of bleeding according to patient characteristics: major bleeding occurred in 0.44% of patients without any identified risk compared with 33% of those with several risk factors. Major bleeding was associated with a higher risk of death.
We found that blood transfusion occurred in 5% of patients, whereas kidney angiography and nephrectomy occurred in 0.4% and 0.1% of patients, respectively. In the meta-analysis of Corapi et al. (2) that included 32 studies and 9456 biopsies, the proportion of patients who had blood transfusion was much lower (0.9%; 95% CI, 0.4% to 1.5%). Whether under-reporting occurred is unknown, but as acknowledged by the authors, the major limit of their study was publication bias (2). The reported risk of major bleeding in 8573 adult patients from 1990 to 2009 in Norway was also lower (2.7%), but the proportion of angiographic interventions/surgery was similar (0.2%; range, 0.1%–0.4%) (20). However, in this study, local nephrologists from 26 different hospitals reported clinical data using a registry notification form, and information regarding complications was lacking in 357 (3.7%) reports (these cases were excluded from the study) (20). Whether reporting bias occurred is possible. In contrast, other recent reports showed more worrying results. A recent study reported a 10% complication rate (including a 2% major complication rate in patients with ultrasound guidance and a 6% major complication rate in those without) among 2138 patients (21). In other recent reports, the proportion of blood transfusion was 5% in major centers in the United States (3) and 9% in Canada (8). Risks of blood transfusion and angiographic intervention were 8% and 2%, respectively, in patients with AKI (22). Finally, our results in real-life conditions explain these heterogeneous findings (2,3,8,20–22). Kidney biopsy procedures in France do not seem different from those performed in most countries in Europe, Canada, and the United States or from current guidelines (1,3,8,23). However, whether similar results would be applicable in other health care systems has to be studied.
We observed that obesity and diabetic kidney disease were associated with a lower risk of bleeding. Accordingly, bleeding was observed in 3% of lean patients, 1% of overweight patients, and 0.9% of obese patients in the study of Lees et al. (9). Whether this lower risk of major bleeding is related to diabetic kidney disease and obesity hypercoagulability is debatable (24–26).
The strength of this study derives from its size and design. This study represents, by far, the largest study focused on this issue. We included all patients who had a percutaneous native kidney biopsy to reduce heterogeneity at a nationwide level. Data are present for the whole population, and there is no selection of patients according to social status, employer, age, or preexisting conditions. There is no selection bias, and there are essentially no missing data because the PMSI database, the French diagnosis related group, includes the whole French population. Of note, percutaneous kidney biopsy is performed in the majority (around >90%) of patients who have a kidney biopsy in France (23). This design also avoided selective or reporting bias and allowed identification of relevant risk factors. Because late complication can occur, we chose to study the risk of bleeding up to 8 days after biopsy. The score was robust and internally validated using a bootstrap procedure.
This study also has some limits. Several parameters, including biologic data, size of gauge, experience and specialty of physicians (nephrologist versus radiologist), and indication of transfusion, were not available for analysis, and our data derived from administrative codes. However, our goal was to assess the risk of bleeding in real-life conditions. Other complications (infections or fistulas) were not studied because we anticipated that most of these complications would not be coded and are not reported in most studies. We did not have information regarding the use of antiplatelet agents or anticoagulants (they are usually stopped before biopsy [23]). Some minor bleedings were certainly missed: in France, postbiopsy imaging is not universally done, especially when the fall in hemoglobin is minor and when no pain is present (23). Finally, we excluded from the analysis kidney transplant recipients and transjugular kidney biopsies.
In conclusion, the risk of bleeding following percutaneous native kidney biopsy is not negligible and is associated with a twofold higher risk of death. It varies widely according to patient characteristics. A bleeding risk score can be helpful to guide the decision of kidney biopsy (including the choice of no biopsy) and the choice of the most adequate procedure (percutaneous versus transjugular and inpatient versus outpatient), and it can be used as a tool for patients and physicians to facilitate shared decision making (27,28).
Disclosures
L. Fauchier reports personal fees from Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
Dr. Jean-Michel Halimi designed the study; Dr. Arnaud Bisson, Dr. Laurent Fauchier, Dr. Leslie Grammatico-Guillon, and Dr. Jean-Michel Halimi performed data analysis; Dr. Julien Herbert performed data management and analysis; and Dr. Christelle Barbet, Dr. Arnaud Bisson, Dr. Matthias Buchler, Dr. Laurent Fauchier, Dr. Philippe Gatault, Dr. Leslie Grammatico-Guillon, Dr. Jean-Michel Halimi, Dr. Hélène Longuet, and Dr. Bénédicte Sautenet wrote the paper.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14721219/-/DCSupplemental.
Supplemental Figure 1. Calibration plots of risk model.
Supplemental Figure 2. Major bleeding risk.
Supplemental Figure 3. Proportion of major bleeding according to simplified risk score and simplified score: area under receiver operating characteristic curve (continuous score).
Supplemental Figure 4. Timing of death after percutaneous kidney biopsy and timing of transfusion after percutaneous kidney biopsy.
Supplemental Table 1. International Classification of Diseases, Tenth Revision codes.
Supplemental Table 2. Simplified score: Parameters associated with major bleeding.
Supplemental Table 3. Risk factors for death within 30 days of kidney biopsy.
References
- 1.Hogan J, Mocanu M, Berns J: The native kidney biopsy: Update and evidence for best practice. Clin J Am Soc Nephrol 11: 354–362, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corapi K, Chen J, Balk E, Gordon C: Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis 60: 62–73, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Whittier W, Korbet S: Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15: 142–147, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ameda S, Kuroda H, Yamada M, Sato K, Miura S, Sakano H, Shibata T, Uemura N, Abe T, Fujii S, Maeda M, Fujita M, Kobune M, Kato J: Thrombotic microangiopathy due to malignant hypertension complicated with late-onset bleeding after renal biopsy. Rinsho Ketsueki 58: 637–642, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Krejčí K, Černá M, Žamboch K, Orság J, Klíčová A, Zadražil J: Late rupture of lumbar artery as an unusual complication after renal biopsy - case report. Urol Int 98: 112–114, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Korbet S, Volpini K, Whittier W: Percutaneous renal biopsy of native kidneys: A single-center experience of 1,055 biopsies. Am J Nephrol 39: 153–162, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Fülöp T, Alemu B, Dossabhoy N, Bain J, Pruett D, Szombathelyi A, Dreisbach A, Tapolyai M: Safety and efficacy of percutaneous renal biopsy by physicians-in-training in an academic teaching setting. South Med J 107: 520–525, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Simard-Meilleur M, Troyanov S, Roy L, Dalaire E, Brachemi S: Risk factors and timing of native kidney biopsy complications. Nephron Extra 4: 42–49, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lees J, McQuarrie E, Mordi N, Geddes C, Fox J, Mackinnon B: Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J 10: 573–577, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantry A, Deneux-Tharaux C, Cans C, Ego A, Quantin C, Bouvier-Colle M; GRACE study group : Hospital discharge data can be used for monitoring procedures and intensive care related to severe maternal morbidity. J Clin Epidemiol 64: 1014–1022, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Lorgis L, Cottenet J, Molins G, Benzenine E, Zeller M, Aube H, Touzery C, Hamblin J, Gudjoncik A, Cottin Y, Quantin C: Outcomes after acute myocardial infarction in HIV-infected patients: Analysis of data from a French nationwide hospital medical information database. Circulation 127: 1767–1774, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Fauchier L, Clementy N, Pelade C, Collignon C, Nicolle E, Lip G: Patients with ischemic stroke and incident atrial fibrillation: A nationwide cohort study. Stroke 46: 2432–2437, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Fauchier L, Chaize G, Gaudin A, Vainchtock A, Rushton-Smith S, Cotté F: Predictive ability of HAS-BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation. A French nationwide cross-sectional study. Int J Cardiol 217: 85–91, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Bisson A, Bodin A, Clementy N, Babuty D, Lip G, Fauchier L: Prediction of incident atrial fibrillation according to gender in patients with ischemic stroke from a nationwide cohort. Am J Cardiol 121: 437–444, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen V, Michel M, Eltchaninoff H, Gilard M, Dindorf C, Iung B, Mossialos E, Cribier A, Vahanian A, Chevreul K, Messika-Zeitoun D: Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol 71: 1614–1627, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski T, Peterson J, Gold J: Validation of a combined comorbidity index. J Clin Epidemiol 47: 1245–1251, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Segal J, Chang H, Du Y, Walston J, Carlson M, Varadhan R: Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care 55: 716–722, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beiser A, D’Agostino R Sr., Seshadri S, Sullivan L, Wolf P: Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med 19: 1495–1522, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones D, Wang T, Leip E, Larson M, Levy D, Vasan R, D’Agostino R, Massaro J, Beiser A, Wolf P, Benjamin E: Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation 110: 1042–1046, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Tøndel C, Vikse B, Bostad L, Svarstad E: Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 7: 1591–1597, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad N, Kumar S, Manjunath R, Bhadauria D, Kaul A, Sharma R, Gupta A, Lal H, Jain M, Agrawal V: Real-time ultrasound-guided percutaneous renal biopsy with needle guide by nephrologists decreases post-biopsy complications. Clin Kidney J 8: 151–156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moledina D, Luciano R, Kukova L, Chan L, Saha A, Nadkarni G, Alfano S, Wilson F, Perazella M, Parikh C: Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol 13: 1633–1640, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollée G, Martinez F, Moulin B, Meulders Q, Rougier J, Baumelou A, Glotz D, Subra J, Ulinski T, Vrigneaud L, Brasseur J, Martin L, Daniel L, Kourilsky O, Deteix P, Sie P, Ronco P, Houillier P: Renal biopsy practice in France: Results of a nationwide study. Nephrol Dial Transplant 25: 3579–3585, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Samad F, Ruf W: Inflammation, obesity, and thrombosis. Blood 122: 3415–3422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrizzo A, Izzo C, Oliveti M, Alfano A, Virtuoso N, Capunzo M, Di Pietro P, Calabrese M, De Simone E, Sciarretta S, Frati G, Migliarino S, Damato A, Ambrosio M, De Caro F, Vecchione C: The main determinants of diabetes mellitus vascular complications: Endothelial dysfunction and platelet hyperaggregation. Int J Mol Sci 19: E2968, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein P, Beemath A, Olson R: Obesity as a risk factor in venous thromboembolism. Am J Med 118: 978–980, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Freidin N, O’Hare A, Wong S: Person-centered care for older adults with kidney disease: Core curriculum 2019. Am J Kidney Dis 74: 407–416, 2019. [DOI] [PubMed] [Google Scholar]
- 28.Woodhouse K, Tremont K, Vachani A, Schapira M, Vapiwala N, Simone C 2nd, Berman A: A review of shared decision-making and patient decision aids in radiation oncology. J Cancer Educ 32: 238–245, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.